Induction of Mandibular Cortical Bone Defects to Study Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Use of Animals for Study
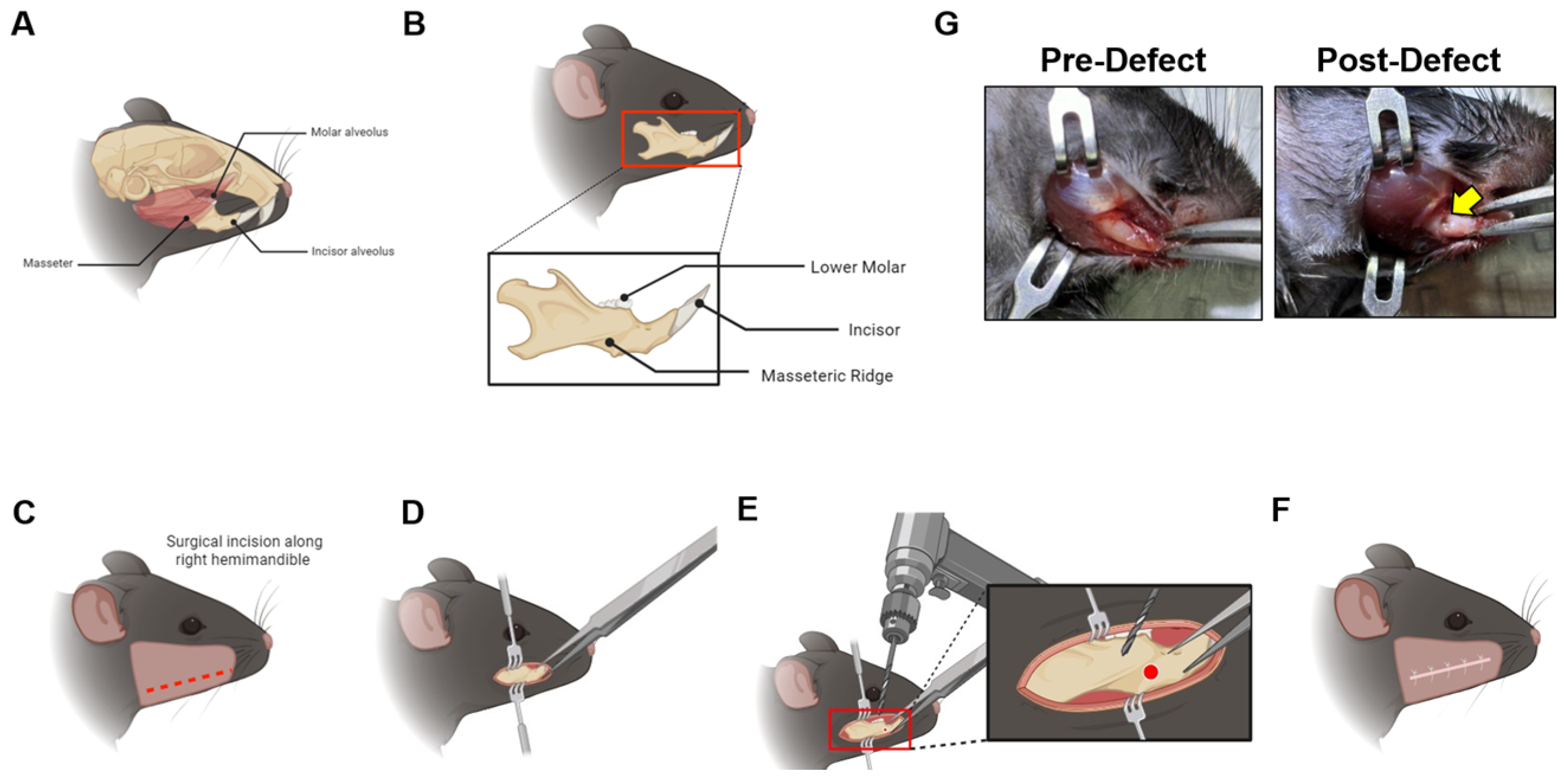
2.2. Mandible Defect Generation
2.3. Micro-Computed Tomography of the Bone Defect Site
2.4. Image Reconstruction of the Mandible
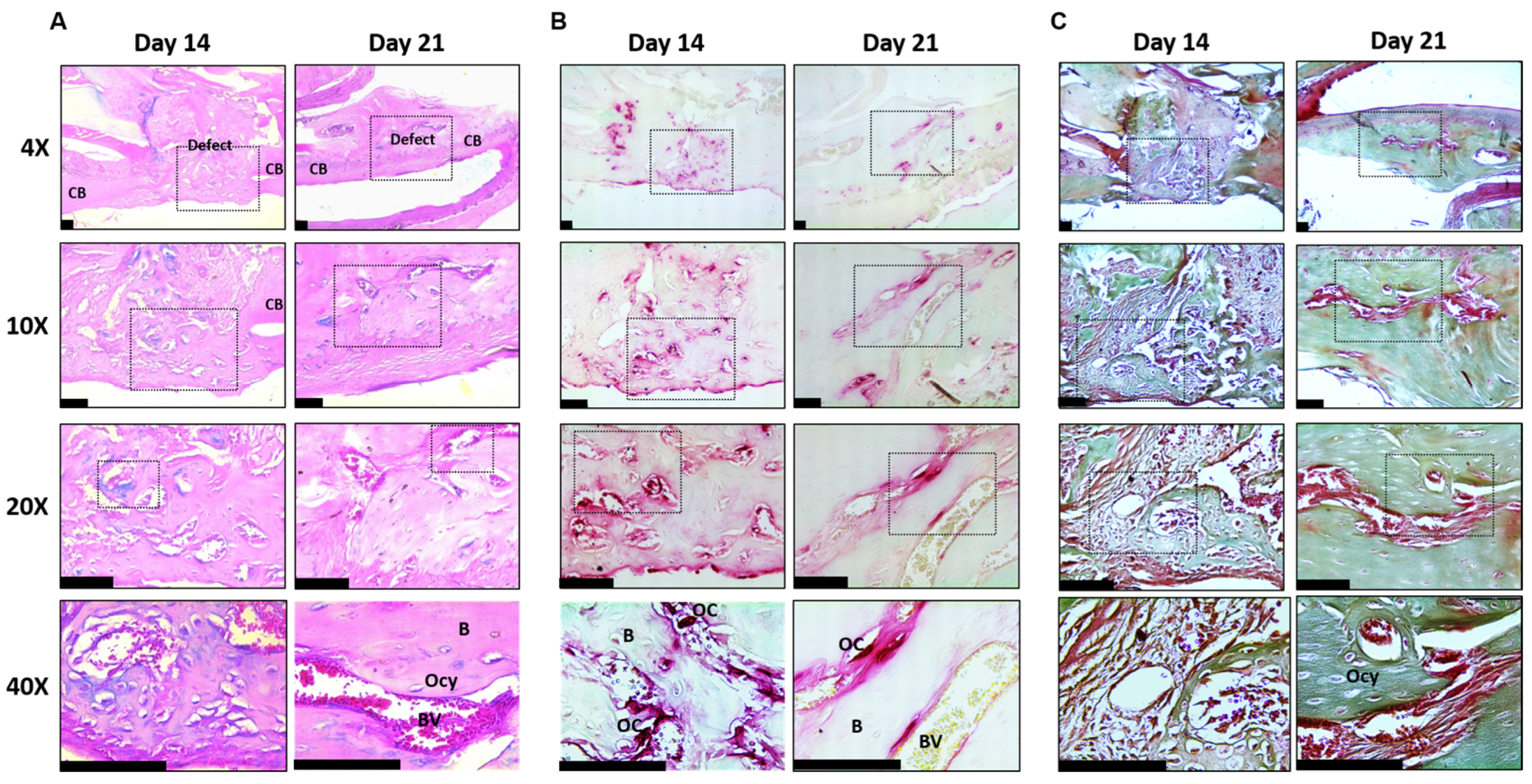
2.5. Mandible Defect Histology
2.6. Statistics
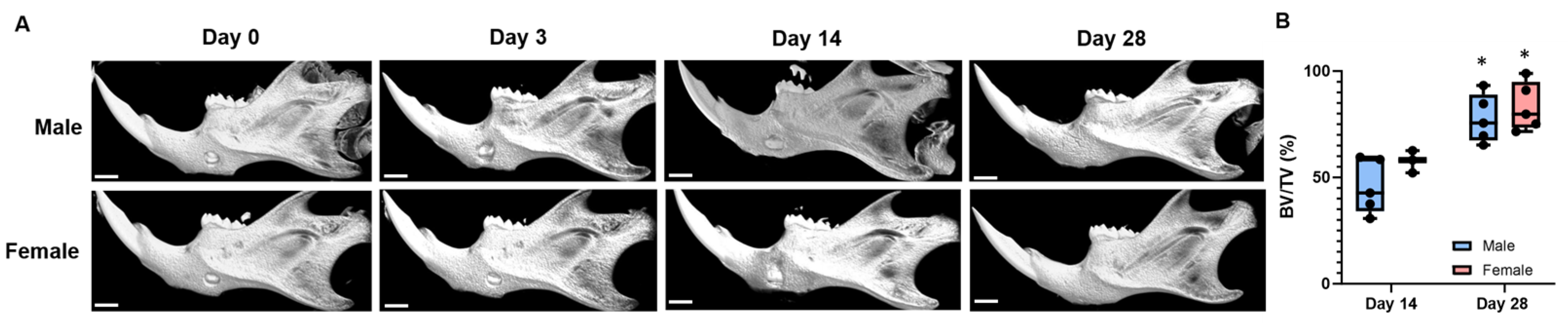
3. Results
Mandible Defects Heal via Intramembranous Ossification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghimire, S.; Miramini, S.; Edwards, G.; Rotne, R.; Xu, J.; Ebeling, P.; Zhang, L. The investigation of bone fracture healing under intramembranous and endochondral ossification. Bone Rep. 2021, 14, 100740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko, F.C.; Sumner, D.R. How faithfully does intramembranous bone regeneration recapitulate embryonic skeletal development? Dev. Dyn. 2021, 250, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, T.H.; Longaker, M.T.; Chan, C.K.F. A Revised Perspective of Skeletal Stem Cell Biology. Front. Cell Dev. Biol. 2019, 7, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bianco, P.; Robey, P.G. Skeletal stem cells. Development 2015, 142, 1023–1027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bok, S.; Sun, J.; Greenblatt, M.B. Are osteoblasts multiple cell types? A new diversity in skeletal stem cells and their derivatives. J. Bone Miner. Res. 2024, 39, 1386–1392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aghali, A. Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy. Cells. 2021, 10, 2993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbay, E.; Aydogan, F. Reconstruction of isolated mandibular bone defects with non-vascularized corticocancellous bone autograft and graft viability. Auris Nasus Larynx 2014, 41, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Covani, U. Maxillary alveolar ridge reconstruction with nonvascularized autogenous block bone: Clinical results. J. Oral Maxillofac. Surg. 2007, 65, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dumanian, Z.P.; Tollemar, V.; Ye, J.; Lu, M.; Zhu, Y.; Liao, J.; Ameer, G.A.; He, T.C.; Reid, R.R. Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS ONE 2017, 12, e0172327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klement, K.A.; Black, J.S.; Denny, A.D. Versatility of Distraction Osteogenesis for the Craniofacial Skeleton. J. Craniofacial Surg. 2016, 27, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Winters, R.; Tatum, S.A. Craniofacial distraction osteogenesis. Facial Plast. Surg. Clin. N. Am. 2014, 22, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.W.; Carpio, L.R.; McGee-Lawrence, M.E.; Castillejo Becerra, C.; Amanatullah, D.F.; Ta, L.E.; Otero, M.; Goldring, M.B.; Kakar, S.; Westendorf, J.J. Phlpp1 facilitates post-traumatic osteoarthritis and is induced by inflammation and promoter demethylation in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karkache, I.Y.; Molstad, D.H.; Vu, E.; Jensen, E.D.; Bradley, E.W. Phlpp1 Expression in Osteoblasts Plays a Modest Role in Bone Homeostasis. JBMR Plus 2023, 7, e10806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattson, A.M.; Begun, D.L.; Molstad, D.H.H.; Meyer, M.A.; Oursler, M.J.; Westendorf, J.J.; Bradley, E.W. Deficiency in the phosphatase PHLPP1 suppresses osteoclast-mediated bone resorption and enhances bone formation in mice. J. Biol. Chem. 2019, 294, 11772–11784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molstad, D.H.H.; Zars, E.; Norton, A.; Mansky, K.C.; Westendorf, J.J.; Bradley, E.W. Hdac3 deletion in myeloid progenitor cells enhances bone healing in females and limits osteoclast fusion via Pmepa1. Sci. Rep. 2020, 10, 21804. [Google Scholar] [CrossRef] [PubMed]
- McGee-Lawrence, M.E.; Razidlo, D.F. Induction of fully stabilized cortical bone defects to study intramembranous bone regeneration. Methods Mol. Biol. 2015, 1226, 183–192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.K.; Kim, Y.S.; Oh, H.S.; Yang, K.H.; Kim, E.C.; Chi, J.G. Prenatal development of the human mandible. Anat. Rec. 2001, 263, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Parada, C.; Chai, Y. Mandible and Tongue Development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behr, B.; Leucht, P.; Longaker, M.T.; Quarto, N. Fgf-9 is required for angiogenesis and osteogenesis in long bone repair. Proc. Natl. Acad. Sci. USA 2010, 107, 11853–11858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.X.; Zhang, G.; Pan, X.H.; Liu, Z.; Zheng, L.Z.; Chan, C.W.; Lee, K.M.; Cao, Y.P.; Li, G.; Wei, L.; et al. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect model. Bone 2011, 48, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Katae, Y.; Tanaka, S.; Sakai, A.; Nagashima, M.; Hirasawa, H.; Nakamura, T. Elcatonin injections suppress systemic bone resorption without affecting cortical bone regeneration after drill-hole injuries in mice. J. Orthop. Res. 2009, 27, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Monfoulet, L.; Rabier, B.; Chassande, O.; Fricain, J.C. Drilled hole defects in mouse femur as models of intramembranous cortical and cancellous bone regeneration. Calcif. Tissue Int. 2010, 86, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tanaka, S.; Sakai, A.; Ninomiya, T.; Arai, Y.; Nakamura, T. Deficiency of vitamin A delays bone healing process in association with reduced BMP2 expression after drill-hole injury in mice. Bone 2010, 47, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Paccione, M.F.; Warren, S.M.; Spector, J.A.; Greenwald, J.A.; Bouletreau, P.J.; Longaker, M.T. A mouse model of mandibular osteotomy healing. J. Craniofacial Surg. 2001, 12, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, L.; Xia, L.; Fang, B. Establishment of a C57BL/6 Mandibular Critical-Size Bone Defect Model. J. Craniofacial Surg. 2021, 32, 2562–2565. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Park, S.Y.; Bradley, E.W.; Mansky, K.; Tasca, A. Mouse mandibular-derived osteoclast progenitors have differences in intrinsic properties compared with femoral-derived progenitors. JBMR Plus 2024, 8, ziae029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kingsmill, V.J.; McKay, I.J.; Ryan, P.; Ogden, M.R.; Rawlinson, S.C. Gene expression profiles of mandible reveal features of both calvarial and ulnar bones in the adult rat. J. Dent. 2013, 41, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yahara, Y.; Nguyen, T.; Ishikawa, K.; Kamei, K.; Alman, B.A. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development 2022, 149, dev199908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, E.K.; Kim, G.; Shimak, M.J.; Karkache, I.Y.; Koroth, J.; Chavez, E.; Mitchell, S.; Clark, R.B.; Mansky, K.C.; Bradley, E.W. Induction of Mandibular Cortical Bone Defects to Study Bone Regeneration. Endocrines 2025, 6, 9. https://doi.org/10.3390/endocrines6010009
Vu EK, Kim G, Shimak MJ, Karkache IY, Koroth J, Chavez E, Mitchell S, Clark RB, Mansky KC, Bradley EW. Induction of Mandibular Cortical Bone Defects to Study Bone Regeneration. Endocrines. 2025; 6(1):9. https://doi.org/10.3390/endocrines6010009
Chicago/Turabian StyleVu, Elizabeth K., Grant Kim, Mitchell J. Shimak, Ismael Y. Karkache, Jinsha Koroth, Emily Chavez, Samuel Mitchell, Rachel B. Clark, Kim C. Mansky, and Elizabeth W. Bradley. 2025. "Induction of Mandibular Cortical Bone Defects to Study Bone Regeneration" Endocrines 6, no. 1: 9. https://doi.org/10.3390/endocrines6010009
APA StyleVu, E. K., Kim, G., Shimak, M. J., Karkache, I. Y., Koroth, J., Chavez, E., Mitchell, S., Clark, R. B., Mansky, K. C., & Bradley, E. W. (2025). Induction of Mandibular Cortical Bone Defects to Study Bone Regeneration. Endocrines, 6(1), 9. https://doi.org/10.3390/endocrines6010009






