How Different Treatments for Acromegaly Modulate Sleep Quality: A Psychometric Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Diagnosis of Acromegaly
Hormonal Assays
2.3. Sleep Assessment
2.4. Study Procedure
2.5. Statistical Analysis
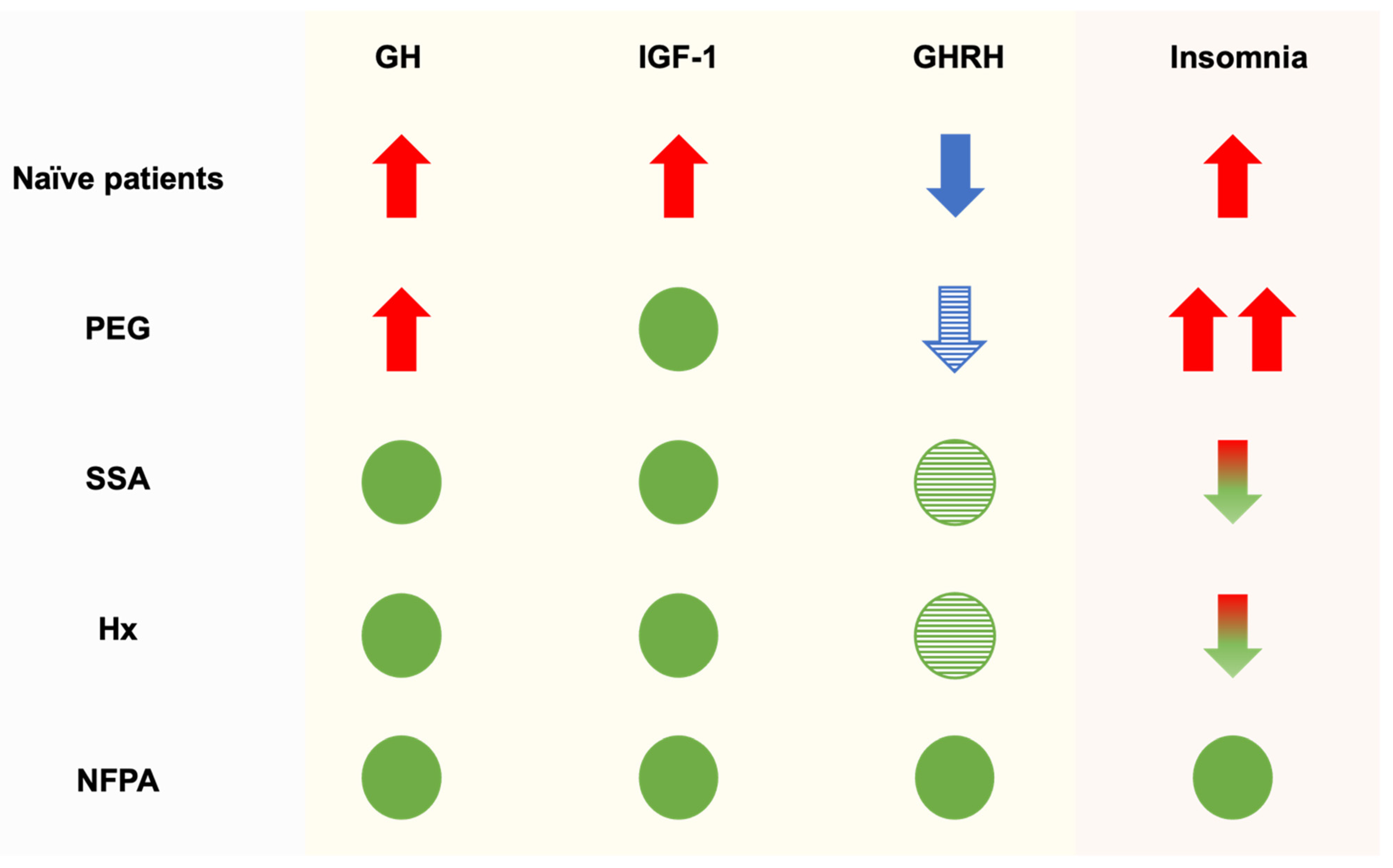
3. Results
3.1. Clinical and Biochemical Features
3.2. Sleep Evaluation: Baseline Sleep Assessment and Group Comparison
3.3. Sleep Evaluation: Treatment-Based Comparison at Last Evaluation
3.4. Sleep Evaluation: Treatment-Based Sleep Characterization
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dineen, R.; Stewart, P.M.; Sherlock, M. Acromegaly—Diagnosis and Clinical Management. QJM Int. J. Med. 2016, 110, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Barkhoudarian, G.; Beckers, A.; Ben-Shlomo, A.; Biermasz, N.; Biller, B.; Boguszewski, C.; Bolanowski, M.; Bollerslev, J.; Bonert, V.; et al. Multidisciplinary management of acromegaly: A consensus. Rev. Endocr. Metab. Disord. 2020, 21, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; Parkinson, C.; Stevens, E.C.; Trainer, P.J. Growth Hormone Receptor Antagonists: Discovery, Development, and Use in Patients with Acromegaly. Endocr. Rev. 2002, 23, 623–646. [Google Scholar] [CrossRef]
- Tritos, N.A.; Chanson, P.; Jimenez, C.; King, D.; Jönsson, P.J.; Klibanski, A.; Biller, B.M.K. Effectiveness of first-line pegvisomant monotherapy in acromegaly: An ACROSTUDY analysis. Eur. J. Endocrinol. 2017, 176, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Bidlingmaier, M.; Bailey, J.; Erickson, D.; Sandroni, P. A Pegylated Growth Hormone Receptor Antagonist, Pegvisomant, Does Not Enter the Brain in Humans. J. Clin. Endocrinol. Metab. 2010, 95, 3844–3847. [Google Scholar] [CrossRef]
- Song, Y.H.; Yoon, J.; Lee, S.H. The role of neuropeptide somatostatin in the brain and its application in treating neurological disorders. Exp. Mol. Med. 2021, 53, 328–338. [Google Scholar] [CrossRef]
- Bray, D.P.; Mannam, S.; Rindler, R.S.; Quillin, J.W.; Oyesiku, N.M. Surgery for acromegaly: Indications and goals. Front. Endocrinol. 2022, 13, 924589. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, N.; Min, K.T.; Kim, E.H.; Oh, H.; Choi, S.H. Sleep disturbance and delirium in patients with acromegaly in the early postoperative period after transsphenoidal pituitary surgery. Medicine 2020, 99, e23157. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Guo, J.; Wang, L.; Zhao, H.; Wang, Y.; Wang, J.; Sun, X.; Jiang, W.; Liu, G.; et al. Sleep quality in acromegaly and changes after transsphenoidal surgery: A prospective longitudinal study. Sleep Med. 2020, 67, 164–170. [Google Scholar] [CrossRef]
- Cho, J.; Kim, J.H.; Kim, Y.H.; Lee, J. Obstructive Sleep Apnea Screening and Effects of Surgery in Acromegaly: A Prospective Study. Endocrinol. Metab. 2024, 39, 641–652. [Google Scholar] [CrossRef]
- Peterfi, Z.; McGinty, D.; Sarai, E.; Szymusiak, R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R147–R156. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Plat, L. Physiology of growth hormone secretion during sleep. J. Pediatr. 1996, 128, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.E.; Locatelli, V.; Cocchi, D. Neuroendocrine Control of Growth Hormone Secretion. Physiol. Rev. 1999, 79, 511–607. [Google Scholar] [CrossRef] [PubMed]
- Parolin, M.; Dassie, F.; Alessio, L.; Wennberg, A.; Rossato, M.; Vettor, R.; Maffei, P.; Pagano, C. Obstructive Sleep Apnea in Acromegaly and the Effect of Treatment: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2020, 105, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.M.; Killick, R.; Keenan, D.M.; Baxter, R.C.; Veldhuis, J.D.; Liu, P.Y. Continuous Positive Airway Pressure Increases Pulsatile Growth Hormone Secretion and Circulating Insulin-like Growth Factor-1 in a Time-Dependent Manner in Men With Obstructive Sleep Apnea: A Randomized Sham-Controlled Study. Sleep 2014, 37, 733–741. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485. [Google Scholar] [CrossRef]
- Gassino, G.; Cicolin, A.; Erovigni, F.; Carossa, S.; Preti, G. Obstructive sleep apnea, depression, and oral status in elderly occupants of residential homes. Int. J. Prosthodont. 2005, 18, 316–322. [Google Scholar]
- Giustina, A.; Barkan, A.; Beckers, A.; Biermasz, N.; Biller, B.M.K.; Boguszewski, C.; Bolanowski, M.; Bonert, V.; Bronstein, M.D.; Casanueva, F.F.; et al. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J. Clin. Endocrinol. Metab. 2020, 105, e937–e946. [Google Scholar] [CrossRef]
- Somatropin WHO International Standard 98/574. Available online: https://nibsc.org/products/brm_product_catalogue/detail_page.aspx?catid=98/574 (accessed on 4 September 2024).
- WHO International Standard Insulin-Like Growth Factor-I, Recombinant, Human, for Immunoassay NIBSC Code: 02/254. Available online: https://nibsc.org/documents/ifu/02-254.pdf (accessed on 4 September 2024).
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford: New York, NY, USA, 1993. [Google Scholar]
- Castronovo, V.; Galbiati, A.; Marelli, S.; Brombin, C.; Cugnata, F.; Giarolli, L.; Anelli, M.M.; Rinaldi, F.; Ferini-Strambi, L. Validation study of the Italian version of the Insomnia Severity Index (ISI). Neurol. Sci. 2016, 37, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Richardson, G.; Roehrs, T.; Scofield, H.; Roth, T. Ford Insomnia Response to Stress Test; American Psychological Association: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Palagini, L.; Bruno, R.M.; Paolo, T.; Caccavale, L.; Gronchi, A.; Mauri, M.; Riemann, D.; Drake, C.L. Association between Stress-Related Sleep Reactivity and Metacognitive Beliefs about Sleep in Insomnia Disorder: Preliminary Results. Behav. Sleep Med. 2016, 14, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Vignatelli, L.; Plazzi, G.; Barbato, A.; Ferini-Strambi, L.; Manni, R.; Pompei, F.; D’Alessandro, R. Italian version of the Epworth sleepiness scale: External validity. Neurol. Sci. 2003, 23, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar] [CrossRef]
- Obal, F.; Krueger, J.M. GHRH and sleep. Sleep Med. Rev. 2004, 8, 367–377. [Google Scholar] [CrossRef]
- Zhang, J.; Obál, F.; Zheng, T.; Fang, J.; Taishi, P.; Krueger, J.M. Intrapreoptic Microinjection of GHRH or Its Antagonist Alters Sleep in Rats. J. Neurosci. 1999, 19, 2187–2194. [Google Scholar] [CrossRef]
- Chennaoui, M.; Léger, D.; Gomez-Merino, D. Sleep and the GH/IGF-1 axis: Consequences and countermeasures of sleep loss/disorders. Sleep Med. Rev. 2020, 49, 101223. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez de Sevilla, M.E.; Fernandez, A.M.; Munive, V.; Martinez-Rachadell, L.; Nuñez, A.; Aleman, I.T. Insulin-like growth factor I modulates sleep through hypothalamic orexin neurons. FASEB J. 2020, 34, 15975–15990. [Google Scholar] [CrossRef]
| Demographic and Clinical Features | Acromegaly (n = 27) | NFPA (n = 24) | p-Value |
|---|---|---|---|
| Demographics | |||
| Sex (female) | 55.55% (n = 15) | 62.50% (n = 15) | 0.61 |
| Age (years) | 55.15 (±10.53) | 51.08 (±11.02) | 0.16 |
| Education (years) | 10.84 (±4.35) | 11.40 (±3.00) | 0.60 |
| Clinical features | |||
| Microadenomas | 25.92% (n = 7) | 100% (n = 24) | 0.001 |
| Random serum GH concentration (µg/L) | 8.30 (±8.29) | 0.98 (±8.62) | 0.001 |
| Serum IGF-1 concentration (µg/L) | 521.34 (±233.72) | 174.11 (±125.26) | 0.01 |
| IGF-1 index | 2.68 (±1.17) | 0.49 (±0.27) | 0.01 |
| Hypopituitarism | 25.92% (n = 7) | 4.16% (n = 1) | 0.01 |
| BMI | 26.5 (±1.70) | ||
| Treatments | |||
| Surgery alone | 18.52% (n = 5) | ||
| Surgery and somatostatin analogues | 7.40% (n = 2) | ||
| Surgery and pegvisomant | 44.44% (n = 12) | ||
| Somatostatin analogues alone | 29.68% (n = 8) | ||
| Evaluation timelines | |||
| Biochemical control (months) | 12 (±1) | ||
| Last psychometric evaluation (months) | 44 (±11) |
| Sleep Questionnaires | Acromegaly (n = 27) | NFPA (n = 24) | p-Value | Effect Size |
|---|---|---|---|---|
| PSQI | 7.79 (±3.92) | 2.88 (±3.09) | 0.001 | 1.381 |
| ISI | 10.79 (±7.18) | 1.67 (±2.55) | 0.001 | 1.654 |
| FIRST | 19.57 (±8.76) | 16.79 (±7.14) | 0.224 | NA |
| ESS | 6.79 (±4.83) | 3.58 (±4.01) | 0.013 | 0.719 |
| Sleep Questionnaires | Acromegaly (n = 27) | NFPA (n = 24) | p-Value |
|---|---|---|---|
| PSQI | 74.07% (n = 20) | 16.66% (n = 4) | <0.001 |
| ISI | 70,37% (n = 19) | 4.16% (n = 1) | <0.001 |
| FIRST | 48.14% (n = 13) | 33.33% (n = 8) | 0.283 |
| ESS | 25.29% (n = 7) | 8.33% (n = 2) | 0.100 |
| Sleep Questionnaires | Sample Comparison | 95% Confidence Interval | |||||
|---|---|---|---|---|---|---|---|
| MD | SD | p-Value | Lower Bound | Upper Bound | |||
| PSQI | PEG | SSA | 5.669 | 6.629 | 0.001 * | 2.414 | 8.924 |
| Hx | 4.735 | 8.010 | 0.016 * | 0.806 | 8.665 | ||
| Hx | SSA | 0.933 | 8.368 | 0.838 | −3.184 | 5.050 | |
| ISI | PEG | SSA | 9.913 | 9.141 | 0.000 * | 5.412 | 14.414 |
| Hx | 11.463 | 10.662 | 0.001 * | 6.214 | 16.712 | ||
| Hx | SSA | −1.550 | 11.425 | 0.770 | −7.171 | 4.071 | |
| FIRST | PEG | SSA | −1.592 | 14.912 | 0.998 | −7.524 | 7.205 |
| Hx | −5.909 | 17.291 | 0.213 | −14.458 | 2.639 | ||
| Hx | SSA | 5.750 | 18.291 | 0.266 | −3.286 | 14.786 | |
| ESS | PEG | SSA | −1.210 | 11.926 | 0.863 | −7.119 | 4.697 |
| Hx | −3.996 | 14.463 | 0.355 | −11.169 | 3.176 | ||
| Hx | SSA | 2.785 | 15.707 | 0.643 | −5.001 | 10.572 | |
| Sleep Questionnaires | First Evaluation | Last Evaluation | p-Value | Effect Size |
|---|---|---|---|---|
| Surgery alone (n = 5) | ||||
| PSQI | 8.20 (±4.76) | 6.60 (±1.34) | 0.456 | NA |
| ISI | 13.80 (±8.95) | 4.20 (±2.75) | 0.038 | 0.913 |
| FIRST | 16.00 (±5.47) | 20.00 (±9.40) | 0.237 | NA |
| ESS | 3.00 (±2.00) | 9.50 (±5.26) | 0.109 | NA |
| Somatostatin analogues (n = 9) | ||||
| PSQI | 6.67 (±4.30) | 5.67 (±3.60) | 0.184 | NA |
| ISI | 11.00 (±7.50) | 5.75 (±3.49) | 0.046 | 0.777 |
| FIRST | 21.50 (±9.81) | 14.25 (±3.53) | 0.076 | NA |
| ESS | 7.75 (±5.28) | 6.25 (±5.06) | 0.358 | NA |
| Pegvisomant (n = 12) | ||||
| PSQI | 8.28 (±3.56) | 11.33 (±2.83) | 0.028 | 1.002 |
| ISI | 9.10 (±4.87) | 15.66 (±4.49) | 0.009 | 1.398 |
| FIRST | 14.94 (±5.31) | 14.09 (±6.23) | 0.627 | NA |
| ESS | 7.62 (±4.93) | 5.50(±4.59) | 0.325 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfì, G.; Menicucci, D.; Ciampa, D.A.; Di Giura, V.; Marconcini, G.; Urbani, C.; Bogazzi, F.; Gemignani, A. How Different Treatments for Acromegaly Modulate Sleep Quality: A Psychometric Study. Endocrines 2024, 5, 408-417. https://doi.org/10.3390/endocrines5030030
Alfì G, Menicucci D, Ciampa DA, Di Giura V, Marconcini G, Urbani C, Bogazzi F, Gemignani A. How Different Treatments for Acromegaly Modulate Sleep Quality: A Psychometric Study. Endocrines. 2024; 5(3):408-417. https://doi.org/10.3390/endocrines5030030
Chicago/Turabian StyleAlfì, Gaspare, Danilo Menicucci, Dalì Antonia Ciampa, Vito Di Giura, Giulia Marconcini, Claudio Urbani, Fausto Bogazzi, and Angelo Gemignani. 2024. "How Different Treatments for Acromegaly Modulate Sleep Quality: A Psychometric Study" Endocrines 5, no. 3: 408-417. https://doi.org/10.3390/endocrines5030030
APA StyleAlfì, G., Menicucci, D., Ciampa, D. A., Di Giura, V., Marconcini, G., Urbani, C., Bogazzi, F., & Gemignani, A. (2024). How Different Treatments for Acromegaly Modulate Sleep Quality: A Psychometric Study. Endocrines, 5(3), 408-417. https://doi.org/10.3390/endocrines5030030








