Abstract
Background: The prevalence of type 2 diabetes mellitus (T2DM) has been steadily increasing over the past few decades, largely due to the rise in obesity rates. Bariatric surgery is a gastrointestinal surgical treatment focused on achieving weight loss in individuals with obesity. A more recent and growing body of literature has shown that improvements in glycemic control and insulin sensitivity and even the remission of T2DM can be seen in patients with obesity and T2DM (“diabesity”), before significant weight loss is achieved, justifying the modification of the terminology from bariatric to metabolic and bariatric surgery (BMS). Main Results: This narrative review provides an overview of the latest literature on BMS for diabesity, discussing key publications and exploring controversial and diverging hypotheses. Robust scientific evidence supporting the use of BMS as a treatment for diabesity has been garnered and new venues are being explored, suggesting the novel and complementary role of the latest generation of incretin-based pharmacotherapy. Conclusions: BMS has emerged as a valuable treatment option for patients with diabesity, offering significant improvements in glycemic control, weight loss, and overall health. The limitations of the currently available and reviewed literature include the flawed knowledge of the mechanisms of action and long-term effects of BMS for the treatment of diabesity. Further studies are also warranted to refine the patient selection criteria and optimal surgical techniques and to evaluate the impact of surgery on T2DM outcomes in diverse populations. Lastly, there is a scarcity of studies investigating the efficacy of BMS against incretin-based pharmacotherapy. The non-systematic, narrative nature of this review and its implicit subjective examination and critique of the body of literature are to be considered additional and intrinsic limitations.
1. Introduction
Type II diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemia, insulin resistance, and relative insulin deficiency [1].
The prevalence of T2DM has been steadily increasing over the past few decades, hence representing a major public health concern at a global level [2]. According to the International Diabetes Federation (IDF), in 2021, diabetes affected 537 million adults worldwide and was responsible for 6.7 million related deaths in the age group of 20 to 79 years old (https://idf.org/about-diabetes/diabetes-facts-figures/; accessed on 3 September 2024). The epidemiological data provided by the World Health Organization (WHO) indicate a parallel trend between obesity and T2DM. According to these data, adult obesity has over doubled from 1990 to 2022, reaching a staggering number of 890 million obese people worldwide (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight; accessed on 3 September 2024).
To note, the WHO defines adult obesity as a condition characterized by a BMI greater than or equal to 30, while an individual is considered overweight if they have a BMI greater than or equal to 25. Based on the latter definition, in 2022, 43% of the adult population aged 18 years or older worldwide fell into the overweight category (Obesity and overweight (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight, accessed on 3 September 2024).
T2DM and obesity are strongly interlinked to the point that they are increasingly grouped under the same term, “diabesity”. This terminology was introduced in the 1970s [3], although it has gained general popularity only in recent years. The “diabesity” terminology adds value because it stresses the robust pathophysiological link between diabetes and excess body weight and thus between the obesity pandemic on one side and the raising prevalence of T2DM on the other. Moreover, the diabesity terminology suggests a more rational therapeutic approach to the management of T2DM that entails not just glycemic control but also weight loss.
Bariatric surgery is a gastrointestinal surgical treatment focused on achieving weight loss in individuals with obesity [4]. The swift metabolic impacts of these surgeries have justified the modification of the terminology from bariatric surgery to bariatric and metabolic surgery (BMS), as improvements in metabolic disorders are frequently seen prior to significant weight loss [5]. Hence, the two terms (bariatric and metabolic) are now used in conjunction to describe a variety of gastrointestinal procedures that lead simultaneously to weight loss and the amelioration or remission of T2DM and other metabolic disorders associated with obesity [5].
Several studies have shown that BMS can lead to significant improvements in glycemic control and insulin sensitivity and even the remission of T2DM in some patients [5].
Hence, BMS is emerging as a treatment for diabesity, although the mechanisms by which BMS exerts its beneficial effects on T2DM have not been fully elucidated yet [6].
The purpose of this narrative review is to provide an overview of the latest literature on BMS for diabesity. We will discuss the current state of the research field, highlight key publications, explore controversial and diverging hypotheses, and discuss the implications for future research and clinical practice.
2. Current State of Research
2.1. Methods
This narrative review was performed through the PubMed database. The timeframe for the search spanned from, 1 January 2000 to 5 October 2024. The search keywords included “bariatric surgery”, “metabolic surgery”, “diabetes mellitus type II”, and “diabesity”. Only articles written in the English language and falling into the following categories were screened for this narrative review: “clinical trial”, “meta-analysis”, “randomized controlled trial”, “review”, and “systematic review”. Additionally, the official websites of internationally recognized nonprofit medical organizations and societies such as the WHO, the IDF, the American Society of Metabolic and Bariatric Surgery (ASMBS), and the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) were used as online resources for the extraction of clinical and surgical definitions, referencing guidelines and epidemiology data.
2.2. Established and Emerging Bariatric and Metabolic Surgery Procedures
The most widely performed metabolic surgeries include vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB). VSG involves the removal of ~80% of the stomach along the greater curvature (Figure 1a). By contrast, RYGB entails the creation of a small gastric pouch out of the most cephalad portion of the stomach (gastric restriction) at a short distance from the gastro-esophageal junction and the rerouting of the intestinal tract, such that ingested food bypasses 95% of the stomach, the duodenum, and the proximal jejunum by emptying directly into the distal jejunum (Figure 1b).

Figure 1.
(a) Vertical sleeve gastrectomy (VSG). (b) Roux-en-Y gastric bypass (RYGB). (c) One anastomosis gastric bypass (OAGB). (d) Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). © Dr Levent Efe, courtesy of IFSO.
The choice of the specific BMS procedure still relies to a great extent on the personal and professional judgment of the BMS surgeon, who must take into account their experience and skills and the health history, the perioperative risk, and (to some extent) the preferences of the patient. The ASMBS has established a Public Education Committee to further the understanding of the different typologies of BMS. Their recommendations represent a valuable tool aiding both medical professionals and potential patients in making good, informed decisions. The information can be easily and publicly accessed online through the ASMBS website (Bariatric Surgery Procedures—American Society for Metabolic and Bariatric Surgery (https://asmbs.org/, accessed on 3 September 2024)). The VSG is a technically simpler and shorter procedure, and it is generally favored for patients with a lower BMI and minimal or no metabolic disease. Otherwise, VSG is often used as a bridging procedure to RYGB or single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) in high-risk patients who have multiple medical comorbidities and/or severe obesity (BMI > 50). RYGB is a technically more complex and longer procedure and tends to be favored over VSG for patients with more pronounced metabolic diseases and/or diabesity and/or a higher BMI or for those patients who have a lower BMI and no or minimal metabolic compromise but do have a pre-existing gastro-esophageal reflux disease, as VSG may worsen this condition (Bariatric Surgery Procedures—American Society for Metabolic and Bariatric Surgery (https://asmbs.org/, accessed on 3 September 2024)).
The laparoscopic gastric band, which, at some point, was the most performed procedure, has eventually lost favor over the past decade due to serious concerns about its long-term efficacy and complications. This has led to a surge in removal and conversion to VSG or other BMS procedures [7].
In recent years, newer BMS procedures have gained widespread acceptance. For instance, the technical difficulty and the risk of developing internal hernias associated with the gold standard RYGB have led to the introduction of the much simpler one anastomosis gastric bypass (OAGB) (Figure 1c). However, the greater simplicity, potential reversibility, and non-inferior outcomes of OAGB compared to RYGB must be weighed against its main pitfall, which is bile reflux [8]. SADI-S (Figure 1d) has been suggested as an alternative to OAGB and RYGB. This procedure has shown similar metabolic efficacy to OAGB and RYGB, with the even greater remission of T2DM and without the bile reflux associated with the former or the need to reconstruct the bowel continuity between the alimentary and biliary limbs associated with the latter. Moreover, SADI-S retains the principles of biliopancreatic diversion along with pylorus preservation. All of the benefits of SADI-S must be weighed against its reported higher rates of early complications and mortality [8]. Thus, further research is needed to determine which will be the next gold-standard procedure. Both procedures (OAGB and SADI-S) have been shown to reduce body weight, improve insulin sensitivity, increase insulin secretion, and lead to the remission of T2DM in a significant proportion of patients [9].
2.3. Key Scientific Evidence Supporting the Use of Bariatric and Metabolic Surgery
Recent randomized controlled trials and meta-analyses have demonstrated that BMS is more effective than medical therapy alone in achieving long-term glycemic control and weight loss in patients with T2DM and obesity, a condition that is being more frequently named “diabesity”. The randomized, controlled, single-center STAMPEDE trial involved 150 obese patients who were assigned, in a 1:1:1 ratio, to one of the three study groups, in which the effects of intensive medical therapy were compared with those of RYGB or VSG. Only 5% of the patients in the medical therapy group met the primary end point (glycated hemoglobin level ≤ 6.0%) at 3 years, as compared with 38% of those in the RYGB group (p < 0.001) and 24% of those in the VSG group (p = 0.01). Furthermore, patients undergoing BMS had greater mean percentage reductions in weight from baseline, with reductions of 24.5 ± 9.1% in the GBP group and 21.1 ± 8.9% in the VSG group, as compared with a reduction of 4.2 ± 8.3% in the medical therapy group (p < 0.001 for both comparisons). Lastly, the quality-of-life rates were significantly better in the BMS groups than in the group being managed with medical therapy [10]. These differences between the two BMS groups and the medical therapy group persisted at 5 years, although the absolute numbers were lower in each group as the criterion for the primary end point was met by two of 38 patients (5%) in the medical group vs. 14 of 49 patients (29%) and 11 of 47 patients (23%) who underwent GBP and VSG, respectively. Patients who underwent BMS had a greater mean percentage reduction from baseline in their glycated hemoglobin levels than did patients who received medical therapy alone (2.1% vs. 0.3%, p = 0.003). At 5 years, the changes from baseline observed in the BMS groups were greater than the changes witnessed in the medical therapy group with respect also to body weight, triglyceride levels, high-density lipoprotein cholesterol levels, the use of insulin, and the quality-of-life measures (p < 0.05 for all comparisons) [11].
The most recent pooled analysis, ARMMS-T2D (Alliance of Randomized Trials of Medicine vs. Metabolic Surgery in Type 2 Diabetes), conducted between May 2007 and August 2013 and including four USA-based single-center randomized trials, confirmed the positive findings of prior randomized trials indicating superior glycemic control with fewer diabetes medications and higher rates of diabetes remission in patients originally randomized to undergo BMS, compared to patients receiving medical/lifestyle interventions only.
The observational follow-up of ARMMS-T2D lasted until July 2022 and included all participants who had been randomized to undergo medical/lifestyle management or BMS (RYGB, VSG, or adjustable gastric banding, respectively) [12]. The change in hemoglobin A1c (HbA1c) from baseline to 7 years was set as the primary outcome for all participants, although data are reported for up to 12 years. A total of 262 out of 305 eligible participants (86%) were enrolled in long-term follow-up for this pooled analysis. At 7 years, HbA1c had decreased by 0.2% (95% CI, −0.5% to 0.2%) from a baseline of 8.2% in the medical/lifestyle group and by 1.6% (95% CI, −1.8% to −1.3%) from a baseline of 8.7% in the BMS group. The between-group difference was −1.4% (95% CI, −1.8% to −1.0%; p < 0.001) at 7 years and −1.1% (95% CI, −1.7% to −0.5%; p = 0.002) at 12 years. The BMS group required fewer antidiabetic medications. Diabetes remission was greater after BMS (6.2% in the medical/lifestyle group vs. 18.2% in the BMS group; p = 0.02) at 7 years and at 12 years (0.0% in the medical/lifestyle group vs. 12.7% in the BMS group; p < 0.001) [12].
Increases in liver, adipose tissue, and muscle insulin sensitivity and in β-cell function are well-known effects of weight loss. However, a large body of scientific evidence has clearly shown that BMS leads to a rapid improvement in glycemic control, enabling the discontinuation of insulin and other glucose-lowering medications, even before substantial weight loss has ensued (Table 1).

Table 1.
Mechanisms sustaining metabolic improvements after BMS.
Hence, additional mechanisms might add to both early and sustained metabolic improvements after BMS [13].
With RYGB, nutrients enter the intestine very rapidly as there is no pylorus or antrum to slow them down. With VSG, the increase in postprandial gastric pressure leads to faster gastric emptying. These changes in nutrient exposure are believed to affect the intestinal structure and function in both surgeries. Intestinal hypertrophy has been demonstrated in both human and rodent studies, with shifts in glucose metabolism towards pathways that support tissue growth and an increase in 18F-fluorodeoxyglucose uptake in multiple intestinal segments in correlation with reduced fasting blood levels of glucose [13].
Increased lipid accumulation in the liver is associated with impaired hepatic insulin sensitivity. BMS is highly effective in lowering the levels of hepatic lipids and is an effective treatment for non-alcoholic fatty liver disease [14]. In humans, reductions in the hepatic levels of triglycerides and fibrosis occur after BMS, which translate into overall improved hepatic insulin sensitivity [13].
Likewise, both short-term and long-term studies find improvements in whole-body insulin sensitivity that are not correlated with a patient’s weight loss, pointing to better adipose and skeletal muscle insulin sensitivity after BMS [13].
Higher circulating levels of glucagon are observed postprandially after BMS compared with the pre-BMS status, suggesting that BMS alters pancreatic α-cell function. However, BMS’ effects are not limited to the α-cells but are related to the β-cells too. After BMS, rapid increases in the postprandial blood levels of glucose, related to the rapid entry of nutrients into the intestine, are paralleled by rapid surges in insulin secretion that quickly return to baseline. Furthermore, fasting insulin and the total insulin output in response to intravenous glucose are reduced after BMS, consistent with the enhanced insulin sensitivity and a change in the dynamic response of insulin to nutrient ingestion.
Another major contributor to the increased postprandial plasma levels of insulin after BMS is represented by the marked increase in the plasma levels of incretin hormones, the gut peptides that are traditionally recognized to be secreted by L cells in the distal gut. The greatest interest has been displayed so far in the incretin hormone glucagon-like peptide 1 (GLP1), whose levels rise over 10-fold after BMS, although there is a host of gut peptides that also increase postprandially in patients who undergo BMS and these are the subjects of further investigation. The dramatic increase in postprandial GLP1 secretion after BMS is widely believed to have a role in mediating the improvements in glucose homeostasis that occur early on after BMS and before significant weight loss has already occurred [15].
Other incretins have also been identified to play a role in generating the beneficial metabolic effects of BMS. For instance, the plasma levels of ghrelin are substantially reduced, while the plasma levels of cholecystokinin (CCK) and gastric inhibitory peptide (GIP) are markedly increased after VSG [16].
The most prominent metabolic effect of ghrelin is the stimulation of appetite via the activation of the orexigenic hypothalamic axis and the food-intake-independent stimulation of lipogenesis, which both lead to an increase in body weight and adiposity. However, recent scientific evidence is emerging that suggests the therapeutic value of the pharmacological inhibition of ghrelin signaling by improving insulin resistance and T2DM. Hence, ghrelin may play a role in the regulation of glucose metabolism as well [17].
CCK has been traditionally regarded as a peptide with the sole purpose of triggering gallbladder contraction and regulating digestion. On the contrary, recent evidence has emerged that supports a role for CCK in the modulation of insulin secretion. For instance, it does appear that the infusion of pharmacological levels (24 pmol/kg h) of CCK in humans will stimulate insulin secretion. Hence, the pharma industry is currently looking at the development of CCK-based pharmacotherapy to treat T2DM [17]. Lastly, like GLP-1, GIP stimulates glucose-dependent insulin release and is also known to inhibit beta-cell apoptosis and promote beta-cell proliferation [18].
The plasma levels of both total and specific bile acids increase significantly after BMS [19]. Bile acids are synthesized in the liver and secreted into the intestine and function as signaling molecules at multiple target organs via FXR (expressed in the intestine, liver, adipose tissue, pancreas, and adrenal gland) and TGR5 (expressed in the gall bladder, ileum, colon, adipose tissue, liver, skeletal muscle, and immune cells) receptors. The activation of TGR5 by bile acids increases GLP-1 secretion. Hence, bile acids represent an attractive target to improve the success of bariatric surgery.
The profound changes in the intestinal anatomy and its function produced by BMS lead to a shift in the composition and diversity of the intestinal microbiome. The improved regulation of bile acid metabolism is, among others, one of the mechanisms suggested to explain the favorable effect of microbiome changes after BMS that contributes to T2DM control [20].
2.4. Optimal Timing of Bariatric and Metabolic Surgery
The optimal timing of surgery during diabetes and the selection criteria for surgery have been a matter of debate over the past decade. Some studies have suggested that earlier intervention with surgery may lead to better outcomes, while others have shown that delaying surgery can still result in significant improvements in diabetes control. Additionally, not all patients with diabesity are suitable candidates for metabolic surgery, and careful patient selection is crucial to ensure favorable outcomes.
In October 2022, the two main governing bodies in the field of BMS—the IFSO and ASMBS—decided to revise the existing 1991 National Institute of Health (NIH) consensus guidelines.
Under the newer IFSO/ASMBS 2022 guidelines, all individuals with a BMI > 35 kg/m2 may be eligible for surgery, regardless of the presence of underlying health problems, while those with a BMI > 30 may be considered for surgery in cases of diabetes (diabesity) or for those who have not been able to maintain long-lasting weight loss [21].
Furthermore, the IFSO and the ASMBS agreed that the BMI thresholds should be adjusted for the Asian population, since individuals of these ethnicities typically suffer negative health outcomes at a lower BMI.
2.5. Bariatric and Metabolic Surgery and Advancements in Pharmacotherapy
In the past few years, we have witnessed some significant advancements in the development of targeted treatments for monogenic obesity that have led to the introduction of a new generation of incretin-based therapies. This novel class of incretin agonists can be grouped into three main categories based on their single or multiple receptor targets (Figure 2) [22].
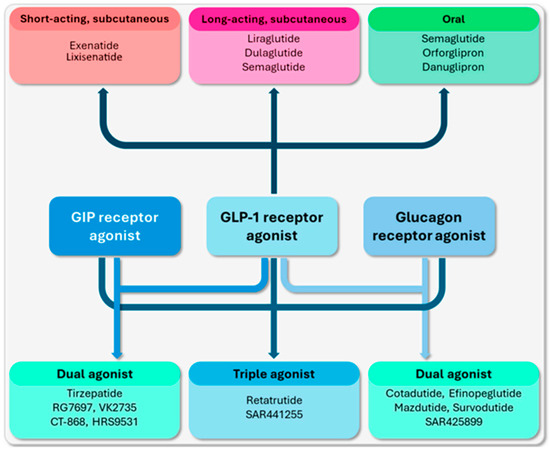
Figure 2.
Incretin-based agents that are commonly used in routine medical practice and are currently under clinical development [22].
2.5.1. Single Incretin Agonists
GLP-1 receptor agonists (GLP-1Ras), such as liraglutide and semaglutide, have been shown to be effective in weight reduction and the improvement of glycemic control by enhancing insulin secretion, delaying gastric emptying, and promoting satiety. Semaglutide can be injected subcutaneously once a week or taken as a once-daily tablet. The STEP clinical trials together provide valuable information about semaglutide’s safety, efficacy, and impact on weight loss and cardiovascular outcomes in various patient populations treated with the once-weekly subcutaneous administration of semaglutide at a dose of 2.4 mg. Roughly 15% weight loss was observed in overweight and obese, non-diabetic adults across various treatment periods, lasting up to 2 years. Conversely, a smaller but significant 9.6% weight loss was observed in patients with diabesity in the STEP-2 trial [23]. Further insights into the efficacy of first-generation GLP-1Ras have been provided very recently by an observational, retrospective cohort study based on data obtained from the electronic medical records of the largest healthcare organization in Israel. Dicker et al.’s study included 6070 subjects aged 24 years or older, who had diabesity and no prior history of ischemic heart disease, ischemic stroke, or congestive heart failure. Patients who underwent BMS and patients who received GLP-1RAs from 1 January 2008 to 31 December 2021 were matched 1:1 by age, sex, and clinical traits and followed for a median of 6.8 years (range 4.1–9.4 years). BMS was associated with greater weight loss and lesser mortality compared with GLP-1RAs among individuals with a diabetes duration of 10 years or less [24]. It is likely that the lower mortality of the surgical group of patients was mediated by the significantly greater weight loss achieved in the same.
2.5.2. Dual Incretin Agonists
GLP-1/GIP Agonists: Dual agonists that target both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors have displayed improved glycemic control and greater weight loss compared to single agonists in various clinical trials. Tirzepatide was approved in the United States and Europe in 2022 for the treatment of T2DM and is the most widely studied representative of this class of double agonists (DAs).
In the Phase 3 SURPASS-1-5 studies, which included >6000 people with T2DM, tirzepatide was associated with clinically meaningful reductions in HbA1c and weight loss when used as a monotherapy, in combination with basal insulin or in comparison to semaglutide, insulin glargine, and insulin degludec [25].
Additionally, tirzepatide significantly reduced weight in obese T2DM patients in a dose-dependent manner; by weeks 40–52 of the trials, a 5 mg dose of tirzepatide resulted in weight loss of 6.2–7.8 kg, the 10 mg dose led to weight loss of 7.8–10.7 kg, and the 15 mg dose caused weight loss of 9.5–12.9 kg [25].
A comparison between tirzapatide and BMS can be extrapolated from a mathematical modeling study that simulated the mean weight and fat loss trajectories in response to dietary restriction, semaglutide 2.4 mg, tirzepatide 10 mg, and RYGB. All interventions except dietary restriction substantially weakened the appetite feedback control circuit, resulting in an extended period of weight loss prior to the plateau. However, the simulated BMS intervention resulted in a persistent magnitude more than three-fold greater than dietary restriction and two-fold greater than tirzepatide and semaglutide. Nevertheless, no comparative data on the effects of the three simulated interventions over T2DM amelioration or remission in obese patients were generated by this study [26].
GLP-1/Glucagon Agonists: Glucagon reduces appetite and food intake while favorably altering lipid metabolism and energy expenditure, making it an attractive option for the treatment of T2DM and obesity, especially when combined with the insulinotropic effects of GLP-1. In recent years, two GLP-1/glucagon agonists underwent phase II clinical trials in human experiments, but only one of the two (cotadutide) has so far completed its development. Cotadutide effectively improved glycemic control and reduced weight in patients with diabesity while increasing insulin secretion and delaying gastric emptying, with the most common side effects being dose-dependent nausea and vomiting [27]. Furthermore, a multicenter study evaluated the effects of subcutaneously administered cotadutide in 834 participants in daily doses of 100 µg, 200 µg, and 300 µg, compared with a placebo and 1.8 mg liraglutide daily [28]. The study lasted for 54 weeks and assessed liver abnormalities and metabolic parameters in patients with diabesity. Every dose of cotadutide and liraglutide significantly reduced the HbA1c levels compared to the placebo. The highest dose of cotadutide resulted in a significantly greater reduction in weight compared to liraglutide and the placebo and a significant improvement in liver enzyme levels and parameters indicative of liver fibrosis compared to liraglutide, setting the basis for the possible use of cotadutide in the treatment of MAFLD. No comparative studies between cotadutide and BMS have so far been implemented.
2.5.3. Triple Incretin Agonists
GLP-1/GIP/Glucagon Agonists: Emerging therapies that target GLP-1, GIP, and glucagon receptors hold promise to further strengthen the metabolic benefits of single and dual therapies. A phase 1b, multicenter, double-blind, placebo-controlled, and randomized trial investigating the novel triple agonist retatrutide in patients with T2DM showed significant reductions in body weight and HbA1c by the end of the 12th week compared to baseline values, while exhibiting a safety and tolerability profile similar to that of other incretin formulations [29]. No comparative data between retatrutide and BMS are currently available.
Altogether, peptide-based therapies (single, double, or triple agonists) hold promise for the treatment of diabesity thanks to their ability to effectively regulate glucose metabolism and body weight. However, peptides often display poor stability and are susceptible to rapid degradation, which limits their therapeutic and clinical application potential.
Even though the preliminary results of the clinical trials investigating the efficacy of these novel anti-obesity medications are encouraging, long-term data on their safety, efficacy, and cardiovascular outcomes are yet to be gathered [22]. At present, the reported weight loss (greater than 10% of overall body weight in more than two thirds of clinical trial participants) are far from reaching the durable weight loss of 25% reported in the long-term studies of BMS, which are also associated with rapid, sustained improvements in complications of obesity.
Likewise, future research and development must address several concerns about the safety profiles of these medications, as the relatively benign and most common side effects associated with their use (gastrointestinal disturbances, severe nausea, and/or vomiting) may be exaggerated by the much more concerning and severe hypoglycemia due to the excessive lowering of blood sugar levels [30]. On the other hand, BMS carries the risk of gastro-intestinal disturbances both perioperatively and in the long term (nutritional deficiencies). Although its associated mortality has been steadily decreasing over time, it is not and likely will never be nil due to the inherent risk associated with a surgical intervention under general anesthesia.
In the near future, we will likely see a rising number of comparative studies exploring the efficacy of these new-generation anti-obesity medications against BMS. There is certainly a great need for this type of comparative research.
Meanwhile, a mounting body of research is suggesting a novel role of pharmacotherapy, not as an alternative but as a complementary therapy to BMS. Medications such as sodium-glucose cotransporter 2 (SGLT2) inhibitors, GLP-1 receptor agonists, and combination therapies are showing potential to maximize weight loss, improve metabolic outcomes, and lower the risk of weight regain after BMS. However, the optimal timing and duration, and which combinations of pharmacotherapy to use together with BMS, are still to be elucidated. Nonetheless, the integration of BMS with pharmacotherapy is emerging as a promising approach to managing diabesity and does provide patients with added options to achieve sustainable weight loss and better their metabolic well-being [31,32].
3. Discussion
Overall, the evidence supporting the use of BMS for diabesity is strong and continues to grow. Several long-term studies have shown that BMS can lead to sustained improvements in glycemic control, reduce the need for diabetes medications, and lower the risk of diabetes-related complications. In some cases, BMS has even resulted in the complete remission of T2DM, with the normalization of blood sugar levels without the need for medication.
The benefits of BMS extend beyond glycemic control to include improvements in cardiovascular risk factors, such as hypertension, dyslipidemia, and obstructive sleep apnea. Weight loss after BMS can also lead to improvements in quality of life, physical function, and mental health. However, it is important to note that BMS is not without risks, and although the perioperative mortality (30 days) has dramatically improved over the past two decades, it presently ranges from 0.03% to 0.2%. Likewise, the 30-day risk of serious adverse events, such as reoperation, prolonged hospitalization, and venous thromboembolism, ranges across studies from 0.8% to 5.6%. The rates of reoperation and readmission are, in general, lower for the most performed BMS (VSG) compared to the second one (RYGB) [33].
Leaks represent the most feared surgical complications and are usually experienced within 30 days, with most of them presenting in the first 7 days after BMS [34]. Leaks can affect the anastomosis (connection) between the intestine and intestine or the intestine and stomach (RYGB, OAGB, and SADI-S) or the gastric staple line (VSG). The incidence of leaks has declined over the years, similarly to the other adverse events following BMS, despite the increasing number of surgical procedures performed over the same period. Data from large, multicenter studies and database analyses indicate leak rates of 0.7–2.4% and 0.4–1.9% for VSG and RYGBP, respectively [35]. Recent advancements in endoscopic treatment have significantly decreased the need for surgical reintervention to treat this dreaded complication, as more and more cases are being treated with endoscopic management strategies such as endoscopic stenting or closure techniques (clips, suturing) by the interventional gastroenterologist or the surgical endoscopist [34].
The alteration of the gut anatomy and the disruption of gut absorption associated with BMS lead invariably to the development of micronutrient (vitamin and mineral) deficiencies, which represent the most frequent, unwanted long-term effects of BMS, requiring routine supplementation and lifelong nutritional follow-up [36].
The available literature shows that approximately one third of BMS patients regain weight or experience suboptimal weight loss within five years post-BMS as result of pre-existing pathological eating styles and psychopathological traits. Thus, interventions focusing on emotion dysregulation before and after BMS can play a pivotal role in improving its clinical outcomes [37].
The recent literature has shown gender differences among candidates for BMS in terms of emotional dysregulation and pathological eating behaviors; for instance, female patients are at higher risk for and have a significantly greater prevalence of emotional eating than their male counterparts [38]. These differences further highlight the importance of individualized care and follow-up to optimize the clinical outcomes of BMS and support the notion that a successful BMS program cannot and should not rely solely on the technical skills and surgical prowess of BMS surgeons. The complex and multi-factorial nature of diabesity and the need for regular and, in some instances, targeted follow-up interventions necessitate a multidisciplinary approach involving not only core members such as obesity physicians, BMS surgeons, dieticians, nutritionists, psychologists, and anesthesiologists but also other specialists available as needed, such as plastic surgeons or physical therapists. Although the evidence in support of the multidisciplinary team approach is still limited, the preliminary literature suggests improved quality of care when BMS is managed through this model [39].
Although the currently available literature offers a valuable body of evidence to support the use of BMS for the treatment of diabesity, it is still burdened by some significant caveats. These include the elucidation of the underlying mechanisms of action, as well as the identification of biomarkers predicting which patients are most likely to benefit from BMS and the detection of the optimal surgical techniques to maximize the outcomes, as well as the development of personalized treatment approaches. These are still a matter of debate and lack sufficient evidence. Another major caveat of the existing literature is the paucity of long-term studies that assess the durability of the metabolic benefits of BMS and evaluate the impact of surgery on diabetes-related microvascular and macrovascular complications.
The latest generation of anti-obesity medications has so far produced very promising results and has overall been accepted by a large portion of the medical community. Paradoxically, though, the great enthusiasm generated around these pharmacological interventions has somewhat limited their potential spectrum of clinical applications, meaning that, so far, they have been developed and are being investigated as a potential replacement for what is considered a more invasive treatment, namely BMS. This is difficult to comprehend, especially in consideration of the fact that the two therapeutic options (pharmacotherapy and BMS) share common mechanistic actions and both have some limitations and thus could benefit from each other. For instance, an area of special interest for the surgical community is represented by those patients who progress well or very well after BMS, but, over time, eventually regain some weight. The newer anti-obesity and anti-diabesity medications should be a critical component of the therapeutic armamentarium adopted by bariatricians and endocrinologists, to whom these patients should be referred by the BMS team. This focus on integrative and combined approaches could represent the ultimate solution to some of the long-standing challenges in achieving lasting weight loss and could virtually eliminate or greatly reduce the need for revisional BMS, which is associated with a significantly higher risk of peri- and postoperative complications in comparison to the initial BMS.
The paucity of studies investigating the efficacy of BMS head-to-head against incretin-based pharmacotherapy represents an additional limitation of the available literature. Future research should focus more on this type of comparative study to better clarify the respective roles of the two therapeutic options (BMS and pharmacotherapy) in the management of diabesity.
Our review has strengths but also some intrinsic limitations.
The founding rationale of our narrative review is based on our intent to provide an encompassing interpretation and critique of a wide variety of studies from both the medical and surgical backgrounds that holds potential for use by medical educators and researchers, as opposed to prior scientific and systematic efforts that focused on a narrow question and were conducted according to a prespecified method of selection of similar studies [40].
To our knowledge, our review is the first to explore diabesity and its interlinking pathophysiologic and epidemiologic implications with obesity, T2DM, and BMS and to discuss concurrently the role of novel incretin-based therapies as alternatives or in conjunction with BMS from a surgical perspective and with a narrative style.
The most recent and comparable scientific effort was carried out in 2018 by El Khoury et al. These authors also had a surgical background, but, with their systematic review, they aimed to examine clinical trials on the long-term effects of BMS in patients with T2DM and to evaluate the potential mechanisms behind the improvement of glycemic control [40]. The concept of diabesity and its link with BMS was not explored, nor was the role of novel incretin-based agents as an alternative or complementary therapy [40].
At the same time, one may argue that the narrative, non-systematic nature and the implicit subjective examination and critique of the reviewed body of literature associated with our review also represent its main limitations. For instance, the identification, interpretation, and analysis of the selected literature may have been biased by the surgical background of the two authors and their professional (surgical) perspective and experience.
The timeframe (20 years) and the search engine (PubMed) chosen by the authors may also be disputed for contrasting reasons. For instance, a shorter timeframe could have potentially enabled the authors to focus on those works specifically investigating the association of diabesity with BMS. Meanwhile, a greater number of search engines could have strengthened the search of the relevant literature and decreased the risk of overlooking one or more relevant articles.
4. Conclusions
BMS is a valuable treatment option for patients with diabesity, offering significant improvements in glycemic control, weight loss, and overall health. While more research is needed to fully understand the mechanisms of action and long-term effects of surgery, the current evidence supports the use of BMS as an effective and sustainable treatment for T2DM. Further studies are warranted to refine the patient selection criteria, optimize the surgical techniques, evaluate the impact of surgery on diabetes outcomes in diverse populations, as well as to better define the role of the novel pharmacotherapy in conjunction with or in comparison to BMS.
Author Contributions
Conceptualization, A.G. and P.B.; methodology, A.G.; writing—original draft preparation, A.G.; writing—review and editing, A.G. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jervell, J. Diabetes i et internasjonalt perspektiv [Diabetes in international perspective]. Tidsskr. Nor. Laegeforen 2000, 120, 2686–2689. (In Norwegian) [Google Scholar] [PubMed]
- Sims, E.A.; Danforth, E., Jr.; Horton, E.S.; Bray, G.A.; Glennon, J.A.; Salans, L.B. Endocrine and metabolic effects of experimental obesity in man. Recent Prog. Horm. Res. 1973, 29, 457–496. [Google Scholar]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Rubino, F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia 2018, 61, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Russel, S.M.; Valle, V.; Spagni, G.; Hamilton, S.; Patel, T.; Abdukadyrov, N.; Dong, Y.; Gangemi, A. Physiologic Mechanisms of Type II Diabetes Mellitus Remission Following Bariatric Surgery: A Meta-analysis and Clinical Implications. J. Gastrointest. Surg. 2020, 24, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, A.; Russel, S.; Patel, K.; Khalaf, H.; Masrur, M.; Hassan, C. Conversion to laparoscopic sleeve gastrectomy after failure of laparoscopic gastric band: A systematic review of the literature and cost considerations. Obes. Res. Clin. Pract. 2018, 12, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Márquez, M.; García-Redondo, M.; Maturana-Ibáñez, V.; Estébanez-Ferrero, B.; Fernández-Alonso, A.; Rubio-Gil, F.; Zamora Soler, J.A.; Ferrer-Ayza, M. Bile reflux and marginal ulcers after one-anastomosis gastric bypass (OAGB). A narrative review. Cir. Esp. 2023, 101 (Suppl. S4), 69–75. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, G.; Leo, S.J.; Sivagnanam, S.T.; Balaji Prasad, S.; Ravindra, C.; Rengan, V.; Arora, E.; Bindal, V. Comparison of Efficacy and Safety Between Roux-en-Y Gastric Bypass (RYGB) vs One Anastomosis Gastric Bypass (OAGB) vs Single Anastomosis Duodeno-ileal Bypass with Sleeve Gastrectomy (SADI-S): A Systematic Review of Bariatric and Metabolic Surgery. Obes. Surg. 2023, 33, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes-3-year outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes-5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Courcoulas, A.P.; Patti, M.E.; Hu, B.; Arterburn, D.E.; Simonson, D.C.; Gourash, W.F.; Jakicic, J.M.; Vernon, A.H.; Beck, G.J.; Schauer, P.R.; et al. Long-Term Outcomes of Medical Management vs Bariatric Surgery in Type 2 Diabetes. JAMA 2024, 331, 654–664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandoval, D.A.; Patti, M.E. Glucose metabolism after bariatric surgery: Implications for T2DM remission and hypoglycaemia. Nat. Rev. Endocrinol. 2023, 19, 164–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldwin, D.; Chennakesavalu, M.; Gangemi, A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg. Obes. Relat. Dis. 2019, 15, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, C.; Bojsen-Møller, K.N.; Jørgensen, N.B.; Jacobsen, S.H.; Kristiansen, V.B.; Naver, L.S.; Hansen, D.L.; Worm, D.; Holst, J.J.; Madsbad, S. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 2013, 56, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Nosso, G.; Griffo, E.; Cotugno, M.; Saldalamacchia, G.; Lupoli, R.; Pacini, G.; Riccardi, G.; Angrisani, L.; Capaldo, B. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: A one-year prospective study. Horm. Metab. Res. 2016, 48, 312–317. [Google Scholar] [CrossRef]
- Poher, A.L.; Tschöp, M.H.; Müller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathak, V.; Flatt, P.R.; Irwin, N. Cholecystokinin (CCK) and related adjunct peptide therapies for the treatment of obesity and type 2 diabetes. Peptides 2018, 100, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.-E.E.; Houten, S.M.; Bianco, A.C.; Bernier, R.; Larsen, P.R.; Holst, J.J.; Badman, M.K.; Maratos-Flier, E.; Mun, E.C.; Pihlajamaki, J.; et al. Serum bile acids are higher in humans with prior gastric bypass: Potential contribution to improved glucose and lipid metabolism. Obesity 2009, 17, 1671–1677. [Google Scholar] [CrossRef]
- Fouladi, F.; Brooks, A.E.; Fodor, A.A.; Carroll, I.M.; Bulik-Sullivan, E.C.; Tsilimigras, M.C.B.; Sioda, M.; Steffen, K.J. The role of the gut microbiota in sustained weight loss following Roux-en-Y gastric bypass surgery. Obes. Surg. 2019, 29, 1259–1267. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2023, 33, 3–14, Erratum in Obes. Surg. 2023, 33, 15–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sztanek, F.; Tóth, L.I.; Pető, A.; Hernyák, M.; Diószegi, Á.; Harangi, M. New Developments in Pharmacological Treatment of Obesity and Type 2 Diabetes-Beyond and within GLP-1 Receptor Agonists. Biomedicines 2024, 12, 1320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Dicker, D.; Sagy, Y.W.; Ramot, N.; Battat, E.; Greenland, P.; Arbel, R.; Lavie, G.; Reges, O. Bariatric Metabolic Surgery vs Glucagon-Like Peptide-1 Receptor Agonists and Mortality. JAMA Netw. Open 2024, 7, e2415392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Block, C.; Bailey, C.; Wysham, C.; Hemmingway, A.; Allen, S.E.; Peleshok, J. Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obes. Metab. 2023, 25, 3–17. [Google Scholar] [CrossRef]
- Hall, K.D. Physiology of the weight-loss plateau in response to diet restriction, GLP-1 receptor agonism, and bariatric surgery. Obesity 2024, 32, 1163–1168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker, V.E.R.; Robertson, D.; Wang, T.; Hornigold, D.C.; Petrone, M.; Cooper, A.T.; Posch, M.G.; Heise, T.; Plum-Moerschel, L.; Schlichthaar, H.; et al. Efficacy, Safety, and Mechanistic Insights of Cotadutide, a Dual Receptor Glucagon-Like Peptide-1 and Glucagon Agonist. J. Clin. Endocrinol. Metab. 2020, 105, dgz047. [Google Scholar] [CrossRef] [PubMed]
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Erratum. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021, 44, 1433–1442, Erratum in Diabetes Care 2022, 45, 3112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urva, S.; Coskun, T.; Loh, M.T.; Du, Y.; Thomas, M.K.; Gurbuz, S.; Haupt, A.; Benson, C.T.; Hernandez-Illas, M.; D’Alessio, D.A.; et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: A phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Lancet 2022, 400, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Glucose-lowering therapies in type 2 diabetes: Opportunities and challenges for peptides. Peptides 2018, 100, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Alabduljabbar, K.; le Roux, C.W. Pharmacotherapy before and after bariatric surgery. Metabolism 2023, 148, 155692. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Firkins, S.A.; Simons-Linares, R. Management of leakage and fistulas after bariatric surgery. Best. Pract. Res. Clin. Gastroenterol. 2024, 70, 101926. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Nasta, A.M.; Goel, M.; Prasad, A.; Jammu, G.; Fobi, M.; Ismail, M.; Raj, P.; Palaniappan, R.; Aggarwal, S.; et al. Complications after bariatric surgery: A multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group. J. Minim. Access Surg. 2021, 17, 213–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dixit, D.; Rodriguez, V.I.; Naumann, A.A.; Kamel, A.Y. Multiple micronutrient deficiencies as a long-term complication of bariatric surgery. BMJ Case Rep. 2023, 16, e254775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belloli, A.; Saccaro, L.F.; Landi, P.; Spera, M.; Zappa, M.A.; Dell’Osso, B.; Rutigliano, G. Emotion dysregulation links pathological eating styles and psychopathological traits in bariatric surgery candidates. Front. Psychiatry 2024, 15, 1369720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benzerouk, F.; Guénin, M.; Gierski, F.; Raucher-Chéné, D.; Barrière, S.; Bertin, E.; Kaladjian, A. Contributing roles of depression, anxiety, and impulsivity dimensions in eating behaviors styles in surgery candidates. J. Eat. Disord. 2021, 9, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bullen, N.L.; Parmar, J.; Gilbert, J.; Clarke, M.; Cota, A.; Finlay, I.G. How Effective Is the Multidisciplinary Team Approach in Bariatric Surgery? Obes. Surg. 2019, 29, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, L.; Chouillard, E.; Chahine, E.; Saikaly, E.; Debs, T.; Kassir, R. Metabolic Surgery and Diabesity: A Systematic Review. Obes. Surg. 2018, 28, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).