1. Introduction
Lowering the serum low-density-lipoprotein cholesterol concentration (LDL-C) by any means has been proven to be beneficial in reducing the incidence of cardiovascular disease (ASCVD) at a rate proportional to the percentage reduction in LDL-C achieved [
1]. The advent of statins, three decades ago, gave birth to an era in which this reduction became more and more efficient, allowing us to evaluate the effects of LDL-C lowering to previously unimaginable degrees. It is noteworthy that, in the past decade or so, large numbers of patients have participated in clinical trials producing LDL-C < 20–30 mg/dL, thus enhancing our understanding of lipids metabolism and its effects on ASCVD risk [
2]. Given the impressive new data, European ASCVD risk management guidelines have become more stringent with regard to lipid lowering reduction goals, especially for those patients found to be at the highest risk for cardiovascular events, i.e., those with a history of ASCVD events [
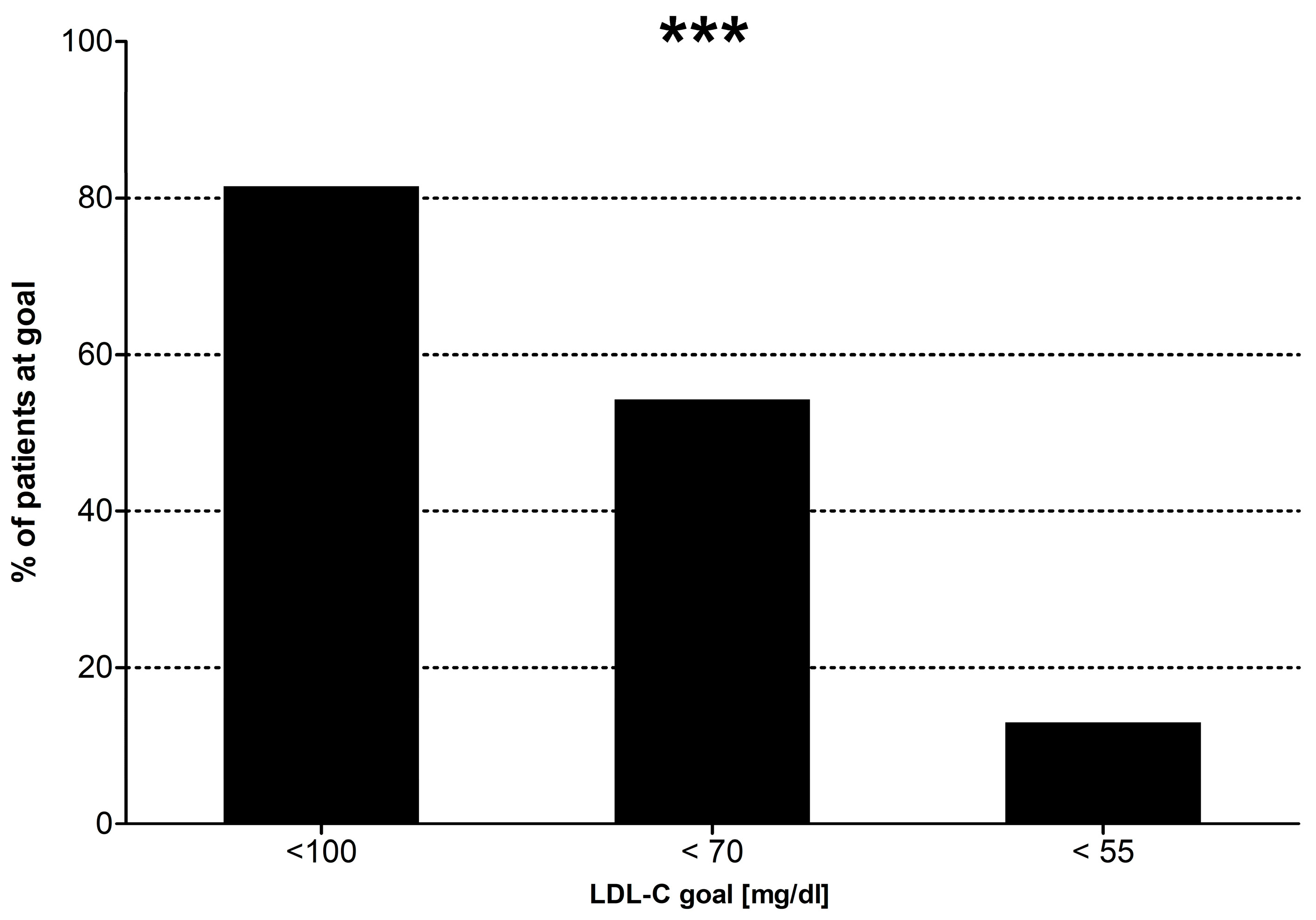
3]. Prior to 2011, LDL-C goals for this population required the achievement of an LDL-C < 100 mg/dL and if possible <80 mg/dL [
4]. This aim changed in 2011, requiring a target of an LDL-C < 70 mg/dL or an LDL-C reduction of >50% from baseline [
5], and again in 2019, when the goal was set to an LDL-C < 55 mg/dL with an LDL-C reduction of >50% from baseline
simultaneously [
3]. The use of higher intensity statins with the addition of ezetimibe and/or PCSK9 inhibitors (PCSK9i), inclisiran or lomitapide have allowed clinicians worldwide to be able to reduce the LDL-C according to the guidelines in many patients, if an adequate LDL-C-lowering medication strength is used. Despite the scientific progress and the wide availability of clinical practice guidelines, researchers in Greece [
6,
7] and globally [
8,
9,
10,
11,
12,
13] have reported poor outcomes with regard to LDL-C goals attainment in high-risk populations. In Greece specifically, the ease of access and the wide availability of most lipid-lowering treatment modalities for high-risk patients (statins, ezetimibe, PCSK9i, inclisiran) should have assisted in achieving these goals. Our clinical observations showed that most patients with a history of ASCVD attending our clinics failed to reach the above-mentioned goals most of the time, without the employment of systematic interventions. Therefore, we designed the present study to address this controversy.
2. Methods
Our Private Practice Clinics are scattered throughout the Greek territory (Athens: 3 sites; Patras: 1 site; Larisa: 1 site; Chania: 1 site; Kavala: 1 site; Alexandroupolis: 1 site) and act as referral centers for many physicians regionally and nationally for the management of all disorders pertaining to the fields of Diabetes, Endocrinology and Metabolism, rarely, though, for the management of lipid disorders alone. Despite this, we have witnessed a continuous flow of patients with a history of established ASCVD (secondary prevention population) referred for the management of other endocrine disorders, such as diabetes mellitus, thyroid disorders, bone metabolic abnormalities, etc. In order to ascertain the patterns in the medical treatment of these patients, with regard to their lipids, we created a combined registry of patients with a history of confirmed ASCVD.
2.1. Eligibility Criteria
For our patients to participate in the present study, they had to have a known form of ASCVD, diagnosed at least 3 months prior to their first ever visit to one of our clinics. ASCVD was defined as one or more of the following: a history of myocardial infarction (MI), comprising ST elevation or non-ST elevation MI; history of stenting of coronary, carotid or peripheral artery (or arteries); history of coronary artery bypass surgery (CABG) or other vascular bypass surgery; identification of an arterial stenosis > 50%, in any major artery, such as the coronary arteries (CAD), carotid arteries (CS) or peripheral arteries (PAD) with doppler ultrasound, coronary or other arterial angiography, axial computerized tomography angiogram (CTA) or magnetic resonance angiogram (MRA). All ASCVD cases’ documentation was reviewed by two of our co-authors (SL and NA) prior to the incorporation of their data in our study.
2.2. Exclusion Criteria
Given the complex nature of vascular thromboembolic disease, we did not include patients whose only manifestation of vascular disease was a stroke of any form, pulmonary or other arterial or venous thromboembolic disease. We also excluded patients with a history of ASCVD events while on oral contraceptives or other medications known to affect clotting factors, patients with an unclear history of ASVCD or inadequate documentation of their diagnosis, those with a known arterial atherosclerotic lesion producing a ≤50% stenosis of the vessel’s lumen and women of reproductive potential or who were interested in achieving a pregnancy. Finally, we excluded the few patients referred to our clinics for lipids management.
2.3. Data Recording
Patients who fulfilled our inclusion criteria were enrolled in the present study, and their past medical records were accessed to retrieve the following information:
Year of their first clinic visit (range 2010–2023), age at that time and gender.
Type(s) of ASCVD, divided in three categories: coronary artery disease (CAD), peripheral arterial disease (PAD) and/or carotid stenosis (CS). Subjects could have more than one vascular disorder if adequate documentation was available at the time of their first encounter at our clinics.
History of diabetes mellitus. When diabetes was present, the form of diabetes (type 1, type 2, LADA or other) was recorded as well.
History of hypertension.
History of tobacco use (current smoker, former smoker, never smoker).
Use of any lipid-lowering medications before their first clinic visit. When medications were being used, the frequency and dosing strength were recorded. When alternate day regimens were used, the mean daily dose was estimated and incorporated into the present analysis.
Lipid panel during our first clinic visit. This included total cholesterol, LDL-C, HDL-C, triglycerides and Lp(a), all measured in mg/dL.
TSH concentration during their first clinic visit (reference range in our clinics 0.40–4.50 IU/mL). This measurement was performed because uncontrolled hypothyroidism, a common referral diagnosis for our patients, is associated with lipid disorders, independent of the use of LLT [
14].
Vital signs were retrieved from their first clinic visit, when available, and they included measurements of blood pressure, heart rate, height and weight. The body mass index (BMI) (kg/m2) was estimated using the NHLBI formula.
The serum measurements of TSH and lipids were performed as part of the usual care of our patients at the time of their first clinic visit, and were analyzed at different commercial clinical laboratories.
2.4. Results Categorization
Our subjects’ LDL-C and lipid-lowering regimens were compared to the European guidelines’ standards from the respective year of their first visit with us. We took into consideration each guideline starting the year after its original publication (i.e., we expected our subjects’ lipids to follow the 2019 ESC standards, when they were seen for the first time at our clinics in 2020 or afterwards). Prior to 2012, subjects who attained an LDL cholesterol concentration < 100 mg/dL were deemed at goal. Between 2012 and 2017, subjects who attained an LDL cholesterol concentration < 70 mg/dL were deemed on target. Between 2017 and 2020, the subjects who either achieved their respective treatment goals for LDL-C or were taking an adequate strength of lipid-lowering medications were deemed to be on a regimen in concordance with the guidelines. For subjects seen for the very first time in 2020 and afterwards, both the presence of an LDL-C at target and the use of an adequate strength of lipid-lowering medications were required to consider the regimen appropriate. Adequate strength of lipid lowering medications is defined as the use of medications achieving an LDL-C reduction > 50%, i.e., atorvastatin ≥ 40 mg, rosuvastatin ≥ 20 mg, combinations of moderate intensity statins with ezetimibe, PCSK9 inhibitors, inclisiran or lomitapide.
2.5. Statistical Analysis
Our main analysis and the generation of images were performed with GraphPad Prism v5.0 (GraphPad Software, Boston, MA, USA). Multivariable logistic regression analysis was performed with MedCalc Statistical Software version 19.2.6 (MedCalc Software bv, Ostend, Belgium;
https://www.medcalc.org; 2020 accessed on 28 December 2023). We compared categorical variables’ rates using the χ
2 or Fischer’s exact test. Continuous variables’ data were assessed for normality with Kolmogorov–Smirnov test and their means were compared with 1-way ANOVA or the non-parametric Kruskal–Walis test. Bonferroni’s or Dunn’s test for multiple comparisons were used accordingly.
p values < 0.05 were deemed significant.
In order to identify factors predictive of treatment success, we performed a backward logistic regression analysis to characterize the effects of age, gender, year of study, LDL-C target, diabetes history, hypertension history, CAD, CS, PAD, tobacco use history, TSH status (normal, low or high) and successful treatment. In addition, an analysis of the effects of these same parameters was performed for each form of ASCVD separately (CAD, PAD, CS) in the form of a logistic regression analysis.
2.6. Ethical Considerations
Institutional Review Board approval was obtained from the Hellenic Endocrine Network Institutional Review Board (IRB approval number Ν2024/0121313). Informed consent was waived due to the retrospective nature of the present work and the minimal risks associated with it, after approval of this waiver by the Institutional Review Board.
3. Results
We reviewed all of the charts of patients attending our Endocrinology, Diabetes and Metabolism clinics. Out of these, n = 2132 charts contained a potential diagnosis of ASCVD, based on ICD-10 coding or reported medical history, but that was confirmed in only n = 1003 subjects, who constituted our study population. The baseline characteristics of our subjects are presented in
Table 1. Overall, a guideline-appropriate treatment strategy was used in 361 (36.0%) subjects, while the remainder were either on no treatment, n = 159 (15.9%), or they were receiving inadequate therapy (low or moderate intensity statins, ezetimibe alone or triglyceride-lowering agents alone), n = 483 (48.2%). The exact type and dose of the medications used, along with the mean LDL-C achieved with each treatment type, are presented in
Supplementary Table S1. Overall, n = 434 (43.3%) subjects were on high-intensity LDL-C-lowering medication(s), n = 361 had achieved the LDL goals set by the year-specific guidelines, which is equivalent to 36.0% of the overall population, or 83.2% of those using high intensity LDL-C-lowering medication(s). Furthermore, we present the efficacy of each treatment strategy regarding the achievement of an LDL-C < 55 mg/dL, which is the one supported by the latest guidelines for patients with documented ASCVD [
3], as shown in
Supplementary Table S1. Of note, n = 100 (9.97%) subjects had an LDL < 55 mg/dL, out of whom 68 were using a high-intensity LLT (68.0%), and notably, 11 of them (11.0%) were on no LLT whatsoever. When our subjects were grouped according to the number of vascular beds involved, those presenting with vascular disease in ≥2 vascular beds had a statistically insignificant, slightly higher likelihood of being on target to reach their treatment goal (72/191, 37.7% vs. 289/812, 35.6%,
p > 0.05). Similarly, when we assessed only those subjects seen since 2020 (treatment goal < 55 mg/dL), n = 48/375 (12.8%) subjects with one vascular bed disease were at their goal, as compared to 12/86 (14.0%) of those with ≥2 vascular beds involved,
p > 0.05.
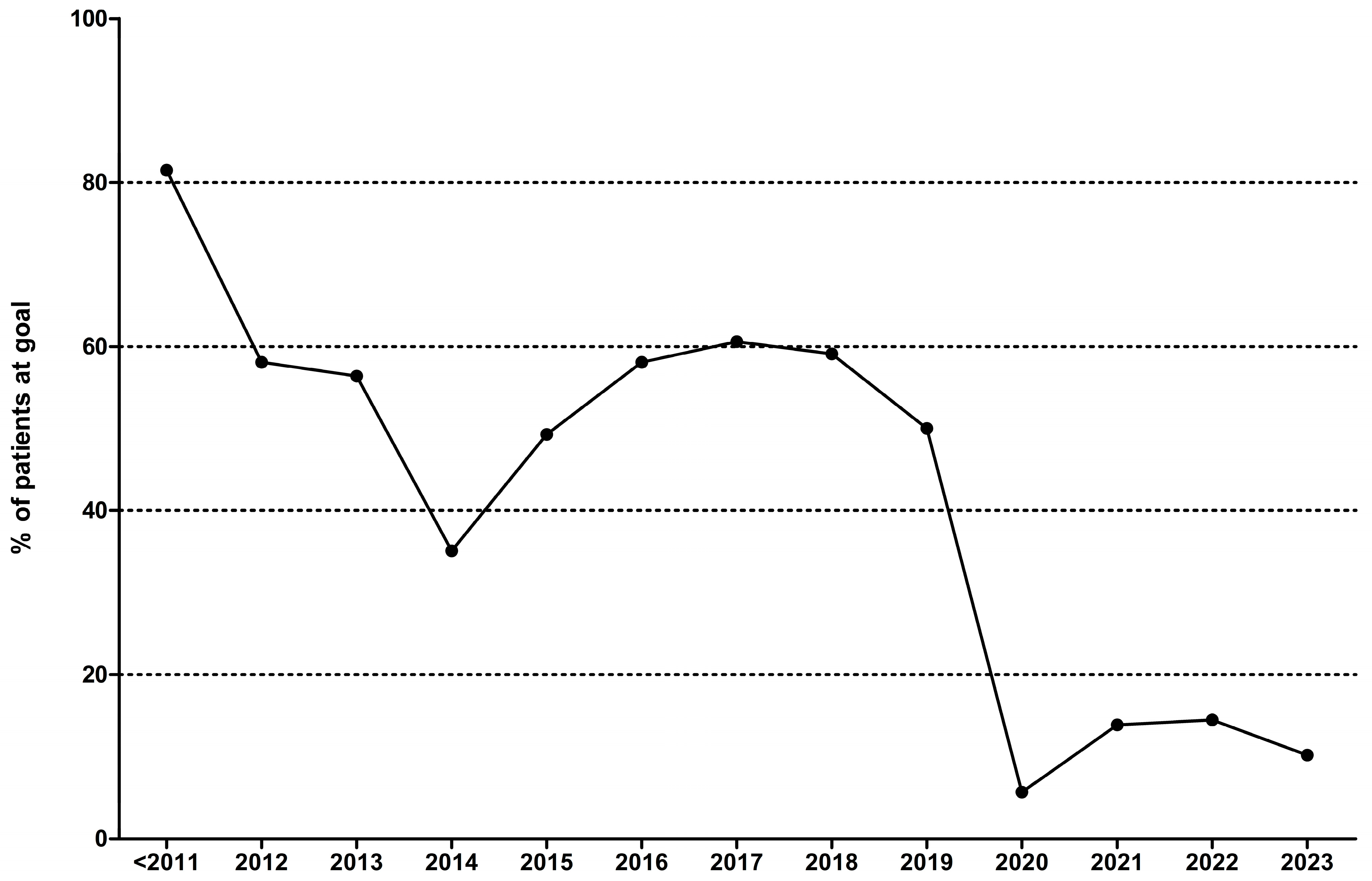
A significant portion of the present work focuses on the changes in the pattern of treatment with LLTs in our nation over time. Given the major changes in international guidelines’ treatment goals, we assessed the number of patients who were placed on treatment strategies in concordance with the respective guidelines and those who achieved these goals. Our results are presented in
Table 2 and depicted in
Figure 1. In brief, the treatment strategies used by the treating physicians seemed somewhat efficient in the past, but this changed dramatically with the introduction of the latest guidelines [
3]. Ever since, the achievement of both an LDL-C < 55 mg/dL and the use of high intensity lipid-lowering therapies were mandated, leading to a very low rate of goal achievement (5.9–14.5%).
Since the current guidelines require such aggressive lipid-lowering goals for patients in secondary ASCVD prevention, we assessed which treatment strategies were the most effective in producing similar reductions. As noted above, n = 100 subjects had an LDL-C < 55 mg/dL (9.97% of all subjects) with 89 of them being on some form of LLT. This was successful in all n = 3 (100%) subjects who were placed on PCSK9 inhibitors, with or without additional lipid lowering agents. Out of the remaining subjects, n = 40 (40.0%) achieved that goal being treated with combination pills, including simvastatin + ezetimibe (n = 8), atorvastatin + ezetimibe (n = 17) and rosuvastatin + ezetimibe (n = 15). In addition, n = 19 (19.0%) subjects on atorvastatin monotherapy achieved an LDL-C < 55 mg/dL, out of whom n = 15 were treated with high-dose atorvastatin—40 mg daily—and the remaining with lower doses (moderate intensity statin therapy). An additional n = 3 (3.0%) subjects receiving low doses of pitavastatin (1 mg n = 1, 2 mg n = 2) and n = 8 (8.0%) subjects receiving simvastatin (20 mg n = 5 and 40 mg n = 3) also achieved an LDL-C < 55 mg/dL, as did n = 15 (15.0%) subjects receiving rosuvastatin (10 mg n = 2, 20 mg n = 12, 40 mg n = 1).
The LDL-C of our population reaches an average of 89.2 ± 34.8 mg/dL, which surpasses by 34.2 mg/dL the <55 mg/dL goal. When we assessed the subjects seen after 2019 alone (2020–2023), the mean LDL-C was not significantly different, at 87.6 ± 37.9 mg/dL, with the distance from goal being 32.6 mg/dL (p > 0.05 for the comparison between those seen < 2020 and those seen afterwards).
Overall n = 662 subjects were on statins alone or a combination with triglyceride-lowering medications and n = 52 (7.9%) out of those achieved an LDL-C < 55 mg/dL. An additional n = 178 subjects were on combination therapies with ezetimibe and n = 44 of them achieved the LDL-C goal of <55 mg/dL (24.7%). Also, n = 2 subjects were on a combination therapy of a statin ± ezetimibe and a PCSK9 inhibitor and they both achieved the LDL-C < 55 mg/dL goal (100.0%). The likelihood of achieving the LDL-C < 55 mg/dL goal was 3.85 (95% CI 2.47–6.00, p < 0.001) with combination therapy as compared to treatment with statins alone.
To evaluate the factors associated with the likelihood of attaining the guidelines-set goals, we assessed several parameters in order to verify whether these impacted treatment outcomes in a significant manner. These are presented in
Table 3 and
Figure 2. In brief, only the guidelines-set treatment goals and the history of tobacco use were statistically significantly associated with the likelihood of achieving the LDL-C goals in a univariate analysis. Specifically, current smoking status and less stringent treatment goals (LDL-C goal of <100 mg/dL or use of high intensity lipid-lowering therapy) are positively associated with treatment success, while a history of diabetes, hypertension, the type of vascular disease, age, gender or serum TSH concentration do not impact this outcome in a statistically significant manner.
In the covariate analysis, our model was highly statistically significant (
p < 0.0001). After excluding insignificant variables, only the use of high-intensity lipid-lowering medications predicted the treatment success in a positive manner, while the stricter LDL-C goals for treatment were strong negative predictors. The results of the multivariate regression analysis are presented in detail in
Table 4. A similar analysis assessing the effects of these factors separately in subjects with CAD, PAD or CS revealed that the only two factors influencing treatment success were the use of high-intensity LLT and the LDL goals, dependent on the year-specific guidelines (
Table 5) in all categories of ASCVD.
4. Discussion
Cardiovascular disease is the leading cause of death globally, accounting for 16% of all deaths, according to a recent World Health Organization report (
https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death accessed on 21 December 2023). Therefore, it is only fair that the global scientific community has devoted plenty of resources to its study and is continuously enhancing its efforts to curb this global pandemic. Luckily, in the past decades, a multitude of medical treatment options emerged, allowing us to design preventive strategies that can have a significant impact on atherosclerotic cardiovascular disease (ASCVD) incidence and progression [
15]. In addition, using our most potent lipid lowering agents, atherosclerotic plaque regression has become feasible as well [
16,
17].
Our understanding of ASCVD is rapidly evolving and the incorporation of newer medical treatments has allowed significant reductions in negative cardiovascular outcomes, where these interventions were followed through adequately. In most countries, especially in the Western world, the availability of statins, ezetimibe and PCSK9-modifying agents (PCSK9i and PCSK9 silencing RNAs) is excellent. Therefore, we would expect a significant decline in the incidence of ASCVD with the routine use of these agents, along with a significant drop in our population’s serum lipid parameters and especially LDL-C. Instead of that, our clinical observation was that most patients attending our eight busy clinics nationwide, were not adequately treated, when their respective ASCVD risk was considered.
In order to better understand this controversy, we designed the present cross-sectional study, in which we reviewed data on lipid-lowering agents use in patients at very high risk for ASCVD events: those who already carry a diagnosis of ASCVD. Our study encompasses data from eight busy solo-practice Endocrinology clinics in major Greek cities, incorporating data from all the patients seen over a continuous period of 4 months. We identified those who were diagnosed with ASCVD
prior to their first visit with us because our special interest in lipids and intensive work on the field could have misled our view of the treatment patterns in the country. We recorded the lipid-lowering strategy used before we intervened, along with the LDL-C attained by each strategy. Furthermore, we separated our patients based on the year when they were first seen in our clinics and the lipid-lowering requirements of that time and estimated the rate of those being adequately treated. Our findings were impressive, in that the rate of LDL-C control became substantially worse with the newer, stricter guidelines and especially with the 2019 guidelines, requiring attainment of an LDL-C < 55 mg/dL and the use of a high potency regimen at the same time [
3]. This finding was expected, in part, since newer studies and changing guidelines require some time before they can be implemented in daily clinical practice. Also, the attainment of such strict goals requires quite aggressive lipid-lowering treatments, which commonly consist of combination therapies [
18]. This is evident in our study as well, where combination therapies almost quadrupled the likelihood of LDL goal attainment when compared to the use of statins alone. Another significant obstacle to the attainment of that goal is polypharmacy, which is a known risk factor for poor treatment adherence and worse clinical outcomes [
19]. Furthermore, clinical inertia has been described as a major obstacle in the performance of cardiovascular disease preventive measures [
20], and it has been recognized as an important factor in the lipids management of patients undergoing secondary ASCVD prevention as well [
21]. Clinical inertia could play a key role in the quality of care provided to the vascular disease population and systematic interventions are required to tackle this global issue.
Additionally, many practitioners could feel less safe using high-potency agents or they could have limited experience with these medications, especially when it comes to injectable treatments, such as the PCSK9 inhibitors. All these factors lead to under-treatment of the actual cardiovascular risk, especially in this vulnerable and at-risk population. Of course, a large proportion of our untreated subjects might have been offered adequate and/or appropriate therapy and they could have stopped taking it against medical advice, because of negligence, lack of adequate follow up and/or poor understanding of the severity of their condition or the benefits of the prescribed treatment.
Irrespective of the cause for this occurrence, only about one third of our subjects were on a high-intensity lipid-lowering treatment and achieved the treatment goals, and that rate decreased significantly when the 2019 guidelines on ASCVD risk reduction were published. In fact, only a small minority of subjects attending our clinics for the first time between 2020 and 2023 had an LDL-C < 55 mg/dL and this rate did not seem to increase substantially during the past 3 years. The type of vascular disease present did not affect the intensity of the regimen used or the success rate of the selected strategy either. It is noteworthy too that the vast majority of those achieving LDL-C goals were on high-dose combination pills (atorvastatin or rosuvastatin high dose + ezetimibe), while very few subjects were treated with PCSK9 inhibitors, despite the ease of access and the whole medication being covered (no copay for this class of medications) by the state insurance, which is the single healthcare provider in Greece. No subject was on inclisiran either, despite it being available in Greece since mid-2022 and being a highly effective treatment as well.
Unsurprisingly, our results concur with those previously reported both in smaller Greek cohorts and internationally. In the DYSIS II trial in 2019, Liberopoulos et al. reported treatment outcomes in 499 patients with ASCVD history and found that the LDL goal was achieved by 16.2% of patients with ASCVD history on study admission, despite a significant portion of that population consisting of patients with a recent onset myocardial infarction [
6]. It is of note, though, that the goal at that time was an LDL < 70 mg/dL, or the use of a high intensity lipid lowering medication, which is overall easier to attain. Even when patients were followed in a focused University Lipid Clinic, the achievement of target goals was inadequate, not surpassing 25% in patients at very high cardiovascular risk [
7].
Of course, this is not a Greek paradox. Indeed, in a large German–Austrian cohort study of over 30,000 patients with diabetes, evaluated in 2020–2021 and stratified by their cardiovascular disease risk, the LDL goal of <70 mg/dL was achieved in 10.97% of patients with type 1 diabetes and 16.25% of patients with type 2 diabetes [
8]. Even lower was the goal attainment of LDL-C < 55 mg/dL (6.16% and 11.81% for type 1 and type 2 diabetes respectively). In that study, PCSK9 inhibitors were used in 0.1% of the population as well, which is very similar to our finding of 0.3% (3/1003). In addition, a recent prospective, multicentric study from the US, aiming to optimize the cardiovascular risk in 1590 patients with ASCVD history, found that only 11% of cases were placed on optimal medical therapy during the two years of the intervention [
9]. An even larger retrospective study from the US evaluated the pharmacy claims between 2018 and 2019 in 601,934 patients with known ASCVD, geographically dispersed in all regions of the country, with a distribution believed to be representative of the general US population [
10]. This immense work reported that only 22.5% of the patients entitled to high-intensity statins and aggressive lipid lowering therapy were on such treatments, while a surprising 49.9% were receiving no lipid lowering medications at all [
10]. Also, a multinational cross-sectional study from all over Europe, reported poor achievement of LDL-C goals as well, when 2794 patients with established ASCVD were evaluated and 42% of those found to be on high-intensity statins, while only 18% were on target to reach their treatment goal as well [
11]. Similarly, only 20.7% of 6954 patients with a confirmed history of ASCVD were on target to reach their treatment goal in the multinational observational study SANTORINI [
12]. Given that a much more robust LDL-C reduction is needed in patients with heterozygous familial hypercholesterolemia, we would expect a higher goal attainment when these are excluded, but this was not the case either [
12].
These outcomes are disappointing, but not unexpected, given that, even in the case of adequate prescribing and patient education and decent patient adherence, most patients at high cardiovascular risk would not achieve LDL-C goals through statin dose optimization alone and would require the addition of second line ezetimibe, and/or a PCSK9 modifying agent [
11,
12,
13]. This is evident in our study as well, where the rates of achievement of treatment goals were significantly higher with the use of the more complex regimens.
4.1. Strengths of the Study
This is the largest study to date assessing the patterns of LLT use and efficacy in patients with a known history of ASCVD—secondary prevention in Greece, including data from 1003 consecutive subjects. It is also the first study to incorporate into the outcomes patients with non-coronary ASCVD, such as PAD and carotid stenosis of a severity requiring vascular intervention(s) or with stenosis of >50%. In addition, the present work is the first to assess the changes in the treatment patterns over time, and especially the only one to assess the effectiveness of treatment strategies regarding the latest, stricter guidelines aiming for a major reduction in LDL-C with the use of effective treatments and the achievement of an LDL-C < 55 mg/dL simultaneously. Furthermore, it is the first study originating from a clinical setting other than a cardiology, vascular surgery, thoracic surgery or lipid clinic, potentially reflecting better the lipid-lowering strategies and their effects as seen in a large part of our patient populations, who do not follow up regularly with these specialists.
4.2. Limitations of the Study
Our present work is limited by some significant parameters which are worth mentioning. Firstly, our data do not contain information on the reason(s) behind the use of low-potency medications in many subjects, or the lack of lipid-lowering therapy use in many more. This could be the effect of previous experience of adverse effects with the use of recommended treatment options, patients being non-adherent to the prescribed treatment, a misunderstanding between patients and their treating physicians, the inadequate supervision of lipid parameters by their treating physicians or other intrinsic difficulties. Of course, a significant number of those subjects might have discontinued their treatment against medical advice or even without notifying their providers at all. Also, our study does not account for the socioeconomic or educational status, their living settings (urban vs. countryside) or other factors which could significantly impact their access or adherence to the necessary therapy, even though patients with a well-defined history of ASCVD, such as those included in this study, have substantial social security benefits in Greece, which are able to fill the care gap adequately. Also, the geography of Greece, the widespread availability of specialist clinics and the relatively low cost of care allow for the vast majority of the population to have access to the care they need. Additionally, our study is limited by the absence of data on ASCVD outcomes in the study population. Naturally, our population presents some bias as compared to the general population with ASCVD, since 64% of them are affected by diabetes, a finding expected though, given the recruitment in Endocrinology, Diabetes and Metabolism Clinics. Finally, our study is limited by its descriptive, retrospective nature, without an active intervention arm, where our care team can potentially propose specific actions in order to attain better adherence and treatment outcomes.