Increased Psychological Symptoms and Autonomic Arousal in Patients with Subclinical Hypothyroidism: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubbs, S.B.; Spangler, R. Hypothyroidism: Causes, killers, and life-saving treatments. Emerg. Med. Clin. N. Am. 2014, 32, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism (Primer). Nat. Rev. Dis. Primers 2022, 8, 30, Erratum in Nat. Rev. Dis. Primers 2022, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Pyun, J.M.; Park, Y.H.; Kim, S. Subclinical Hypothyroidism and Cognitive Impairment. J. Alzheimers Dis. 2022, 88, 757–762. [Google Scholar] [CrossRef]
- Samuels, M.H. Cognitive function in subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2010, 95, 3611–3613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almeida, C.; Vaisma, M.; Costa, A.J.; Reis, F.A.; Reuters, V.; Teixeira, P.; Ferreira, M.; Teixeira, L.B.d.M.; de Araújo, G.R.; Brasil, M.A. Are neuropsychological changes relevant in subclinical hypothyroidism? Arq. Bras. Endocrinol. Metabol. 2007, 51, 606–611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Jongh, R.T.; Lips, P.; van Schoor, N.M.; Rijs, K.J.; Deeg, D.J.H.; Comijs, H.C.; Kramer, M.H.H.; Vandenbroucke, J.P.; Dekkers, O.M. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur. J. Endocrinol. 2011, 165, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Brasil, M.A.; Costa, A.J.; Reis, F.A.A.; Reuters, V.; Teixeira, P.; Ferreira, M.; Marques, A.M.; Melo, B.A.; Teixeira, L.B.B.d.M.; et al. Subclinical hypothyroidism: Psychiatric disorders and symptoms. Braz. J. Psychiatry 2007, 29, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Tremont, G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007, 32, 49–65. [Google Scholar]
- Soheili-Nezhad, S.; Sprooten, E.; Tendolkar, I.; Medici, M. Exploring the Genetic Link Between Thyroid Dysfunction and Common Psychiatric Disorders: A Specific Hormonal or a General Autoimmune Comorbidity. Thyroid 2023, 33, 159–168, Erratum in Thyroid 2023, 33, 656. [Google Scholar] [CrossRef]
- Mahajan, A.S.; Lal, R.; Dhanwal, D.K.; Jain, A.K.; Chowdhury, V. Evaluation of autonomic functions in subclinical hypothyroid and hypothyroid patients. Indian. J. Endocrinol. Metab. 2013, 17, 460–464. [Google Scholar] [CrossRef]
- Davis, J.D.; Stern, R.A.; Flashman, L.A. Cognitive and neuropsychiatric aspects of subclinical hypothyroidism: Significance in the elderly. Curr. Psychiatry Rep. 2003, 5, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Ergür, A.T.; Taner, Y.; Ata, E.; Melek, E.; Bakar, E.E.; Sancak, T. Neurocognitive functions in children and adolescents with subclinical hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2012, 4, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Behera, J.K.; Sood, S.; Rajput, R.; Satpal Praveen, P. Study of cognitive functions in newly diagnosed cases of subclinical and clinical hypothyroidism. J. Nat. Sci. Biol. Med. 2014, 5, 63–66. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR); American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Gulseren, S.; Gulseren, L.; Hekimsoy, Z.; Cetinay, P.; Ozen, C.; Tokatlioglu, B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch. Med. Res. 2006, 37, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Peng, Y.Y.; Qi, C.C.; Chen, F.H.; Zhou, J.N. Depression-like behavior in subclinical hypothyroidism rat induced by hemi-thyroid electrocauterization. Endocrine 2014, 45, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Dayan, C.M.; Panicker, V. Hypothyroidism and depression. Eur. Thyroid. J. 2013, 2, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Demartini, B.; Masu, A.; Scarone, S.; Pontiroli, A.E.; Gambini, O. Prevalence of depression in patients affected by subclinical hypothyroidism. Panminerva Med. 2010, 52, 277–282. [Google Scholar] [PubMed]
- Reuters, V.S.; Almeida, C.d.P.; Teixeira, P.d.F.; Vigário, P.d.S.; Ferreira, M.M.; de Castro, C.L.N.; Brasil, M.A.; da Costa, A.J.L.; Buescu, A.; Vaisman, M. Effects of subclinical hypothyroidism treatment on psychiatric symptoms, muscular complaints, and quality of life. Arq. Bras. Endocrinol. Metabol. 2012, 56, 128–136. [Google Scholar] [CrossRef]
- Foley, C.M.; McAllister, R.M.; Hasser, E.M. Thyroid status influences baroreflex function and autonomic contributions to arterial pressure and heart rate. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2061–H2068. [Google Scholar] [CrossRef]
- Heemstra, K.A.; Burggraaf, J.; van der Klaauw, A.A.; Romijn, J.A.; Smit, J.W.; Corssmit, E.P. Short-term overt hypothyroidism induces sympathovagal imbalance in thyroidectomized differentiated thyroid carcinoma patients. Clin. Endocrinol. 2010, 72, 417–421. [Google Scholar] [CrossRef]
- Galetta, F.; Franzoni, F.; Fallahi, P.; Tocchini, L.; Braccini, L.; Santoro, G.; Antonelli, A. Changes in heart rate variability and QT dispersion in patients with overt hypothyroidism. Eur. J. Endocrinol. 2008, 158, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Cacciatori, V.; Gemma, M.L.; Bellavere, F.; Castello, R.; De Gregori, M.; Zoppini, G.; Thomaseth, K.; Moghetti, P.; Muggeo, M. Power spectral analysis of heart rate in hypothyroidism. Eur. J. Endocrinol. 2000, 143, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid 2000, 10, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Galetta, F.; Franzoni, F.; Fallahi, P.; Rossi, M.; Carpi, A.; Rubello, D.; Antonelli, A.; Santoro, G. Heart rate variability and QT dispersion in patients with subclinical hypothyroidism. Biomed. Pharmacother. 2006, 60, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Turan, N.; Kosar, F.; Taskapan, C.; Gunen, H. Evaluation of autonomic activity in patients with subclinical hypothyroidism. J. Endocrinol. Investig. 2005, 28, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Crown, S.; Crisp, A.H. Crown-Crisp Experiential Index; Organizzazioni Speciali: Firenze, Italy, 1979. [Google Scholar]
- Birtchnell, J.; Evans, C.; Kennard, J. The total score of the Crown-Crisp Experiential Index: A useful and valid measure of psychoneurotic pathology. Br. J. Med. Psychol. 1988, 61 Pt 3, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.D. Biofeedback Methods and Procedures in Clinical Practice; Biofeedback Press: San Francisco, CA, USA, 1979. [Google Scholar]
- Monzani, F.; Del Guerra, P.; Caraccio, N.; Pruneti, C.; Puccil, E.; Luisit, M.; Baschieri, L. Subclinical hypothyroidism: Neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin. Investig. 1993, 71, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, J.; Komulainen, K.; García-Velázquez, R.; Määttänen, I.; Gluschkoff, K.; Savelieva, K.; Jokela, M. Subclinical hypothyroidism and symptoms of depression: Evidence from the National Health and Nutrition Examination Surveys (NHANES). Compr. Psychiatry 2021, 109, 152253. [Google Scholar] [CrossRef]
- Bode, H.; Ivens, B.; Bschor, T.; Schwarzer, G.; Henssler, J.; Baethge, C. Association of Hypothyroidism and Clinical Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1375–1383. [Google Scholar] [CrossRef]
- Allen, L.A.; Escobar, J.I.; Lehrer, P.M.; Gara, M.A.; Woolfolk, R.L. Psychosocial treatments for multiple unexplained physical symptoms: A review of the literature. Psychosom. Med. 2002, 64, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Fekete, E.M.; Antoni, M.H.; Schneiderman, N. Psychosocial and behavioral interventions for chronic medical conditions. Curr. Opin. Psychiatry 2007, 20, 152–157. [Google Scholar] [CrossRef]
- World Health Organization. Summary Reports on Proceedings Minutes and Final Acts of the International Health Conference held in New York from 19 June to 22 July 1946. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/85573 (accessed on 15 January 2024).
- Monzani, F.; Pruneti, C.A.; De Negri, F.; Simoncini, M.; Neri, S.; Di Bello, V.; Baschieri, L. Preclinical hypothyroidism: Early involvement of memory function, behavioral responsiveness and myocardial contractility. Minerva Endocrinol. 1991, 16, 113–118. [Google Scholar] [PubMed]
- Parle, J.; Roberts, L.; Wilson, S.; Pattison, H.; Roalfe, A.; Haque, M.S.; Heath, C.; Sheppard, M.; Franklyn, J.; Hobbs, F.D.R. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: The Birmingham Elderly Thyroid study. J. Clin. Endocrinol. Metab. 2010, 95, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Biondi, B. Subclinical thyroid disease. Lancet 2012, 379, 1142–1154. [Google Scholar] [CrossRef]
- Pruneti, C.; Innocenti, A.; Cosentino, C.; Monzani, F.; Guccini, I. Subclinical Hypothyroidism: Behavioral and psychophysiological characteristics. A pilot study. Int. J. Adv. Res. 2016, 4, 249–255. [Google Scholar]
| Variable | Patients Group (n = 50) | Control Group (n = 50) | t or χ2 | p |
|---|---|---|---|---|
| Age, M (SD) | 23.90 (5.20) | 28.10 (8.40) | t (99) = 7.65 | n.s. |
| Sex, N (%) | χ2 (1, N = 99) = 0.55 | n.s. | ||
| Male | 17 (34%) | 22 (44%) | ||
| Female | 33 (66%) | 28 (56%) | ||
| Marital status, N (%) | χ2 (2, N = 99) = 2.18 | n.s. | ||
| Married/cohabitant | 12 (24%) | 16 (32%) | ||
| Unmarried | 38 (76%) | 32 (64%) | ||
| Separated/divorced | 0 (0%) | 2 (4%) | ||
| Education Level, N (%) | χ2 (2, N = 99) = 3.33 | n.s. | ||
| Middle school graduation | 5 (10%) | 13 (26%) | ||
| High school graduation | 30 (60%) | 25 (50%) | ||
| University | 15 (30%) | 12 (24%) | ||
| Current Occupation, N (%) | χ2 (2, N = 99) = 9.25 | n.s. | ||
| Student | 35 (70%) | 28 (56%) | ||
| Employed | 15 (30%) | 20 (40%) | ||
| Unemployed/retired | 0 (0%) | 2 (4%) | ||
| Thyroid Hormonal Dosages | ||||
| TSH (mg/µL) | 9.50 (5.70) | 1.90 (2.20) | 1.92 | <0.001 |
| TT3 (ng/dL) | 12.80 (2.50) | 13.10 (4.70) | −0.08 | n.s. |
| TT4 (µg/dL) | 6.90 (1.40) | 8.20 (3.30) | −0.55 | n.s. |
| FT3 (pg/mL) | 3.10 (0.70) | 4.20 (3.10) | −0.58 | n.s. |
| FT4 (pg/mL) | 6.20 (1.60) | 8.90 (4.90) | −0.83 | n.s. |
| Patients Group (n = 50) | Control Group (n = 50) | t Test | p | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Crown–Crisp Experiential Index | ||||||
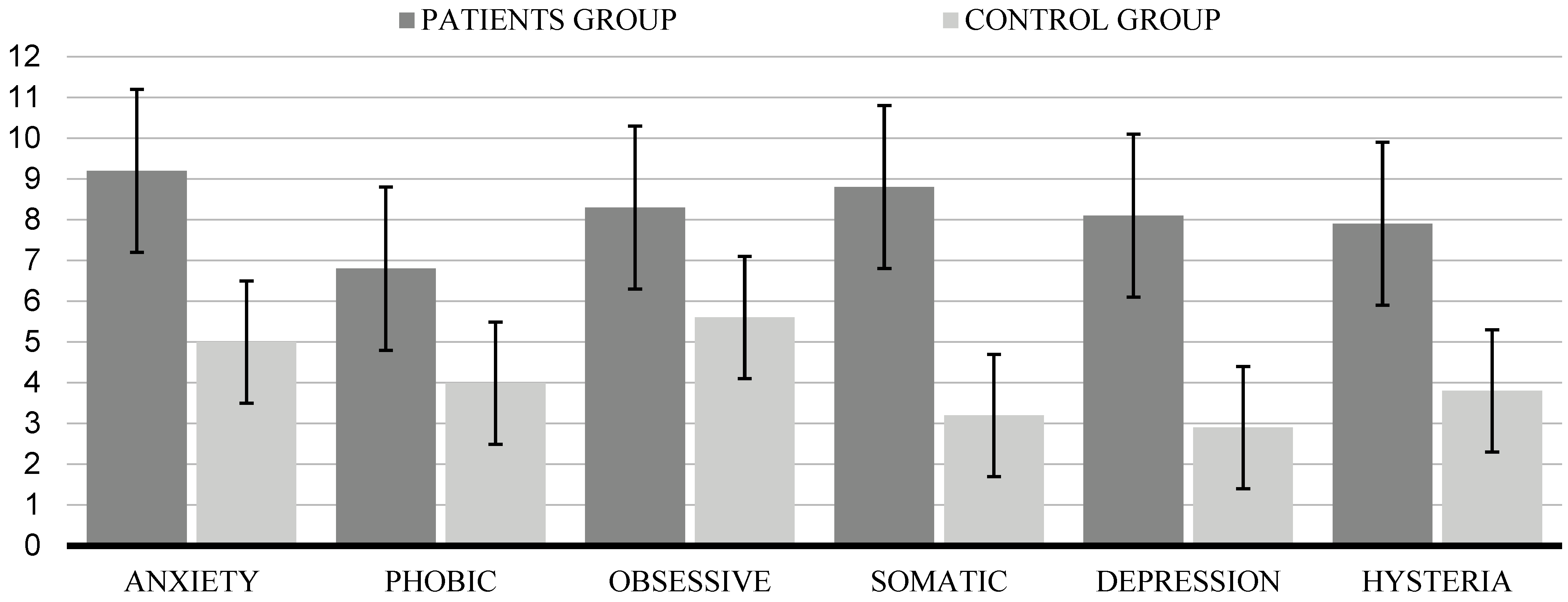
| Anxiety | 9.20 | 2.40 | 5.00 | 3.20 | 7.40 | <0.001 |
| Phobic | 6.80 | 4.10 | 3.99 | 2.20 | 5.53 | n.s. |
| Obsessive | 8.30 | 2.20 | 5.60 | 3.10 | 1.02 | n.s. |
| Somatic | 8.80 | 1.70 | 3.20 | 1.80 | 3.20 | <0.001 |
| Depression | 8.10 | 1.90 | 2.90 | 1.10 | 3.47 | <0.001 |
| Hysteria | 7.90 | 3.20 | 3.80 | 2.30 | 1.49 | <0.001 |
| Psychophysiological Assessment | ||||||
| Surface Electromiography | 4.60 | 1.20 | 2.20 | 1.60 | 1.71 | <0.001 |
| Skin Conductance | 11.90 | 3.60 | 4.60 | 2.50 | 2.39 | <0.001 |
| Heart Rate | 84.60 | 11.40 | 74.10 | 8.10 | 1.08 | <0.001 |
| Peripheral Temperature | 29.90 | 2.20 | 34.00 | 2.90 | −1.61 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidotti, S.; Innocenti, A.; Cosentino, C.; Monzani, F.; Guccini, I.; Pruneti, C. Increased Psychological Symptoms and Autonomic Arousal in Patients with Subclinical Hypothyroidism: A Case–Control Study. Endocrines 2024, 5, 186-196. https://doi.org/10.3390/endocrines5020013
Guidotti S, Innocenti A, Cosentino C, Monzani F, Guccini I, Pruneti C. Increased Psychological Symptoms and Autonomic Arousal in Patients with Subclinical Hypothyroidism: A Case–Control Study. Endocrines. 2024; 5(2):186-196. https://doi.org/10.3390/endocrines5020013
Chicago/Turabian StyleGuidotti, Sara, Augusto Innocenti, Chiara Cosentino, Fabio Monzani, Irene Guccini, and Carlo Pruneti. 2024. "Increased Psychological Symptoms and Autonomic Arousal in Patients with Subclinical Hypothyroidism: A Case–Control Study" Endocrines 5, no. 2: 186-196. https://doi.org/10.3390/endocrines5020013
APA StyleGuidotti, S., Innocenti, A., Cosentino, C., Monzani, F., Guccini, I., & Pruneti, C. (2024). Increased Psychological Symptoms and Autonomic Arousal in Patients with Subclinical Hypothyroidism: A Case–Control Study. Endocrines, 5(2), 186-196. https://doi.org/10.3390/endocrines5020013








