Modulatory Effects of Ethinyl Estradiol Plus Drospirenone Contraceptive Pill on Spontaneous and GnRH-Induced LH Secretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Assay
2.2. Pulse Analysis
2.3. Statistical Analysis
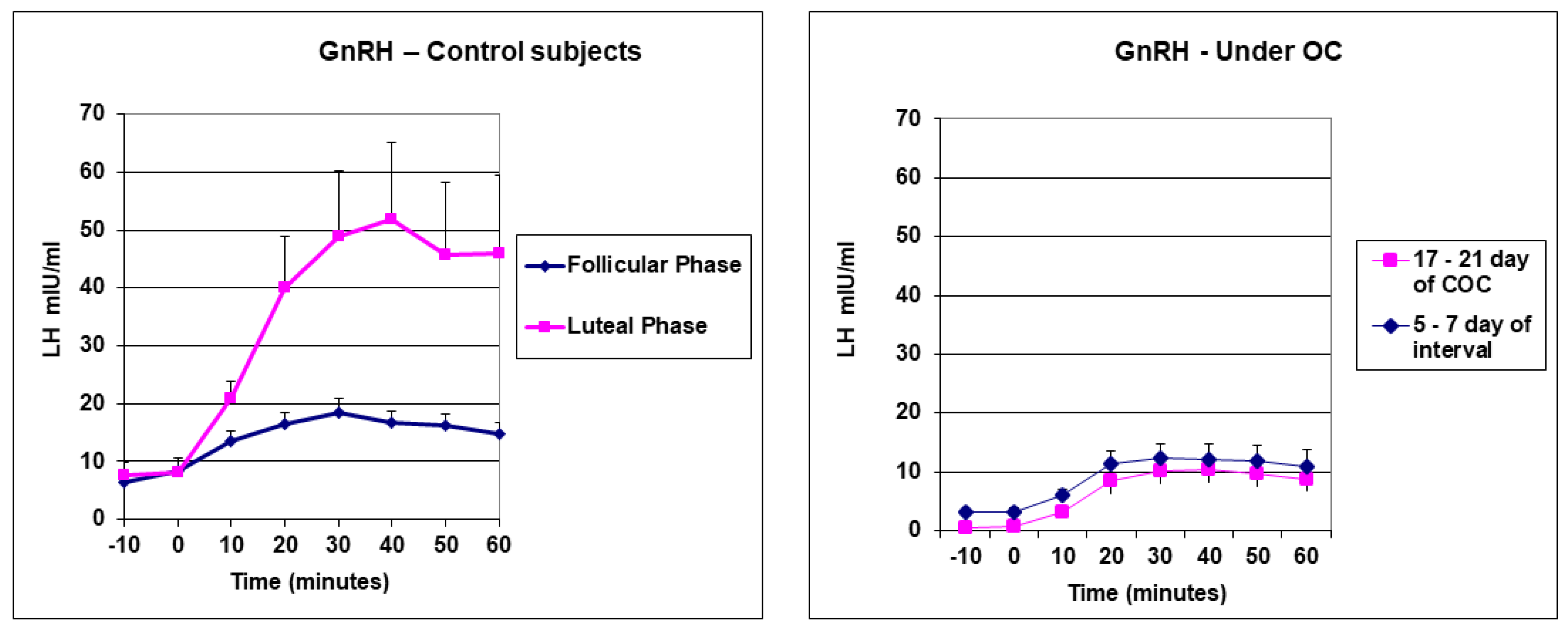
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, J. Neuroendocrine Control of the Menstrual Cycle. In Yen and Jaffe’s Reproductive Endocrinology, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 139–154. [Google Scholar]
- Constantin, S. Progress and Challenges in the Search for the Mechanisms of Pulsatile Gonadotropin-Releasing Hormone Secretion. Front. Endocrinol. 2017, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Garner, K.L.; Voliotis, M.; Tsaneva-Atanasova, K.; McArdle, C.A. Mathematical modeling of gonadotropin-releasing hormone signaling. Mol. Cell. Endocrinol. 2017, 449, 42–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bilezikjian, L.M.; Justice, N.J.; Blackler, A.N.; Wiater, E.; Vale, W.W. Cell-Type Specific Modulation of Pituitary Cells by Activin, Inhibin and Follistatin. Mol. Cell. Endocrinol. 2012, 359, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Seekallu, S.; Toosi, B.; Zeigler, A.; Rawlings, N. Effects of estradiol and progesterone on circulating LH and FSH secretion, and ovarian antral follicle growth in anestrous ewes. Small Rumin. Res. 2010, 91, 178–185. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Guardabasso, V.; Petraglia, F.; Genazzani, A.R. Specific concordance index defines the physiological lag between LH and progesterone in women during the midluteal phase of the menstrual cycle. Gynecol. Endocrinol. 1991, 5, 175–184. [Google Scholar] [CrossRef]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef]
- Harter, C.J.L.; Kavanagh, G.S.; Smith, J.T. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 2018, 238, R173–R183. [Google Scholar] [CrossRef]
- Chabbert-Buffeta, N.; Skinner, D.C.; Caraty, A.; Bouchard, P. Neuroendocrine effects of progesterone. Steroids 2000, 65, 613–620. [Google Scholar] [CrossRef]
- Ferin, M. The Menstrual Cycle: Physiology, Reproductive Disorders, and Infertility; Oxford University Press: New York, NY, USA, 1993; 250p. [Google Scholar]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef]
- Uenoyama, Y.; Tsuchida, H.; Nagae, M.; Inoue, N.; Tsukamura, H. Opioidergic pathways and kisspeptin in the regulation of female reproduction in mammals. Front. Neurosci. 2022, 16, 958377. [Google Scholar] [CrossRef]
- Predieri, B.; Luisi, S.; Casarosa, E.; Farinelli, E.; Antoniazzi, F.; Wasniewska, M.; Bernasconi, S.; Petraglia, F.; Iughetti, L.; Italian Society of Pediatric Endocrinology and Diabetology-Study Group of Puberty. Allopregnanolone levels decrease after gonadotropin-releasing hormone analog stimulation test in girls with central precocious puberty. J. Endocrinol. Investig. 2011, 34, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.D.; Histed, S.N.; Srouji, S.S.; Yang, J.; Lee, H.; Hall, J.E. Estrogen Negative Feedback on Gonadotropin Secretion: Evidence for a Direct Pituitary Effect in Women. J. Clin. Endocrinol. Metab. 2010, 95, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Son, W.Y.; Das, M.; Shalom-Paz, E.; Holzer, H. Mechanisms of follicle selection and development. Minerva Ginecol. 2011, 63, 89–102. [Google Scholar] [PubMed]
- Spona, J.; Elstein, M.; Feichtinger, W.; Sullivan, H.; Lüdicke, F.; Müller, U.; Düsterberg, B. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception 1996, 54, 71–77. [Google Scholar] [CrossRef] [PubMed]
- van Heusden, A.M.; Fauser, B.C. Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception 1999, 59, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Goldzieher, J.W.; de la Pena, A.; Chenault, C.B.; Cervantes, A. Comparatives studies of the ethynil estrogens used in oral contraceptives. III. Effects on plasma gonadotropins. Am. J. Obstet. Gynecol. 1975, 122, 625–636. [Google Scholar] [CrossRef]
- Goldzieher, J.W.; Maqueo, M.; Chenault, C.B.; Woutersz, T.B. Comparative studies of the ethynyl estrogens used in oral contraceptives. I. Endometrial response. Am. J. Obstet. Gynecol. 1975, 122, 615–618. [Google Scholar] [CrossRef]
- Bastianelli, C.; Farris, M.; Rosato, E.; Brosens, I.; Benagiano, G. Pharmacodynamics of combined estrogen-progestin oral contraceptives 3. Inhibition of ovulation. Expert. Rev. Clin. Pharmacol. 2018, 11, 1085–1098. [Google Scholar] [CrossRef]
- Oerter, K.E.; Guardabasso, V.; Rodbard, D. Detection and characterization of peaks and estimation of instantaneous secretory rate for episodic pulsatile hormone secretion. Comput. Biomed. Res. 1986, 19, 170–191. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Pluchino, N.; Begliuomini, S.; Pieri, M.; Centofanti, M.; Freschi, L.; Casarosa, E.; Luisi, M. Drospirenone increases central and peripheral beta-endorphin in ovariectomized female rats. Menopause 2007, 14, 63–73. [Google Scholar] [CrossRef]
- Pluchino, N.; Cubeddu, A.; Giannini, A.; Merlini, S.; Cela, V.; Angioni, S.T.; Genazzani, A.R. Progestogens and brain: An update. Maturitas 2009, 62, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Rodbard, D.; Forti, G.; Petraglia, F.; Baraghini, G.F.; Genazzani, A.R. Estimation of instantaneous secretory rate of luteinizing hormone in women during the menstrual cycle and in men. Clin. Endocrinol. 1990, 32, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Inaudi, P.; Reymond, M.J.; Rey, F.; Genazzani, A.D.; Lemarchand-Béraud, T. Pulsatile secretion of gonadotropins and prolactin during the follicular and luteal phases of the menstrual cycle: Analysis of instantaneous secretion rate and secretory concomitance. Fertil. Steril. 1992, 58, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Henzl, M.R. Evolution of Steroids and Their Contraceptive and Therapeutic Use. In Contraception; Shoupe, D., Haseltine, F.P., Eds.; Clinical Perspectives in Obstetrics and Gynecology; Springer: New York, NY, USA, 1993; pp. 1–16. [Google Scholar] [CrossRef]
- Mendoza, N.; Simoncini, T.; Genazzani, A.D. Hormonal contraceptive choice for women with PCOS: A systematic review of randomized trials and observational studies. Gynecol. Endocrinol. 2014, 30, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Rapkin, A.J.; Korotkaya, Y.; Taylor, K.C. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): Current perspectives. Open Access J. Contracept. 2019, 10, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Hemrika, D.J.; Slaats, E.H.; Kennedy, J.C.; de Vries Robles-Korsen, T.J.; Schoemaker, J. Pulsatile luteinizing hormone patterns in long term oral contraceptive users. J. Clin. Endocrinol. Metab. 1993, 77, 420–426. [Google Scholar]
- De Berardis, D.; Serroni, N.; Salerno, R.M.; Ferro, F.M. Treatment of premenstrual dysphoric disorder (PMDD) with a novel formulation of drospirenone and ethinyl estradiol. Ther. Clin. Risk Manag. 2007, 3, 585–590. [Google Scholar]
- Yoshino, O.; Suzukamo, Y.; Yoshihara, K.; Takahashi, N. Quality of Life in Japanese Patients with Dysmenorrhea or Endometriosis-Associated Pelvic Pain Treated with Extended Regimen Ethinylestradiol/Drospirenone in a Real-World Setting: A Prospective Observational Study. Adv. Ther. 2022, 39, 5087–5104. [Google Scholar] [CrossRef]

| Group A (n = 6) | LH mlU/mL | FSH mlU/mL | PRL ng/mL | E2 pg/mL | P ng/mL | A ng/mL | T ng/dL | 17OHP ng/mL |
|---|---|---|---|---|---|---|---|---|
| Follicular phase (day 4–6) | 6.9 ± 1.7 | 4.4 ± 0.4 | 10.6 ± 1.5 | 72 ± 20.7 | 1 ± 0.1 | 182.8 ± 35.1 | 64.2 ± 10.1 | 1 ± 0.2 |
| Luteal phase (day 17–21) | 9.4 ± 2.1 | 2.9 ± 0.3 | 15.7 ± 2.8 | 155.5 ± 23.8 | 16.8 ± 3.8 | 310.6 ± 46.3 | 69.8 ± 7.3 | 3.6 ± 0.3 |
| p level vs. follicular phase | 0.003 | 0.02 | 0.00004 | 0.0006 | 0.003 | 0.001 |
| Group B (n = 6) | LH mlU/mL | FSH mlU/mL | PRL ng/mL | E2 pg/mL | P ng/mL | A ng/mL | T ng/dL | 17OHP ng/mL |
|---|---|---|---|---|---|---|---|---|
| Day 17–21 of treatment | 1.1 ± 0.3 | 0.95 ± 0.3 | 14.4 ± 0.3 | 15.5 ± 4.2 | 0.8 ± 0.07 | 135.1 ± 19.3 | 43.5 ± 5.0 | 0.4 ± 0.09 |
| p level vs. follicular phase (Table 1) | 0.005 | 0.00001 | 0.007 | 0.01 | ||||
| Day 5–7 of interval | 4.2 ± 0.8 | 5.7 ± 0.7 | 10.4 ± 1.9 | 53 ± 9.8 | 1.1 ± 0.2 | 197.8 ± 23.8 | 57 ± 6.3 | 0.7 ± 0.09 |
| p level vs. follicular phase (Table 1) | n.s | n.s | n.s | n.s | n.s | n.s | n.s | n.s |
| p level vs. Day 17–21 | 0.004 | 0.0007 | 0.007 | 0.04 | 0.03 | 0.03 |
| Group A (n = 6) | LH Spontaneous Secretion | LH ISR on Spontaneous Secretion | GnRH Test—Raw Data | GnRH Test—ISR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Integrated Mean mIU/mL | N Pulse/180 min | Amplitude mIU/mL | N Pulse/180 min | Amplitude mIU/mL | Amplitude mIU/mL | Duration min | Amplitude mIU/mL | Duration min | |
| Follicular phase (Day 4–6) | 5.82 ± 1.2 | 2.6 ± 0.3 | 1.8 ± 0.3 | 2.0 ± 0.3 | 1.6 ± 0.3 | 14.9 ± 1.8 | 120 ± 5 | 7.4 ± 1.4 | 50.6 ± 6.3 |
| p. vs. ISR on GnRH | 0.005 | 0.0000003 | |||||||
| Luteal phase (Day 17–21) | 6.5 ± 1.7 | 1.7 ± 0.3 | 4.9 ± 1.2 | 1.3 ± 0.2 | 2.3 ± 0.2 | 51.2 ± 12.3 | 120 ± 5 | 26.9 ± 6.5 | 51.6 ± 6.6 |
| p. vs. follicular phase | 0.01 | 0.05 | 0.05 | 0.02 | 0.01 | ||||
| p. vs. ISR on GnRH | 0.01 | 0.00009 | |||||||
| Group B (n = 6) | LH Spontaneous Secretion | LH ISR on Spontaneous Secretion | GnRH Test—Raw Data | GnRH Test—ISR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Integrated Mean mIU/mL | N Pulse/180 min | Amplitude mIU/mL | N Pulse/180 min | Amplitude mIU/mL | Amplitude mIU/mL | Duration min | Amplitude mIU/mL | Duration min | |
| Day 17–21 of treatment | 0.6 ± 0.1 | - | - | - | - | 9.1 ± 2.2 | 85 ± 3.7 | 5.4 ± 1.3 | 40 ± 4.9 |
| p. vs. ISR on GnRH | 0.007 | 0.0004 | |||||||
| p. vs. Fol phase | 0.05 | 0.000001 | |||||||
| Day 5–7 of interval | 3.4 ± 0.5 | 2.3 ± 0.2 | 1.4 ± 0.5 | 1.5 ± 0.2 | 1.1 ± 0.2 | 7.5 ± 2.9 | 80.5 ± 7.3 | 6.0 ± 1.6 | 53.3 ± 3.1 |
| p. vs. Day 17–21 | 0.0003 | ||||||||
| p. vs. ISR on GnRH | 0.03 | 0.00001 | |||||||
| p. vs. Fol phase (Table 3) | 0.05 | 0.00001 | |||||||
| p. vs. Luteal phase (Table 3) | 0.05 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genazzani, A.D.; Sponzilli, A.; Mantovani, M.; Fusilli, E.; Ricciardiello, F.; Semprini, E.; Simoncini, T.; Battipaglia, C. Modulatory Effects of Ethinyl Estradiol Plus Drospirenone Contraceptive Pill on Spontaneous and GnRH-Induced LH Secretion. Endocrines 2024, 5, 36-45. https://doi.org/10.3390/endocrines5010003
Genazzani AD, Sponzilli A, Mantovani M, Fusilli E, Ricciardiello F, Semprini E, Simoncini T, Battipaglia C. Modulatory Effects of Ethinyl Estradiol Plus Drospirenone Contraceptive Pill on Spontaneous and GnRH-Induced LH Secretion. Endocrines. 2024; 5(1):36-45. https://doi.org/10.3390/endocrines5010003
Chicago/Turabian StyleGenazzani, Alessandro D., Alessandra Sponzilli, Marcello Mantovani, Emma Fusilli, Francesco Ricciardiello, Elisa Semprini, Tommaso Simoncini, and Christian Battipaglia. 2024. "Modulatory Effects of Ethinyl Estradiol Plus Drospirenone Contraceptive Pill on Spontaneous and GnRH-Induced LH Secretion" Endocrines 5, no. 1: 36-45. https://doi.org/10.3390/endocrines5010003
APA StyleGenazzani, A. D., Sponzilli, A., Mantovani, M., Fusilli, E., Ricciardiello, F., Semprini, E., Simoncini, T., & Battipaglia, C. (2024). Modulatory Effects of Ethinyl Estradiol Plus Drospirenone Contraceptive Pill on Spontaneous and GnRH-Induced LH Secretion. Endocrines, 5(1), 36-45. https://doi.org/10.3390/endocrines5010003






