Abstract
Adenomyosis (ADM) is a multifaceted uterine pathology characterized by the ectopic infiltration of endometrial tissue into the myometrium, affecting approximately 20% of women in the reproductive age group seeking gynecological care. This condition manifests as a range of debilitating symptoms, including dysmenorrhea, menorrhagia, impaired fertility, and heightened susceptibility to miscarriage and obstetric complications. Substantial research has been dedicated to exploring its underlying molecular mechanisms and developing non-invasive precision medical therapies. ADM is primarily characterized by a dysregulation in sex steroid hormone homeostasis, particularly estrogen and progesterone. However, emerging evidence suggests that additional endocrine mediators and disruptors may play contributory roles in the etiology of ADM. Genetic and epigenetic alterations of endocrine signaling pathways have been implicated as prevailing mechanisms underlying the development and progression of the disease. The present review aims to provide an updated and comprehensive overview of the current understanding of the pathophysiology of ADM, with a particular emphasis on the dysregulated hormonal milieu and the potential involvement of endocrine disruptors. By elucidating these intricate molecular mechanisms, this review seeks to pave the way for novel research directions in the development of targeted therapeutic strategies for ADM management.
1. Introduction
Adenomyosis (ADM) is a benign, chronic, hormonal, and inflammatory uterine disorder, affecting between 8.8% and 61.5% of patients undergoing hysterectomy for diverse medical indications. This important variability is primarily attributed to the absence of consensus on histopathological diagnostic criteria. Image screening, potentially superior for pre-menopausal women compared to hysterectomy, lacks standardized diagnostic criteria. The prevalence of ADM also varies widely among subgroups of women with concurrent uterine-related conditions, including leiomyomas, prolapse, abnormal uterine bleeding, infertility, and endometriosis [1]. The most common clinical manifestations are bleeding, dysmenorrhea, chronic pelvic pain, and infertility [2]. ADM increases the risk of miscarriage and obstetric complications, but the underlying biological mechanisms remain unclear [3]. Historically categorized as “endometriosis interna”, ADM shares similarities with endometriosis in terms of the ectopic presence of functional endometrial-like tissue. However, the diseases differ in clinical profiles since endometriosis is often identified in nulligravid, younger women during infertility investigations, while ADM is prevalent in parous, relatively older women [4,5].
The ruling histological definition of ADM was given in 1972 by Bird, stating “Adenomyosis may be defined as the benign invasion of endometrium into the myometrium, producing a diffusely enlarged uterus which microscopically exhibits ectopic non-neoplastic, endometrial glands and stroma surrounded by the hypertrophic and hyperplastic myometrium” [6]. However, ADM diagnosis by medical imaging remains a challenge. Because recent evidence revealed that ADM also impacts younger women, uterus-sparing diagnostic methods are needed [7]. Transvaginal ultrasound and magnetic resonance imaging (MRI) have emerged as equally effective noninvasive diagnostic alternatives [8]. ADM can hence be divided into focal, diffuse, or rarely cystic adenomyoma, depending on the distribution of the lesion pattern within the myometrium [9,10,11]. Further genetic studies are needed in order to define a molecular signature of ADM in endometrium and blood-based biomarkers. All things considered, there is potential for better diagnosis, or even prediction, of ADM [12].
While a consensus regarding the classification of ADM is yet to be reached, the classification proposed by Kishi et al. in 2012 has gained recognition and can aid in distinguishing various origins of the lesions. This classification system delineates four distinct subtypes. Subtype I, referred to as intrinsic ADM, originates from the inner layer of the uterus without impacting the outer structures. Subtype II, known as extrinsic ADM, arises from the outer layer of the uterus, leaving the inner structures unaffected. Subtype III, termed intramural ADM, exists independently and lacks direct continuity with structural components. Lastly, subtype IV represents an indeterminate type, requiring further investigation to determine its specific characteristics [13]. Unfortunately, this classification is not associated with typical clinical symptoms; therefore, it does not help in choice of treatment [14].
Despite the widespread occurrence and adverse effects on quality of life of ADM, the absence of management guidelines or standardized treatments for this condition is likely attributable to an insufficient understanding of its pathophysiology and etiology [15].
The present manuscript aims to elucidate the underlying mechanisms governing the development of ADM with a focus on the role of hormones to enhance our understanding of the endocrine aspects of the disease and potential therapeutic opportunities. We will discuss the different theories and mechanisms with an insight into the endocrine mediators. Specifically, the influence of hormones, as well as external endocrine disruptors, on ADM will be considered. Additionally, we will address the potential hormonal role behind infertility associated with ADM. Finally, this review will discuss the traditional, as well as the emerging, hormonal therapies and other medical options for managing ADM.
2. Pathogenesis
In the past 15 years, an expanding body of research suggests a potential shared origin between ADM and endometriosis. Recently, Leyendecker and colleagues introduced that both conditions originate from the “archimetra”, which constitutes the endometrial–sub-endometrial unit. Their theory suggests that the two illnesses are essentially the same and proposes a new term, “archimetrosis”. Similar preventive measures could be explored, such as early intervention to suppress hypercontractility shortly after menarche onset. However, it is essential to note that several distinctions exist between these two conditions, particularly concerning their triggers. As with all advancing theories, this one requires further substantiating evidence [5,16].
Even though theories have emerged to clarify the mechanisms driving ADM development, none of them have been accepted yet. The three theories proposed are (a) the invagination of basalis endometrial cells in the myometrium caused by the activation of tissue injury and repair (TIAR) mechanism, (b) metaplasia from Mullerian rests, and (c) retrograde menstruation of adult stem cells [17].
2.1. Theory of Invagination of Endometrial Basalis in the Myometrium
Regarding the etiology of ADM, the prevailing hypothesis suggests that the endometrial myometrial junction zone (JZ), susceptible to steroid hormonal influences, undergoes trauma from myometrial contractions. As a result, the disjunction of this ”barrier” allows the endometrial basalis cells to enter the myometrium [17]. Supporting the myometrium’s participation in pathogenesis is the presence of myometrial cell nests embedded within the endometrium in a model of induced ADM [18]. That said, the reason for their presence is vague. Therefore, two major assumptions are made to explain the origin of the vicious cycle present in ADM [19,20].
The first theory relies on the mechanism of tissue injury and repair (TIAR). It posits that repeated chronic hyperperistalsis of the uterus under the influence of estrogen induces trauma and activates this system in return [5,16]. This biomechanical stress leads to the localized release of additional estrogen. Evidence shows that, due to the injury at the JZ, inflammatory cascade reactions will lead to the elevation of interleukin 1B (IL-1B). This local elevation in IL-1B subsequently triggers the production of prostaglandin E2 (PGE2) by cyclooxygenase-2 (COX-2). Then, an increase in P450 aromatase levels leads to upregulation of the steroidogenic acute regulatory protein. This in turn activates estradiol (E2) to increase the expression of estrogen receptor (ER) beta isoform (ERβ). Hyperestrogenism from aberrant levels of ER-b is hypothesized to initiate the growth of adenomyotic lesions [21]. This can spur a vicious circle: estrogen-mediated contractions > auto-traumatization > wound-healing > inflammation > more local estrogen production [20,22]. The tissue repair mechanisms also involve macrophages, platelets, and their secreted cytokines, leading to chronic inflammation at the JZ and facilitating endometrial attachment, as well as infiltration. This theory elegantly elucidates the fibroblast-to-myofibroblast trans-differentiation and the recruitment of heterogenous cells with ADM-specific signatures, contributing to local changes in the extracellular matrix [23].
The second theory, endometrial–myometrial interface disruption (EMID), states that the injury can be iatrogenic because of mechanical or thermal stress. These disruptions may be induced surgically, for example. In 2020, the EMID theory was demonstrated in mice [24]. Various mechanisms are theorized to be at play, including hypoxia, epithelial–mesenchymal transition (EMT), the recruitment of bone-marrow-derived stem cells, and increased survival of endometrial cells that have been dispersed and displaced due to iatrogenic procedures. At the molecular level, on one hand, activated platelets induce the expression of hypoxia-inducible factor-1 (HIF1), potentially influenced by transforming growth factor-beta (TGF-β) signaling. On the other hand, tissue damage increases substance P levels, triggering the hypothalamic–pituitary–adrenal axis to release adrenaline and noradrenaline, subsequently leading to a reduction in cell-mediated immunity [25]. A link between the TIAR and EMID theories offers a possible explanation for epidemiological observations. Indeed, these theories suggest that multiparity and uterine surgeries such as curettages or caesarian sections may be risk factors for ADM due to their potential to disrupt the integrity of the JZ [26]. Numerous factors, such as inflammation, heightened nociceptive sensitivity, biomechanical strain, chemical exposure, neurogenic distress, pain awareness, and oxidative stress, possess the capacity to trigger this tissue response [27,28]. Repeated tissue trauma interrupts the sectarian vascularization, leading to hypoxia and therefore platelet aggregation, increased estrogen production, induction of angiogenic factors, and overexpression of COX2 and PGE2. This all intensifies the stress, resulting in the invagination process described hereinabove [29].
2.2. De Novo Development Theories
The EMID theory claims that adenomyotic lesions arise de novo, autonomously from metaplasia of misplaced embryonic pluripotent Müllerian remnants in the myometrium [30]. These ducts, composed of surface epithelium and urogenital mesenchyme, can potentially develop into endometrial glands and stroma. Within adult myometrial tissue, residual cells have the capacity to undergo metaplastic changes, giving rise to new ectopic endometrial tissue [31,32].
The last theory, the retrograde hypothesis, is based on the transformation of multipotent adult stem cells in the myometrium. It is thought that these stem cells settle in the endometrial basalis, within niches, ensuring cell regeneration in healthy endometrium [33,34,35]. However, they can also promote unregulated proliferation that can extend beyond the endometrium into the myometrium [36]. Adult stem cells may (a) be deposited in the uterus after retrograde menstruation and differentiate into endometrial glands and stroma or (b) be activated by tissue injury altering the niche and permitting their differentiating progeny to move toward the myometrium rather than toward the endometrial functionalis resulting in focal ADM [17,34,37]. Also known as the “outside to inside invasion” theory, this notion is strongly supported by the established association between posterior focal ADM (extrinsic) and deep infiltrating endometriosis nodules in the posterior compartment [11,38].
Based on recent evidence, these de novo theories account more for the external subtype of ADM. A recent article showed that the immunohistological pattern of Ber-EP4-stained glands and CD10-stained stromal cells of extrinsic ADM is like that of coexistent DIE lesions. In contrast, the pattern of the gland and stromal cells resembled the endometrium in the cases with intrinsic ADM. In addition to these theories, the possibility that extrinsic ADM may also arise directly from coexistent DIE warrants further study [39].
3. Endocrine Pathogenic Mediators and Molecular Mechanisms
ADM involves a multitude of mechanisms, and this review focuses on elucidating the endocrine aspects of the disease in the pursuit of identifying potential therapeutic opportunities. The other mechanisms, including dysregulation of cell proliferation, resistance to apoptosis, inflammatory responses, neurogenesis, angiogenesis, and fibrosis, have been recently examined in separate reviews [25,37]. In this section, we examine (1) intrinsic endocrine factors, including steroid imbalances, pituitary influences, genetic and epigenetic contributions, and (2) environmental implications, in particular, the role of endocrine disruptors (Figure 1).
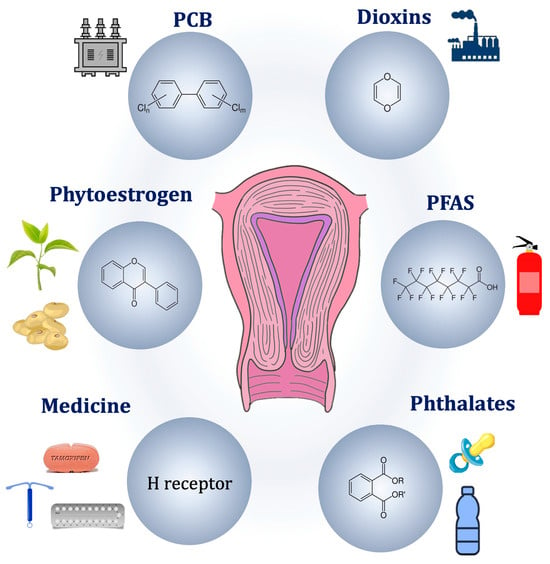
Figure 1.
Potential endocrine pathogenic causes of adenomyosis.
3.1. Intrinsic Hormonal Dysregulation
3.1.1. The Imbalance of Sex Steroid Hormones
Pronounced estrogen dependency is a unique characteristic of the disease and is central to its development. Indeed, the lesions develop in a hyperestrogenic environment, exhibit distinct patterns of ERs, and manifest signs of localized estrogenic effects, namely an estrogen-responsive uterine contractility. It has been three decades since elevated E2 levels were observed in the menstrual blood of a limited cohort of ADM patients, showing notably higher concentrations compared to patients with endometriosis [40]. Interestingly, increased E2 was limited to the lesions, while circulating levels were unaffected. Consequently, the localized estrogen synthesis in ectopic endometrial cells has been attributed to high estrone (E1) sulfatase levels, which activates circulating sulfated steroids and local activity of aromatase, an enzyme that converts androgens into estrogens [41]. In the context of ADM, sulfatases and sulfotransferases also contribute to the activation of steroids, particularly in the modulation of estrogen levels. Sulfatases, such as estrone sulfatase, play a role in local estrogen synthesis in ectopic endometrial cells by activating circulating sulfated steroids. Additionally, sulfotransferases are involved in the conjugation of sulfate groups onto steroids, affecting their bioavailability and enhancing their activity [42,43]. This explanation was further supported by a specific polymorphism of aromatase cytochrome P450 found in the eutopic endometrium of ADM [44,45]. The role of aromatase in ADM remains a topic of debate, with another group failing to identify a significant contribution of aromatase in endometriosis [46]. Most recently, however, clinical evidence has demonstrated the effectiveness of low-dose aromatase inhibitors in improving symptoms of ADM, such as menorrhagia, hemoglobin levels, and lesion size [47]. Using a recent transgenic murine model, Heinosalo et al. demonstrated that overexpression of the human estrogen biosynthetic enzyme hydroxysteroid, 17-beta-hydroxysteroid dehydrogenase type 1 (17β-HSD1), catalyzing the last step in estrogen activation, leads to the development of an ADM-like phenotype [48]. Conversely, the activity of 17β-HSD2, which deactivates E2 to E1, is downregulated in their eutopic and ectopic endometrium [17,49]. Two components are hypothesized to be responsible for the hyperestrogenic status observed in patients with ADM. An increased local aromatization process and a decreased local estrogen metabolism within both the eutopic and ectopic endometrium are thought to give rise to this occurrence.
Effects of High Estrogen Concentration
The local increase in estrogen concentration with normal peripheral E2 levels may cause hyperperistalsis of the uterus [50]. These specific steroids seem to create a paracrine effect, presumably mediated by the endometrial oxytocin (OT) signaling. This estrogen dominance is considered the “primum movens” in the chain of key events [37].
Hypersensitivity to Estrogen
The higher risk of developing ADM is associated with hypersensitivity to estrogen via specific polymorphism and relatively increased expression of ERα. A decreased expression of progesterone receptor isoform B (PR-B) is also understood to intensify the risk of developing ADM. Concerning the cognate receptors, ERα (NR3A1) and ERβ (NR3A2), membrane ERα and β, and G protein-coupled ER (GPER) were expressed significantly more in ADM than in normal myometrium. These findings are the same as those in endometriosis. The ERα isoform plays dominant roles in uterine development and estrogen sensitivity during the early proliferative phase and differential subtype expression in later phases [51,52]. Accordingly, ERα mediates the E2-induced uterine epithelial cell proliferation of human endometrial cells [53]. Given the central role of this isoform, an in vivo study discovered specific PvuII restriction fragment-length polymorphisms (PP, Pp, pp genotypes) of the ERα gene in ADM patients. The findings suggested a protective characteristic of the P allele, as well as how the local estrogenic effect is more potent with the P allele than with the p allele. Even if it is still unclear how the ERα gene polymorphisms influence its protein function, the authors suggested a possible explanation by a modulation of the ligand estrogen [54]. The ERβ isoform was described as upregulated in adenomyotic lesions and was proposed to be responsible for inflammation in ADM [55]. In regard to the GPER receptor, it enhances contractile responses to OT in the myometrium, seemingly supporting the TIAR theory in ADM [55,56].
Progesterone Resistance
Also proposed is the notion that progesterone resistance may contribute to the hormonal imbalance theory in ADM, as with endometriosis [57]. With this in mind, the proliferative effect driven by hyperestrogenism is not enough when counteracted by progesterone during the secretory phase of the cycle. As a result, hyperproliferation of the endometrium is promoted [58].
The predominance of ERβ over ERα leads to the suppression of PR-B expression and thus the development of progesterone resistance. PR-B and PR-A, two isoforms of the nuclear receptor PR, have dynamic cellular localization, influencing the effect of progesterone. PR-B can promote uterine epithelial cell proliferation but only when not repressed by PR-A. In ADM, PR-B was reportedly suppressed by DNA hypermethylation. Conversely to endometriosis, however, a recent study did not find decreased expression of progesterone membrane receptors in ADM, suggesting molecular differences between ADM and endometriosis [55].
Summary on Sex Steroid Dysregulation
In short, the highly localized concentrations of estrogen combined with altered expression of steroid receptors are central mechanisms leading to ADM (Figure 2). The local conversion of androgens and estrone sulfate into estradiol is catalyzed by increased activity of CYP19A1, STS (steroid sulfatase), and 17βHSD1 enzymes. Additionally, an altered expression pattern of steroid receptors (Erβ >> Erα > PRA > PRB) contributes to ERβ hyperactivation and progesterone resistance. These characteristics lead to reduced decidualization, increased proliferation of endometrial and myometrial smooth muscle cells, and endometrial angiogenesis. Together, these are key elements in the onset and progression of ADM lesions [17].
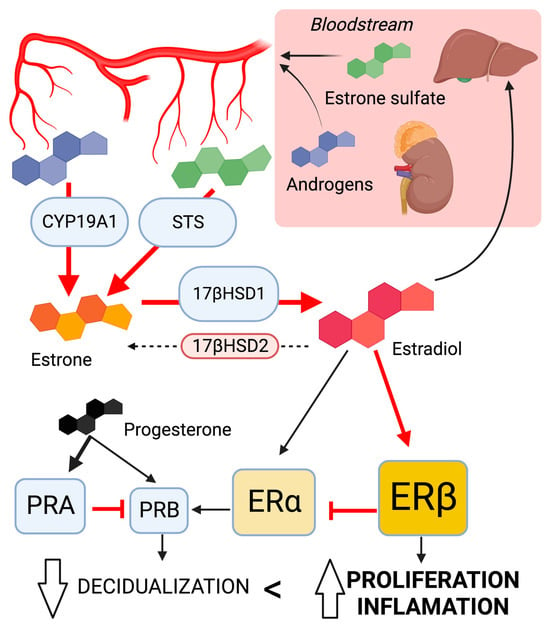
Figure 2.
The dysregulation of sex steroid signaling; involvement in the development of ADM lesions leads to local hyperestrogenism. Changes in key enzyme activity promote estradiol synthesis. The altered pattern of steroid receptor expression enhances estrogen response while suppressing progesterone response. Ellipses represent enzymes; object size correlates with activity. Squares represent sex steroid receptor; object size correlates with expression levels. Arrows indicate the predominant direction in ADM lesions. Created with biorender.com.
3.1.2. The Pituitary Gland Influence
Prolactin
Several in vivo studies identified the role of prolactin (PRL) in ADM. In 1981, an ectopic anterior pituitary gland transplantation into the uterine lumen was sufficient in inducing ADM in mice. Circulating levels of PRL were consistently higher in pituitary isograft mice than in control mice with submaxillary gland grafts. This emphasizes the significant role of PRL in synergy with ovarian steroid hormones. Indeed, steroid supplements were necessary in pituitary grafts of ovariectomized mice to induce ADM [59]. Similar results were obtained after the administration of PRL or dopamine antagonists, inducing hyperprolactinemia. Moreover, induced ADM mice exhibited a significant upregulation of the messenger ribonucleic acid (mRNA) coding for PRL receptors [59,60]. These early findings were confirmed when observed in spontaneously occurring bovine ADM, where the levels of PRL and its receptors were found to be abnormally high during necropsies. When cells were isolated in vitro, E2 decreased the expression levels of PRL receptors in non-adenomyotic stromal cells and adenomyotic myometrial cells while increasing the secretion of PRL in adenomyotic myometrial cells [61]. In a murine model of ADM exposed to a potent suppressor of pituitary prolactin secretion, the treatment resulted in the absence of adenomyosis in all 39 experimental mice at 12 months of age, while 46.9% of 32 control mice developed the condition. These findings indicate a potential protective role of dopamine agonists within the context of ADM [61,62]. Validating this preclinical data, serum PRL is reported to be higher in patients with ADM than controls. A correlation was even suggested between the rise of PRL levels as an adverse effect of serotonin reuptake inhibiting antidepressants and the development of ADM [63]. The hyperprolactinemic state leads to the invasion of endometrial stromal cells and then glands into the myometrium. This coincides with the overall loosening and disruption of the myometrial layer and the disintegration of individual muscle cells [64]. Moreover, PRL enhances E2 actions in the uterus and stimulates ER expression in the endometrium. This initiates a vicious cycle within the ADM myometrial cells [61]. If not a sufficient explanation for the whole process by itself, the surgical act of grafting mice may cause mechanical disruption, aiding the invasion of endometrial tissue [65]. The intravaginal administration of bromocriptine, a dopamine agonist reducing PRL level, proved significant in decreasing menstrual bleeding and pain [66].
Oxytocin
Through estrogen-mediated contractions in the inner myometrium, OT is suspected to intervene in the microtrauma of the JZ event. It provokes hyperperistalsis of the myometrium via the cognate OT receptor (OTR), resulting in the TIAR effect. OTRs are expressed both in normal endometrium and myometrium, but their expression fluctuates with the cycle phase [67]. However, high non-cyclic expression was observed in a histological biopsy from ADM patients [68]. OTR expression at the JZ is higher in the fundal region of ADM uteri compared to control during the proliferative phase. Moreover, expression of the OTR was lower in the isthmic region than in the fundus region of ADM uteri during the proliferative phase, which is the opposite of control uteri. The dysperistalsis event can be explained by the overexpression of the fundal myometrial OTR in pathological uteri. The opposite expression pattern between fundus and isthmus may even interfere with fertility and the sperm track by disturbing the direction of the JZ contractions [69]. Furthermore, relative overexpression of the OTR in the myometrial cells of ADM patients combined with high-amplitude muscle cell contractility was positively correlated with the severity of dysmenorrhea in patients with ADM. Thus, treatments known to reduce OTR expression, like deacetylase inhibitors and andrographolides, hold potential in treating ADM [70].
Insulin-like Growth Factor 1
Insulin-like growth factor 1 (IGF1) is a multifaceted factor that plays a pivotal role in regulating growth and development. In the uterus, IGF1 is essential for adequate decidualization of the endometrium and directly affects fertility. Significant hormonal crosstalk exists between estrogen and IGF1. E2 enhances IGF1 synthesis and IGF1 potentiates the effects of estrogen [71]. In the endometrium, IGF1 expression and secretion are prominent in stromal cells, while IGF1 receptors are expressed in epithelial cells. In patients with ADM, the expression of IGF1 receptors is significantly elevated, contributing to the aberrant growth of endometrial tissue within the uterine wall [72]. IGF1 may exacerbate inflammation and fibrosis. Furthermore, the interplay between IGF1 and estrogen could further promote lesion formation. These findings warrant greater research to fully elucidate the precise mechanisms by which IGF1 contributes to the development and progression of ADM.
3.1.3. Genetics and Epigenetics Alteration of Endocrine Signaling
Genetic variants influence enzyme activity and increase the risk of estrogen dependency in ADM. In particular, cytochrome P450 (CYP) and catechol-O-methyltransferase (COMT) gene variants are involved [73]. When comparing ADM to disease-free patients, a recent study found an increased frequency of the C allele in T/C and C/C genotypes of the CYP1A1 gene (CYP1A1 M1 polymorphism), the A allele in C/A and A/A genotypes of the CYP1A2 gene (CYP1A2*1F polymorphism), and the T allele in C/T and C/C genotypes of the CYP19 gene (Arg264Cys polymorphism). The study also found a decreased frequency of the mutant allele and heterozygous and mutant homozygous genotype of the CYP1A2 gene in ADM patients. These results suggest that ADM is triggered by the active hormones and their metabolites’ higher concentration due to enzymes under these polymorphisms’ influence [73].
Another presumption is that the KRAS gene mutation is part of the genetic predisposition to ADM. Among micro-dissected eutopic samples, KRAS mutations were observed in 55.6% of those with ADM, 50% of those with endometriosis, and only 29.1% of the disease-free cohort [74]. KRAS-activating mutations stimulate signaling pathways to enhance cell survival and proliferation and are associated with progesterone resistance in ADM [75]. Recent findings support genetics as a driver in the pathogenesis of ADM through alterations in gene functions, governing not only the steroid function but also the extracellular cell matrix dysregulation, angiogenesis, TIAR mechanism, and inflammatory mediators. This was thoroughly reviewed by Zhai et al. and will not be detailed in the present review [17].
Evidence also reports several types of epigenetic alterations in ADM. In relation to the deoxyribonucleic acid, increased expression of deoxyribonucleic acid methyltransferases DNMT1 and DNMT3B were found in the ectopic endometrium of ADM patients [76]. These enzymes catalyze the transfer of a methyl group to DNA for gene silencing [76,77]. Therefore, they are a candidate for explaining the hypermethylated status of the PR gene observed in ADM and its progesterone resistance [78]. Histone epigenetic modification is a second potential mechanism of the disease. Aberrant expression and localization of class I histone deacetylases (HDACs) was demonstrated in the endometrium, and the immunoreactivity of HDAC1 and HDAC3 was elevated in the adenomyotic endometrium (both ectopic and eutopic) [79]. Hence, the use of valproic acid, a well-known HDAC inhibitor, has been proposed as an effective treatment in refractory disease based on murine observations [80]. In patients with ADM, alterations in epigenetic modifications were detected in RNA molecules. A decrease in RNA methyl regulators and, specifically, lower levels of N6-methyladenosine was observed in the pathological endometrium compared to controls. This contributes to an increase in the expression of various factors, including IGF1 [81]. Finally, the expression of regulatory microRNAs was dysregulated in the eutopic endometrium of ADM patients, including namely members of the miRNA-200 family pivotal in EMT, and Let-7 involved in cell cycle control. The detailed discussion of those examples can be found in the review by Khan et al. and will not be included here [26]. Although there is continuing evidence from several studies that support the involvement of the epigenetic system in ADM, additional research is needed to conclusively pinpoint epigenetic aberrations as a mechanism of disease upgrowth.
3.2. Extrinsic Factors
3.2.1. Medical Therapies
A possible correlation between the use of hormonal contraceptives and the occurrence of ADM has been considered, but a consensus has not been reached yet. Templeman et al. proposed a positive association between past hormonal contraceptive use and ADM onset, yet ambiguity persists regarding whether subjects primarily employed contraception for birth control or symptom management [82]. It is plausible that the use of the hormonal contraceptive was preferred by the patients already experiencing ADM, rather than the contraceptives being a risk factor for the development of the condition. Conversely, Parazzini et al. could not establish significant association between ADM and a history of hormonal contraceptive use [83,84]. While heightened exposure to exogenous estrogen through hormonal contraceptives may contribute to the development of lesions, such exposure could lead to a reduction in endogenous estrogen production, thereby lowering the risk of ADM [1]. Continued research is necessary to clarify the specific relationship between hormonal contraceptive use and ADM, considering the various reasons for contraceptive use among individuals.
Tamoxifen (TAM) is the prototypical selective ER modulator (SERM), a class of non-steroidal drugs exhibiting agonist and/or antagonist effects given the target tissue. TAM displays anti-estrogenic effects in breast tissue and pro-estrogenic activity in uterine tissue. Due to its demonstrated impact on endometrial tissue, the use of TAM has been identified as a risk factor for ADM [1]. Clinical findings showed that women undergoing treatment for breast cancer with involving TAM are at a higher likelihood of developing ADM than control patients (53% compared to 18%) [85]. Mice studies complemented the human data by revealing an association between TAM exposure and ADM development and progression as mice aged. Interestingly, the study uncovered significant contributions of platelets in the development of TAM-induced lesions, affirming their involvement in the disease and indicating that TAM induces ADM through the TIAR mechanism [86,87].
3.2.2. Endocrine Disrupting Chemicals
Endocrine disrupting chemicals (EDCs) are exogenous compounds that interfere with the endocrine functions, potentially affecting health and promotion of disease [88]. EDCs can be divided into persistent and non-persistent organic pollutants (POPs, nonPOPs), based on their lipid solubility [89]. Both groups are lipophilic, but nonPOPs have a lower lipid solubility, resulting in a short half-life in humans. On the other hand, POPs are not readily biodegradable. Their bioaccumulation in the adipose tissue and slow release in the bloodstream account for the long-term effects [90]. Unfortunately, industrialization has made exposure to EDCs inevitable, either through packaged consumer products or contaminated foods. Since the end of 20th century, warnings have been issued against the harmful effects of EDCs, prompting the ban of polychlorinated biphenyls (PCBs), dichloro-diphenyl-trichloroethane (DDT), and diethylstilbestrol (DES) [91].
Animal studies conclusively demonstrated that EDCs are sufficient to induce ADM or endometriosis. Specifically for ADM, murine studies contributed to the understanding of the role and toxicity of EDCs [18,92,93]. The significant clinical coexistence of both conditions within a patient strongly supports a high probability of a causal relationship between EDC exposure and the initiation of endometrium-related diseases [90]. The probable association of early life exposure to EDCs with ADM offers valuable insights for therapeutic approaches and essentially its prevention [94].
Persistent Organic Pollutants
POPs are a group of synthetic chemicals resistant to environmental degradation, such as PCBs (mainly used as insulant), perfluoroalkyl substances (PFAS, notably in firefighting foams and non-stick cookware), tetrachlorodibenzo-p-dioxin (TCDD, present in Agent Orange), and dichlorodiphenyltrichloroethane (DTT, insecticide). They persist in the environment for decades, or even centuries, and can bioaccumulate in trophic networks. The hydroxylated metabolites of POPs can also have estrogenic activity.
Multiple epidemiological studies investigated the involvement of PCBs in the context of endometriosis [94]. Reflecting potential prenatal exposure, a Chinese study reported a notable correlation between a shorter anogenital distance amongst patients diagnosed with endometriosis or ADM [94]. Moreover, the sum of PCB levels was significantly higher in patients with rectovaginal ADM compared to patients with endometriosis and controls.
The ENDO study found that two types of PFAS were associated with increased incidence of endometriosis diagnosis. No association has been reported for ADM yet [95].
Association studies established a link between TCDD and endometriosis [96,97]. TCDD has the capacity to modulate signaling pathways mediated by the steroid hormones in the normal uterine physiology [98]. Bruner-Tran et al. conducted a retrospective investigation to identify mice with ADM-like lesions resulting from any type of exposure to TCDD over multiple generations [18,99]. Deep adenomyotic lesions were detected in more than half of the mice with a history of either direct (F1-F2) or indirect (F3) exposure [18]. Nonetheless, further studies are needed to assess a potential link between TCDD and ADM.
Non-Persistent Organic Pollutants
NonPOPs, such as glyphosate, phtalates, bisphenol, and pyrethroids, can degrade and break down relatively quickly in the environment, often within days to months. They are typically less likely to bioaccumulate in organisms than POPs and have lower potential for long-range transport through air and water.
Clinical studies address the potential role of gene–environment interactions in the context of ADM. Huang et al. revealed an increased risk for ADM in individuals who carry the glutathione S-transferase M1 polymorphism (GSTM1) and are exposed to high levels of phthalates compared to those unexposed [100]. Although rare cases of EDCs induce genetic mutations, most EDCs are unable to alter DNA sequences. Conversely, an association between epigenetic modifications and EDC exposure has been expressed. Some toxic agents in the environment were also proven to generate epigenetic modifications within the germline, hence causing multi- and transgenerational repercussions [101]. Despite a lack of evidence regarding the exact bond between EDCs and epigenetics, it seems like they act with two mechanisms: gene-specific and global action [102].
Phthalates are EDCs characterized by an anti-androgenic and pro-estrogenic effect [103]. The concentration of phthalates in endometriosis patients’ blood is significantly higher than in control patients [104,105]. Other studies showed an increase in urinary phthalates in patients suffering from endometriosis [100,106]. Similar results were seen in an additional study from this group that identified a modest increase in urinary phthalates in patients with either endometriosis or ADM [107]. In a case–control study, urinary levels of phthalates, particularly MEHP (the primary metabolite of DEHP), were higher and strongly associated with significantly increase in risk for ADM (OR = 10.4; 95% CI, 1.26–85.0) [100].
Bisphenols are estrogen-mimicking molecules that maintain a low concentration of PRs, eventually leading to uterine cyclicity disruption [108]. Using a murine model, Newbold et al. identified a correlation between BPA neonatal exposure and suggested a link between parental EDC exposure and the onset of endometriosis and ADM in the female offspring [109]. In 2010, another research group demonstrated that prenatal exposure to BPA in mice caused development of endometrial glands and stroma within adipose tissue neighboring the reproductive tract, accompanied by the expression of ERs and HOX-A10 [92]. However, the substantiating evidence linking BPA to ADM remains relatively scant in comparison to its association with endometriosis.
Diethylstillbestrol (DES) is a synthetic potent estrogen that was given to mitigate the risk of pregnancy loss but was prohibited in the 1970s following the disclosure of significant morbidities to females and their female offspring [110]. Further studies highlighted a link between in utero exposure to DES and the risk of endometriosis [111]. Although epidemiology studies have not identified a link between DES exposure and ADM in humans, mice studies suggested a positive correlation between DES and ADM [112,113].
3.2.3. Natural Endocrine Disruptors
Phytoestrogens can function as endocrine disruptors by binding to ERs and either mimicking or blocking the effects of natural estrogen. These actions can result in hormonal imbalances. They can exert ER-independent mechanisms of action, such as altering hormone-binding globulin levels. Furthermore, some phytoestrogens inhibit aromatase and other enzymes involved in the synthesis of steroid hormones. Several studies have suggested that phytoestrogens may be involved in the development or progression of uterine diseases, such as endometrial cancer [114,115].
3.2.4. Mode of Action of Endocrine Disruption
Most endocrine disruptors act gene-specifically by interfering with NR function, but global action is considered. NRs regulate gene-specific chromatin states by engaging histone modifiers and recruiting DNMTs and thymine DNA glycosylases (TDGs) to specified genomic loci [116]. This is supported by different studies in mice, though still not demonstrated in humans [93,117]. EDCs act globally on DNMTs by downstream regulation of messenger RNA and/or microRNA expression by defective receptors [118,119]. Moreover, studies showed a dysregulation of DNA demethylases and histone-modifying enzymes by EDC exposure [120,121].
Based on current scientific knowledge, endocrine disruptors can have an agonist or antagonist effect on hormone receptors or alter hormone receptor expression, as described with the TCDD and decreased PR expression in mice uteri [122]. EDCs, with the help of EDC co-factors, are also linked to a perturbation of both nuclear steroid receptors and cell surface membrane receptors, causing a dysregulation of downstream signal transduction [123,124]. Furthermore, they can alter the activity of hormone-responsive cells by interfering with hormonal transport [123].
4. Infertility in ADM
ADM could exert detrimental effects on fertility and perinatal outcomes. Inflammation, immune modulation, oxidative stress, extracellular matrix remodeling, and aberrant angiogenesis have all been implicated as factors contributing to altered oocyte development, uterine receptivity, implantation, and successful maintenance of pregnancy.
A recent prospective randomized study called ADENOFERT (NCT05937490) analyzes the association of ADM with fertility outcomes in relation to different GnRH agonist protocols of assisted reproductive technology (ART). The study started in July 2023 and will last until 2025. The goal of this clinical trial is to not only investigate the pregnancy and neonatal outcomes of women under different protocols in ART but also how the endometrial interface stimulates decidualization markers in response to treatments in ART. Via an in vitro study, ADENOFERT also plans to evaluate the immune changes during the pregnancy [125].
Both medical and surgical treatments of ADM have positive results on fertility [126]. However, fertility outcomes were better in focal ADM than diffuse ADM treated by surgical and/or medical treatment [127]. Despite many studies on the pathogenesis of fertility failure in ADM, there is a lack of correlation between their results and treatments. Therefore, more evidence is needed to explain the real association between ADM and infertility. This will allow a standardized protocol to be established for fertility treatment in ADM.
Various mechanisms are suspected to be part of the fertility disturbance in ADM patients. The local inflammation during the endometrial cell infiltration process is suggested to be a central cause of infertility. The platelet aggregation and hypoxia preceding the production of cytokines/prostaglandins and local estrogen may prompt both uterine hyperperistalsis through estrogen receptor induction of OT signaling and fibrosis [128]. The biological mechanisms suspected have been reviewed by Szubert et al. in 2021. However, there is still a lack of conclusive data in humans that prevents us from drawing conclusions [129].
In general, endometriosis and ADM are associated with a negative impact on fertility. All things considered, the clinical weight given to the different effects of endometriosis and ADM on fertility is still uncertain, with only weak associations based on a limited number of studies and events [128].
5. Therapeutics Options
5.1. Conventional Hormonal Treatments
Because of the strong estrogen dependency of ADM, like endometriosis, several hormonal mechanisms have been studied to control the hormonal medium [44]. Although no drug is specifically approved for the treatment of ADM, some off-label treatments show promise for clinical management [15] (Figure 3).
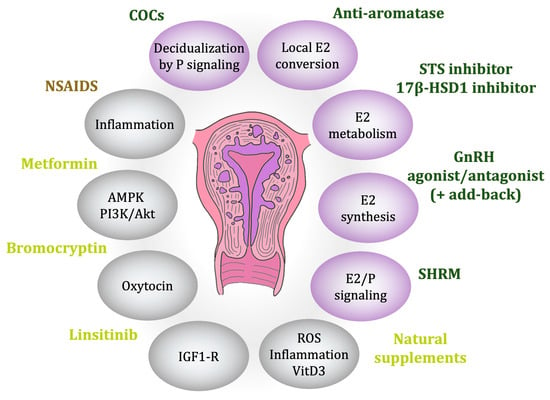
Figure 3.
Existing and promising therapies. Ellipses denote the targeted action mode, with purple indicating steroid-related functions, primarily regarding estrogen. Family compound colors: brown represents family compounds with at least one approved drug for symptom management, green denotes at least one approved for disease control, and yellow signifies drugs currently under investigation.
5.1.1. Non-Steroidal Anti-Inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are used to relieve pain without treating the pathology. In fact, they are believed to relieve the hypercontractility of the uterus by inhibiting endometrial prostaglandin production through cyclooxygenase enzyme inhibition. They also have a direct analgesic effect at the central nervous system level [130,131]. Recent research evaluated the effects of the selective COX-2 inhibitor celecoxib on the development of uterine adenomyosis in mice. Celecoxib significantly reduced disease severity by inhibiting infiltration into the myometrium, decreasing estrogen levels, reversing epithelial–mesenchymal transition, and relieving fibrosis. Further studies are needed to validate these findings and to determine the optimal dosage, duration, and potential side effects [86].
5.1.2. Combined Oral Contraceptives and Progestin
Combined oral contraceptives (COCs) inhibit LH and FSH and subsequently block follicle development and endometrial proliferation [132]. They are proven to be beneficial in treating dysmenorrhea, regardless of the underlying cause [133,134]. Regarding ADM, two clinical trials found that both the levonorgestrel-releasing intrauterine device (LNG-IUD) and dienogest were more effective in managing associated pain and bleeding [135,136]. A recent meta-analysis of prospective studies by Abbas et al. demonstrated that LNG-IUDs effectively reduced symptom severity, uterine volume, and endometrial thickness while also improving laboratory outcomes [137]. The proposed mechanism of action is based on the decidualization and atrophy of the endometrium and downregulation of ERs due to high progestin release [138]. There is ongoing debate regarding the optimal duration of treatment for ADM, with efficacy reported for durations between 1 and 6 years [139]. For ADM patients with contraception desires, LNG-IUDs are considered preferable to other hormonal therapies due to their direct action on the uterus, low systemic hormone levels, and long-acting user-independent administration. Despite encouraging results from several clinical trials, progestin efficacity in ADM is still debated. Moreover, metrorrhagia was a very frequent side effect that led patients to discontinue their treatment [139].
The primary drawback of progestins is the significant proportion of non-responders, which is likely due to a progesterone resistance mechanism, previously documented in both ADM and endometriosis [139,140]. A case report on hormonal receptor expression suggested that the abnormal expression of ER and PR isoforms in ADM uteri contributes to disease pathogenesis, symptomatology, and resistance to medical treatments [58].
5.1.3. Gonadotropin-Releasing Hormone Agonists and Antagonists
Gonadotropin-releasing hormone (GnRH) agonists possess greater potency and a longer half-life compared to native GnRH and initially stimulate pituitary gonadotrophs, known as the initial flare-up. With continued non-pulsatile administration, their therapeutic effect is achieved by binding to and sequestering natural GnRH receptors, inhibiting gonadotropin secretion, and ultimately reducing E2 concentration [15]. Despite promising results regarding pain, amenorrhea, uterine volume [141], and JZ thickness [142], they are associated with more adverse effects due to the induced hypoestrogenism effect. One of note is a reduction in bone mineral density that limits the duration of the treatment without add-back therapy. Furthermore, symptoms tend to reappear upon treatment cessation [143]. On the other hand, encouraging results, named in the review of Stratopoulou et al. in 2021, suggest pre-treatment with GnRH analog therapy before in vitro fertilization (IVF) [139].
Promising results were reported for GnRH antagonists as a potential drug to treat ADM. GnRH antagonists allow dose-dependent control of E2 levels and are characterized by the absence of a flare-up effect and rapid reversibility. Two studies were published by Donnez’s team on the subject, concluding that an initial course of 200 mg/day linzagolix for 12 weeks and further treatment with either 100 mg/day linzagolix or 200 mg/day linzagolix with add-back therapy should be evaluated for long-term management [144]. Clinical randomized trials are suggested to certify these encouraging findings.
5.1.4. Hormonal Targeting Therapies
Selective Hormonal Receptor Modulators
The development of multiple pharmaceutical agents that selectively target hormonal receptors (selective hormonal receptor modulator, SHRM), with a particular focus on ERs, arises from the strong dependence of endometriosis and ADM on estrogen [145]. Despite extensive efforts and investments, this therapeutic strategy experienced a series of unfortunate clinical failures, as reviewed by Guo and Groothuis [146].
Raloxifene, another SERM binding both ERα and ERβ, displays tissue-specific activities based on the expression of ERs, and co-activators or repressors. For example, it has an estrogenic agonist effect on bone but an antagonist effect in both the breast and the uterus. In a murine model, raloxifene effectively reversed the implantation of endometriosis lesions. One proposed mechanism suggests that raloxifene counteracts the EMT process and impedes the migration of epithelial cells. However, a phase 2 clinical trial revealed a potential drawback, as raloxifene exposure was associated with a faster recurrence of pain symptoms and an elevated risk of venous thromboembolism as compared with a placebo [146].
In 1981, mifepristone (or RU-486) was synthesized as the first selective PR modulator (SPRM). A preclinical study showed that mifepristone inhibits the development of ADM in mice [147]. Mifepristone downregulates the expression of various genes (CDK1/CDK2/Cyclin B/Cyclin E/CXCR4) in the endometrium, inhibiting the proliferation, migration, and invasion of endometrial cells through the JZ. Individuals diagnosed with ADM who received treatment with mifepristone 5 mg/day experienced a reduction in uterine volume, restoration of hemoglobin levels, and a significant reduction in dysmenorrhea [148]. Laboratory investigation of the treated patients showed lower secretion of interleukin-6 and tumor necrosis factor from endometrial epithelial and stromal cells, restricted infiltration, and degranulation of mast cells in eutopic and ectopic endometrium. In 2019, a multicenter, placebo-controlled, double-blind, randomized clinical trial was conducted to further investigate the effectiveness of mifepristone in treating ADM. With dysmenorrhea as the primary endpoint, significant clinical improvement was achieved with acceptable tolerability after a 12-week treatment of mifepristone at a dosage of 10 mg/day [149].
Ulipristal acetate (UPA), another potent SPRM capable of delaying ovulation and endometrial maturation, is indicated for emergency contraception; the management of uterine fibroids; and refractory, severe heavy menstrual bleeding [150,151]. In patients with ADM and concomitant fibroids, UPA impressively alleviates bleeding symptoms, but pain exacerbation in over half the patients limits its utility [152]. Worsening pelvic pain, bulk symptoms, and imaging features following UPA treatment are frequently reported and warrant discontinuation for ADM. Notably, UPA serves as a diagnostic tool for adenomyosis when fibroids remain unresponsive to UPA therapy [153].
Aromatase Inhibitors
In 2007, the efficacy of anti-aromatase therapy was noticed through a case study involving the concurrent administration of GnRH agonists. This study observed a decrease in uterine volume following the combined treatment, but it could not demonstrate the contribution of each drug [154]. A prospective randomized controlled trial (RCT) was thus granted to compare the efficacy of aromatase inhibitors (letrozole at a dosage of 2.5 mg/day) to that of GnRH agonists (goserelin at a dosage of 3.6 mg/month). In their report, the authors established similar results in reducing the volume of adenomyoma and improving the symptoms [155]. Most recently, Sharma et al. reported in their RCT that a lower dose of letrozole (2.5 mg, 3 times weekly) resulted in regular menstrual cycles, improved hemoglobin concentrations, and sonographic feature enhancements, especially in cases of diffuse ADM and adenomyoma [47]. It appeared to be more cost-effective and had no noticeable side effects compared to GnRH agonist therapy. Nevertheless, further research is needed to establish definitive conclusions due to the undecided debate regarding the contribution of aromatase in ADM.
Sulfatase Inhibitors
Sulfatase inhibitors, exemplified by danazol, are substances that impede the activity of sulfatase enzymes, thereby preventing the conversion of sulfated steroids into their active, biologically potent forms. In ADM, these inhibitors are explored for their therapeutic potential in addressing the hyperestrogenic environment; however, despite their ability to inhibit sulfatase and potentially alleviate symptoms, the recurrence of ADM after discontinuing treatment suggests challenges in achieving sustained relief [156].
Danazol is a synthetic androgen and gonadotropin inhibitor that exhibits significant sulfatase inhibitory activity, reducing the conversion of sulfated steroids into their active forms. Researchers investigated its potential to mitigate the hyperestrogenic environment in ADM. Danazol also proved to inhibit aromatase activity in ADM lesions [45]. However, symptoms tend to reappear within a few menstrual cycles after discontinuing treatment, suggesting that danazol alone may not be sufficient to provide long-lasting relief from ADM symptoms [15]. Furthermore, danazol therapy is associated with potential side effects, such as masculinization, weight gain, acne, and liver abnormalities.
17β-Hydroxysteroid Dehydrogenase Type 1 Inhibitors
Inhibitors of the enzyme 17β-HSD1 involved in converting E1 to E2 have been suggested as a potential therapeutic approach to reduce E2 levels. This strategy is promising for the treatment of other estrogen-dependent conditions such as endometriosis and breast cancer. A research group successfully synthesized a series of novel C15-substituted derivatives. Extensive in vivo tests of one compound showed selective inhibition of recombinant human 17β-HSD1 (IC50 at 10 nM) with no effect on 17β-HSD2 activity [157]. The efficacy and safety of Organon Finland’s reversible inhibitor is being assessed in a phase 2 double-blind RCT, the Elena study (NCT05560646), for the treatment of moderate to severe endometriosis [158]. Poirier’s team tested a non-estrogenic and steroidal covalent irreversible inhibitor of 17β-HSD1 (named PBRM) in endometriosis models with promising results [159].
Currently, information regarding the side effects, efficacy, and tolerability of 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1) inhibitors appears to be limited. The details shared focus on the synthesis and testing of specific inhibitors, such as those developed by Organon Finland and Poirier’s team, with an emphasis on their potential in treating conditions like endometriosis. However, there is no explicit mention of comprehensive studies detailing the side effects or tolerability of these inhibitors [159]. To provide a thorough comparison, additional data from clinical trials or research studies evaluating the efficacy, safety profile, and adverse effects of 17β-HSD1 inhibitors would be required. As of now, the available information underscores the need for further investigations and studies to ascertain the overall impact of these inhibitors on patient health and their suitability for therapeutic applications [160].
5.2. Unconventional Treatments and Ongoing Research
The scarce and disappointing outcomes of clinical trials in adenomyosis highlight the shortcomings of past research methodologies. Considering recent advancements in understanding the natural history of ectopic endometrium, a reassessment of previous research and novel treatment strategies is warranted [146]. Multiple treatment options have been reported for endometriosis and ADM, such as anti-platelet, anti-inflammatory, and anti-Let7-miRNA therapies and minimally invasive interventions like uterine artery embolization, high-intensity focused ultrasounds, and radiofrequency ablation [161]. The present review will solely focus on endocrine and dietary innovations.
5.2.1. Bromocriptine
We already discussed the role of bromocriptine as a new potential studied treatment in Section 3.1.2. for PRL. The pilot study in 2018 of Andersson et al. lasting 6 months showed a significant reduction in the symptoms, as well as the JZ thickness, in a small number of patients reflected in radiological appearance [162]. Despite preclinical observations proposing an association between high uterine concentration of PRL and ADM, limited evidence prevents the establishment of a causal relationship. Additional research investigating aberrant PRL signaling is necessary to evaluate the therapeutic potential of bromocriptine for treating ADM [66,163]. A recent publication found that bromocriptine effectively suppresses the proliferation and migration of endometrial cells amongst individuals with ADM uteri. The proposed mechanism of action involves the suppression of specific gene expression through the activation of several miRNAs, namely members of the miRNA-200 family and Let-7, as well as the enrichment of signaling pathways associated with cell proliferation and apoptosis. Moreover, bromocriptine demonstrated the highest sensitivity towards these specific microRNAs already identified in the context of ADM, underlying key regulatory roles for PRL in the disease [164]. Further research and validation are necessary to confirm and expand upon these initial findings.
5.2.2. Oxytocin Antagonists
As stressed above, OT is suspected to contribute to ADM via overexpression of the OTR in uteri suffering from ADM [68]. Non-peptide oxytocin antagonists, distinct from peptide-based counterparts, offer a potential avenue for addressing ADM. By targeting oxytocin receptors through alternative chemical structures, these antagonists hold promise in mitigating pain, dysperistalsis, and inflammation associated with ADM, providing a diverse range of therapeutic options for this condition. Several compounds were developed such as Barusiban, Nolasiban, Retosiban, and Epelsiban. A phase I trial on Epelsiban, initially tested for embryo transfer, proved it to be well tolerated and with no significant safety concerns [165]. Its efficacy was investigated in phase II (NCT02794467), but results were inconclusive as the trial was halted by the sponsor for priority reasons, rather than safety concerns or regulatory interactions [166]. Nevertheless, further trials are needed to evaluate its efficacy [167].
5.2.3. Metformin
Metformin is a biguanide drug widely used in type 2 diabetes and, in some cases, polycystic ovary syndrome, where it is used as an ovulation induction agent. In muscle, adipose tissue, and liver, metformin suppresses gluconeogenesis and reduces blood sugar by activating adenosine monophosphate-activated protein kinase (AMPK) and may protect from liver fibrosis by suppressing the TGF-β pathway. The first comparative in vitro study investigating the potential therapeutic effect of metformin in ADM biopsies revealed the involvement of AMPK and PI3K/AKT signaling pathways. This founding study showed higher AMPK expression levels in endometrial cells from ADM patients than in disease-free patients. The authors demonstrated that metformin inhibits the proliferation of ADM endometrial cells via activation of AMPK and inhibition of PI3K/AKT pathways, hence identifying a potential therapeutic target in ADM [168]. Further investigations are needed to establish whether metformin is a viable treatment option for ADM.
5.2.4. Linsitinib
Linsitinib is a specific inhibitor of the IGF1 receptor with modest activity against the insulin receptor, primarily designed for cancer applications. It demonstrates the ability to reverse pain behavior in an animal model of endometriosis at 40 mg/kg [169].
5.2.5. Nutritional Supplements
While dietary supplements have already been studied in the context of endometriosis, research on their use specifically for ADM is limited. Given the potential relationship between these two conditions, particularly in cases of deep nodular endometriosis and ADM, exploring the efficacy of the same nutritional additives in ADM is worthwhile. Dietary supplements offer an interesting natural alternative because of their antioxidant, anti-inflammatory, anti-proliferative, and immune-modulatory properties.
Epigallactocatechin-3-Gallate
Epigallactocatechin-3-gallate (EGCG) is a bioactive plant-based compound specifically found in green tea. It possesses inhibitory effects on estrogen-related activation, proliferation, and vascular endothelial growth factor (VEGF) expression in endometrial cell cultures. Animal studies provide further evidence that green tea can downregulate the VEGF signaling pathway, leading to a reduction in the formation of endometriotic implants [170]. Another study examined the potential of EGCG in relieving dysmenorrhea in mice with tamoxifen-induced ADM. The researchers observed that the induction of the disease led to an elevation in plasma corticosterone levels, which could be attributed to the stress and hyperalgesia caused by the lesions. In this model, the administration of EGCG demonstrated positive effects in reducing plasma corticosterone levels, alleviating uterine contractility, and suppressing the infiltration of myometrial cells, suggesting its clinical potential for symptom management [171]. In a subsequent study conducted by the same group, there was a significant reduction in inhibitory gamma-aminobutyric acid (GABA)ergic neurons in the raphe magnus nucleus following the induction of ADM, indicative of hyperalgesia. Administration of EGCG restores the number of these neurons, suggesting a potential attenuation of the ADM-associated hyperalgesia [172]. Collectively, these findings provide insights into the potential beneficial effects of green tea, specifically EGCG, for ADM. Continued investigation is warranted to explore and validate the therapeutic potential of green tea in managing ADM-related symptoms.
Vitamin D
Vitamin D (VitD), a lipophilic vitamin found in fatty fish, liver, egg yolk, and cheese, plays a crucial role in various reproductive processes. It presents a role in both the development and suppression of proliferative processes in reproductive organs with its antiproliferative effects and ability to stimulate differentiation. Its active form, calcitriol (VitD3), improves the hypothalamic–pituitary system, immune system, steroidogenesis, folliculogenesis, and endometrial function. Association studies proposed hypovitaminosis D in the pathogenesis of endometriosis, while a higher dietary intake of VitD has been found to lower the risk [170]. As a biological explanation, endometriosis epithelial and stromal cells exhibit elevated VitD metabolism activity, approximately 10-fold greater than that of control cells, which plays a crucial role in regulating cellular motility and invasion [169]. In a recent publication of over 150 patients, a daily dose of at least 2000 IU of VitD for 3 months is recommended for management of ADM. The authors stressed the potential to correct the metabolic process in ADM via a repeated course of treatment [173].
Curcumin
Evidence demonstrates that curcumin exhibits hormonal regulatory properties in addition to its anti-inflammatory, anti-angiogenic, and antioxidant effects. In vitro, curcumin potentially enhances folliculogenesis in cases of endometriosis. Additionally, curcumin reduces estrogen production, and high doses are used to inhibit cell proliferation and counteract the stimulatory effect of exogenous estrogen seen in estrogen-dependent breast cancers. Animal studies further demonstrate that curcumin treatment results in a regression of endometriotic lesions. These findings highlight the potential therapeutic implications of curcumin, although further research is needed to validate and elucidate the underlying mechanisms [170]. Evidence supports that a dietary curcumin supplement, alongside standard therapies for patients with endometrial disorders, leads to optimal outcomes and a significant reduction in pain-related symptoms without any indications of systemic toxicity [174]. That being said, curcumin’s clinical application is hindered by its instability and low bioavailability. Loading curcumin into exosomes enhances its solubility, stability, and activity. While not yet studied for endometriosis or ADM, exosome-loaded curcumin shows promise for future research [175].
Quercetin
Quercetin, a prominent dietary compound found in vegetables and fruits, possesses anti-inflammatory, antioxidant, and hormonal modulation properties. Regarding endometriosis, quercetin shows various beneficial effects by inhibiting the proliferation of endometriotic cells and inducing cell cycle arrest. Additionally, it promotes apoptosis by stimulating the production of reactive oxygen species. Animal studies reveal that quercetin exhibits both anti-estrogenic and anti-progestogenic effects. When administered at a dose of 10 mg/kg in conjunction with steroids, it exerts an anti-estrogenic effect on uterine weight. However, at a higher dose of 100 mg/kg, quercetin demonstrates a potent estrogenic effect [176]. These findings suggest that incorporating quercetin into the natural therapeutic arsenal may offer an alternative treatment option for individuals with endometriosis and ADM, complementing existing approaches [170].
6. Conclusions and Perspectives
The etiology of ADM remains partially unresolved, with numerous factors likely contributing to its development. However, the role of the endocrine system has been established, particularly estrogen and progesterone dysfunction, immune response, inflammation, and altered effects of OT and PRL on uterine hyperperistalsis. Estrogen-mimetic EDCs are suspected to play a role in initiating these changes. Various hormonal and non-hormonal therapies have been proposed to alleviate symptoms and preserve fertility. Targeting sex steroid hormones and exploring other pathogenetic endocrine pathways are potential means of action. The growing demand for personalized and natural therapies warrants the investigation of novel approaches and dietary supplements for ADM. Clinical trials are essential for determining effective treatment strategies. Specific attention should be directed towards understanding the consequences of environmental EDC exposure and establishing preventive measures. In conclusion, continued investigation into the molecular, genetic, and hormonal mechanisms underlying the development and progression of ADM is necessary to deepen our understanding of the disease and to discover novel therapeutic options.
Author Contributions
Conceptualization, D.D.M., G.E.C., J.d., M.d.C. and R.O.; methodology, J.d. and M.d.C.; validation, D.D.M., D.P.-G., G.E.C., M.d.C., P.P. and R.O.; writing—original draft preparation, J.d.; writing—review and editing, G.E.C., P.P. and R.O.; supervision, D.D.M., G.E.C., M.d.C. and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Individual researcher funding includes grants from the National Researcher Incentive Program (PRONII) of the Paraguayan National Council for Research and Technology (CONACYT) awarded to D.D.M. (level I).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Victoria Torok (B.Sc.) for revising the English language of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Upson, K.; Missmer, S.A. Epidemiology of Adenomyosis. Semin. Reprod. Med. 2020, 38, 89–107. [Google Scholar] [CrossRef]
- Taran, F.A.; Stewart, E.A.; Brucker, S. Adenomyosis: Epidemiology, Risk Factors, Clinical Phenotype and Surgical and Interventional Alternatives to Hysterectomy. Geburtshilfe Frauenheilkd. 2013, 73, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Taniguchi, F.; Harada, T. Increased Risk of Obstetric Complications in Patients with Adenomyosis: A Narrative Literature Review. Reprod. Med. Biol. 2022, 21, e12473. [Google Scholar] [CrossRef] [PubMed]
- Benagiano, G.; Brosens, I.; Habiba, M. Structural and Molecular Features of the Endomyometrium in Endometriosis and Adenomyosis. Hum. Reprod. Update 2014, 20, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L.; Laschke, M.W.; Mall, G. Archimetrosis: The Evolution of a Disease and Its Extant Presentation: Pathogenesis and Pathophysiology of Archimetrosis (Uterine Adenomyosis and Endometriosis). Arch. Gynecol. Obstet. 2023, 307, 93–112. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I. History of Adenomyosis. Best. Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 449–463. [Google Scholar] [CrossRef]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F.; Professor, A.; Doctor, T. The Impact of Adenomyosis on Women’s Fertility. Obstet. Gynecol. Surv. 2016, 71, 557–568. [Google Scholar] [CrossRef]
- Sam, M.; Raubenheimer, M.; Manolea, F.; Aguilar, H.; Mathew, R.P.; Patel, V.H.; Low, G. Accuracy of Findings in the Diagnosis of Uterine Adenomyosis on Ultrasound. Abdom. Radiol. 2020, 45, 842–850. [Google Scholar] [CrossRef]
- Van Den Bosch, T.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; et al. Terms, Definitions and Measurements to Describe Sonographic Features of Myometrium and Uterine Masses: A Consensus Opinion from the Morphological Uterus Sonographic Assessment (MUSA) Group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Classification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.W.; Just, P.A.; Noël, J.C.; et al. Diagnosing Adenomyosis: An Integrated Clinical and Imaging Approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Antero, M.F.; Ayhan, A.; Segars, J.; Shih, I.M. Pathology and Pathogenesis of Adenomyosis. Semin. Reprod. Med. 2020, 38, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four Subtypes of Adenomyosis Assessed by Magnetic Resonance Imaging and Their Specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef] [PubMed]
- Moawad, G.; Fruscalzo, A.; Youssef, Y.; Kheil, M.; Tawil, T.; Nehme, J.; Pirtea, P.; Guani, B.; Afaneh, H.; Ayoubi, J.M.; et al. Adenomyosis: An Updated Review on Diagnosis and Classification. J. Clin. Med. 2023, 12, 4828. [Google Scholar] [CrossRef]
- Vannuccini, S.; Luisi, S.; Tosti, C.; Sorbi, F.; Petraglia, F. Role of Medical Therapy in the Management of Uterine Adenomyosis. Fertil. Steril. 2018, 109, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Benagiano, G.; Guo, S.W. An Appraisal of the Tissue Injury and Repair (TIAR) Theory on the Pathogenesis of Endometriosis and Adenomyosis. Biomolecules 2023, 13, 975. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Duleba, A.J.; Taylor, H.S.; Osteen, K.G. Developmental Toxicant Exposure Is Associated with Transgenerational Adenomyosis in a Murine Model. Biol. Reprod. 2016, 95, 73. [Google Scholar] [CrossRef]
- Donnez, J.; Donnez, O.; Dolmans, M.M. Introduction: Uterine Adenomyosis, Another Enigmatic Disease of Our Time. Fertil. Steril. 2018, 109, 369–370. [Google Scholar] [CrossRef]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of Uterine Adenomyosis: Invagination or Metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Huang, J.H.; Duan, H.; Wang, S.; Wang, Y.Y. Estrogen 17βestradiol Accelerates the Proliferation of Uterine Junctional Zone Smooth Muscle Cells via the Let7a/Lin28B Axis in Adenomyosis. Mol. Med. Rep. 2021, 23, 337. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L.; Leyendecker, G. A New Concept of Endometriosis and Adenomyosis: Tissue Injury and Repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, Y.; Liu, H.; Liang, N.; Xu, L.; Li, Y.; Yao, W.; Shi, W.; Liu, Z. Single-Cell Profiling Uncovers the Roles of Endometrial Fibrosis and Microenvironmental Changes in Adenomyosis. J. Inflamm. Res. 2023, 16, 1949–1965. [Google Scholar] [CrossRef]
- Hao, M.; Liu, X.; Guo, S.W. Adenomyosis in Mice Resulting from Mechanically or Thermally Induced Endometrial–Myometrial Interface Disruption and Its Possible Prevention. Reprod. Biomed. Online 2020, 41, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Vannuccini, S.; Capezzuoli, T.; Fambrini, M.; Vannuzzi, V.; Donati, C.; Petraglia, F. Mechanisms and Pathogenesis of Adenomyosis. Curr. Obstet. Gynecol. Rep. 2022, 11, 95–102. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Mori, T. Pathogenesis of Human Adenomyosis: Current Understanding and Its Association with Infertility. J. Clin. Med. 2022, 11, 4057. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Inoue, T.; Tateishi, S.; Fujishita, A.; Nakashima, M.; Masuzaki, H. Additive Effects of Inflammation and Stress Reaction on Toll-like Receptor 4-Mediated Growth of Endometriotic Stromal Cells. Hum. Reprod. 2013, 28, 2794–2803. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Imamura, T.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Toll-like Receptor 4-Mediated Growth of Endometriosis by Human Heat-Shock Protein 70. Hum. Reprod. 2008, 23, 2210–2219. [Google Scholar] [CrossRef]
- Guo, S.W. The Pathogenesis of Adenomyosis Vis-à-Vis Endometriosis. J. Clin. Med. 2020, 9, 485. [Google Scholar] [CrossRef]
- Bergeron, C.; Amant, F.; Ferenczy, A. Pathology and Physiopathology of Adenomyosis. Best. Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 511–521. [Google Scholar] [CrossRef]
- Spencer, T.E.; Hayashi, K.; Hu, J.; Carpenter, K.D. Comparative Developmental Biology of the Mammalian Uterus. Curr. Top. Develop Biol. 2005, 68, 85–122. [Google Scholar] [CrossRef]
- Spencer, T.E.; Dunlap, K.A.; Filant, J. Comparative Developmental Biology of the Uterus: Insights into Mechanisms and Developmental Disruption. Mol. Cell. Endocrinol. 2012, 354, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Nguyen, H.P.T.; Ye, L. Endometrial Regeneration and Endometrial Stem/Progenitor Cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E. Uterine Stem Cells: What Is the Evidence? Hum. Reprod. Update 2007, 13, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Deane, J.A.; Masuda, H.; Maruyama, T.; Gargett, C.E. Stem Cells in Endometrial Physiology. Semin. Reprod. Med. 2015, 33, 326–332. [Google Scholar] [CrossRef]
- Hufnagel, D.; Li, F.; Cosar, E.; Krikun, G.; Taylor, H.S. The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis. Semin. Reprod. Med. 2015, 33, 333–340. [Google Scholar] [CrossRef]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of Adenomyosis: An Update on Molecular Mechanisms. Reprod. BioMed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef]
- Chapron, C.; Tosti, C.; Marcellin, L.; Bourdon, M.; Lafay-Pillet, M.C.; Millischer, A.E.; Streuli, I.; Borghese, B.; Petraglia, F.; Santulli, P. Relationship between the Magnetic Resonance Imaging Appearance of Adenomyosis and Endometriosis Phenotypes. Hum. Reprod. 2017, 32, 1393–1401. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Koshiba, A.; Kuroboshi, H.; Mori, T.; Ogi, H.; Itoh, K.; Nakashima, M.; Kitawaki, J. Biological Differences between Intrinsic and Extrinsic Adenomyosis with Coexisting Deep Infiltrating Endometriosis. Reprod. BioMed. Online 2019, 39, 343–353. [Google Scholar] [CrossRef]
- Takahashi, K.; Nagata, H.; Kitao, M. Clinical Usefulness of Determination of Estradiol Level in the Menstrual Blood for Patients with Endometriosis. Nihon Sanka Fujinka Gakkai Zasshi 1989, 41, 1849–1850. [Google Scholar] [PubMed]
- Yamamoto, T.; Noguchi, T.; Tamura, T.; Kitawaki, J.; Okada, H. Evidence for Estrogen Synthesis in Adenomyotic Tissues. Am. J. Obstet. Gynecol. 1993, 169, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of Aromatase Expression in Estrogen-Responsive Breast and Uterine Disease: From Bench to Treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Reed, M.J. Regulation of Estrogen Synthesis in Postmenopausal Women. Steroids 2002, 67, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, J. Adenomyosis: The Pathophysiology of an Oestrogen-Dependent Disease. Best. Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 493–502. [Google Scholar] [CrossRef]
- Kitawaki, J.; Noguchi, T.; Amatsu, T.; Maeda, K.; Tsukamoto, K.; Yamamoto, T.; Fushiki, S.; Osawa, Y.; Honjo, H. Expression of Aromatase Cytochrome P450 Protein and Messenger Ribonucleic Acid in Human Endometriotic and Adenomyotic Tissues but Not in Normal Endometrium. Biol. Reprod. 1997, 57, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Colette, S.; Lousse, J.C.; Defrère, S.; Curaba, M.; Heilier, J.F.; Van Langendonckt, A.; Mestdagt, M.; Foidart, J.M.; Loumaye, E.; Donnez, J. Absence of Aromatase Protein and MRNA Expression in Endometriosis. Hum. Reprod. 2009, 24, 2133–2141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, S.; Roychoudhury, S.; Padmaja Bhattacharya, M.; Hazra, S.; Majhi, A.K.; Oswal, K.C.; Chattopadhyay, R. Low-Dose Letrozole À an Effective Option for Women with Symptomatic Adenomyosis Awaiting IVF: A Pilot Randomized Controlled Trial. Reprod. BioMed. Online 2023, 47, 84–93. [Google Scholar] [CrossRef]
- Heinosalo, T.; Rytkönen, K.T.; Saarinen, N.; Järvensivu, P.; Damdimopoulou, P.; Strauss, L.; Orasniemi, S.; Horshauge, P.; Gabriel, M.; Koskimies, P.; et al. Overexpression of Human Estrogen Biosynthetic Enzyme Hydroxysteroid (17beta) Dehydrogenase Type 1 Induces Adenomyosis-like Phenotype in Transgenic Mice. Int. J. Mol. Sci. 2022, 23, 4815. [Google Scholar] [CrossRef]
- Kitawaki, J.O.; Koshiba, H.; Ishihara, H.; Kusuki, I.; Tsukamoto, K.; Honjo, H. Progesterone Induction of 17-Hydroxysteroid Dehydrogenase Type 2 during the Secretory Phase Occurs in the Endometrium of Estrogen-Dependent Benign Diseases But Not in Normal Endometrium. J. Clin. Endocrinol. Metab. 2000, 85, 3292–3296. [Google Scholar] [CrossRef]
- Urabe, M.; Yamamoto, T.; Kitawaki, J.; Honjo, H.; Okada, H. Estrogen Biosynthesis in Human Uterine Adenomyosis. Acta Endocrinol. 1989, 121, 259–264. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Winuthayanon, W.; Korach, K.S. What’s New in Estrogen Receptor Action in the Female Reproductive Tract. J. Mol. Endocrinol. 2016, 56, 55–71. [Google Scholar] [CrossRef]
- Oehler, M.K.; Greschik, H.; Fischer, D.C.; Tong, X.; Schuele, R.; Kieback, D.G. Functional Characterization of Somatic Point Mutations of the Human Estrogen Receptor α (HERα) in Adenomyosis Uteri. Mol. Hum. Reprod. 2004, 10, 853–860. [Google Scholar] [CrossRef][Green Version]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, J.; Obayashi, H.; Ishihara, H.; Koshiba, H.; Kusuki, I.; Kado, N.; Tsukamoto, K.; Hasegawa, G.; Nakamura, N.; Honjo, H. Oestrogen Receptor-Alpha Gene Polymorphism Is Associated with Endometriosis, Adenomyosis and Leiomyomata. Hum. Reprod. 2001, 16, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Sztachelska, M.; Ponikwicka-Tyszko, D.; Martínez-Rodrigo, L.; Bernaczyk, P.; Palak, E.; Półchłopek, W.; Bielawski, T.; Wołczyński, S. Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy. J. Clin. Med. 2022, 11, 4407. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.G.; Park, J.Y.; Chong, G.O.; Lee, Y.H.; Lee, H.J.; Shinn, J.U.; Lee, Y.S.; Seong, W.J. Transmembrane G Protein-Coupled Receptor 30 Gene Polymorphisms and Uterine Adenomyosis in Korean Women. Gynecol. Endocrinol. 2019, 35, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Donnez, J. Peritoneal Endometriosis, Ovarian Endometriosis, and Adenomyotic Nodules of the Rectovaginal Septum Are Three Different Entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Mehasseb, M.K.; Panchal, R.; Taylor, A.H.; Brown, L.; Bell, S.C.; Habiba, M. Estrogen and Progesterone Receptor Isoform Distribution through the Menstrual Cycle in Uteri with and without Adenomyosis. Fertil. Steril. 2011, 95, 2228–2235.e1. [Google Scholar] [CrossRef]
- Mori, T.; Nagasawa, H.; Takahashi, S. The induction of adenomyosis in mice by intrauterine pituitary isografts. Life Sci. 1981, 29, 1277–1282. [Google Scholar] [CrossRef]
- Singtripop, T.; Mori, T.; Park, M.K.; Sakamoto, S.; Kawashima, S. Development of Uterine Adenomyosis after Treatment with Dopamine Antagonists in Mice. Life Sci. 1991, 49, 201–206. [Google Scholar] [CrossRef]
- Łupicka, M.; Socha, B.M.; Szczepańska, A.A.; Korzekwa, A.J. Prolactin Role in the Bovine Uterus during Adenomyosis. Domest. Anim. Endocrinol. 2017, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Mori, T. Stimulation of mammary tumorigenesis and suppression of uterine adenomyosis by temporary inhibition of pituitary prolactin secretion during youth in mice (41492). Proc. Soc. Exp. Biol. Med. 1982, 171, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Sharma, A.; Mazumdar, G.; Banerjee, I.; Tripathi, S.K.; Bagchi, C.; Das, N. The Possible Role of Fluoxetine in Adenomyosis: An Animal Experiment with Clinical Correlations. J. Clin. Diagn. Res. 2013, 7, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ohta, Y.; Nagasawa, H. Ultrastructural changes in uterine myometrium of mice with experimentally-induced adenomyosis. Experientia 1984, 40, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.M.; Jeong, J.W.; Fazleabas, A.T. Animal Models of Adenomyosis. Semin. Reprod. Med. 2020, 38, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.K.; Khan, Z.; Weaver, A.L.; Vaughan, L.E.; Gemzell-Danielsson, K.; Stewart, E.A. Vaginal Bromocriptine Improves Pain, Menstrual Bleeding and Quality of Life in Women with Adenomyosis: A Pilot Study. Acta Obstet. Gynecol. Scand. 2019, 98, 1341–1350. [Google Scholar] [CrossRef]
- Takemura, M.; Nomura, S.; Kimura, T.; Inoue, T.; Onoue, H.; Azuma, C.; Saji, F.; Kitamura, Y.; Tanizawa, O. Expression and localization of oxytocin receptor gene in human uterine endometrium in relation to the menstrual cycle. Endocrinology 1993, 132, 1830–1835. [Google Scholar] [CrossRef]
- Mechsner, S.; Grum, B.; Gericke, C.; Loddenkemper, C.; Dudenhausen, J.W.; Ebert, A.D. Possible Roles of Oxytocin Receptor and Vasopressin-1α Receptor in the Pathomechanism of Dysperistalsis and Dysmenorrhea in Patients with Adenomyosis Uteri. Fertil. Steril. 2010, 94, 2541–2546. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, P.; Sun, F.; Li, T.C.; Cheng, J.M.; Duan, H. Expression of Oxytocin Receptors in the Uterine Junctional Zone in Women with Adenomyosis. Acta Obstet. Gynecol. Scand. 2015, 94, 412–418. [Google Scholar] [CrossRef]
- Guo, S.W.; Mao, X.; Ma, Q.; Liu, X. Dysmenorrhea and Its Severity Are Associated with Increased Uterine Contractility and Overexpression of Oxytocin Receptor (OTR) in Women with Symptomatic Adenomyosis. Fertil. Steril. 2013, 99, 231–240. [Google Scholar] [CrossRef]
- Sekulovski, N.; Whorton, A.E.; Shi, M.; Hayashi, K.; MacLean, J.A. Insulin Signaling Is an Essential Regulator of Endometrial Proliferation and Implantation in Mice. FASEB J. 2021, 35, e21440. [Google Scholar] [CrossRef] [PubMed]
- Konopka, B.; Skasko, E.; Kluska, A.; Goluda, M.; Janiec-Jankowska, A.; Paszko, Z.; Ujec, M. Changes in the concentrations of receptors of insulin-like growth factor-I, epithelial growth factor, oestrogens and progestagens in adenomyosis foci, endometrium and myometrium of women during menstrual cycle. Eur. J. Gynaecol. Oncol. 1998, 19, 93–97. [Google Scholar] [PubMed]
- Artymuk, N.; Zotova, O.; Gulyaeva, L. Adenomyosis: Genetics of Estrogen Metabolism. Horm. Mol. Biol. Clin. Investig. 2019, 37, 20180069. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T. Uterine Adenomyosis Is an Oligoclonal Disorder Associated with KRAS Mutations. Nat. Commun. 2019, 10, 5785. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.J. Adenomyosis Pathogenesis: Insights from next-Generation Sequencing. Hum. Reprod. Update 2021, 27, 1086–1097. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.W. Aberrant Immunoreactivity of Deoxyribonucleic Acid Methyltransferases in Adenomyosis. Gynecol. Obstet. Investig. 2012, 74, 100–108. [Google Scholar] [CrossRef]
- Cedar, H.; Bergman, Y. Linking DNA Methylation and Histone Modification: Patterns and Paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X.; Guo, S.W. Promoter Hypermethylation of Progesterone Receptor Isoform B (PR-B) in Adenomyosis and Its Rectification by a Histone Deacetylase Inhibitor and a Demethylation Agent. Reprod. Sci. 2010, 17, 995–1005. [Google Scholar] [CrossRef]
- Liu, X.; Nie, J.; Guo, S.W. Elevated Immunoreactivity against Class i Histone Deacetylases in Adenomyosis. Gynecol. Obstet. Invest. 2012, 74, 50–55. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.W. Valproic Acid Alleviates Generalized Hyperalgesia in Mice with Induced Adenomyosis. J. Obstet. Gynaecol. Res. 2011, 37, 696–708. [Google Scholar] [CrossRef]
- Zhai, J.; Li, S.; Sen, S.; Opoku-Anane, J.; Du, Y.; Chen, Z.J.; Giudice, L.C. M6A RNA Methylation Regulators Contribute to Eutopic Endometrium and Myometrium Dysfunction in Adenomyosis. Front. Genet. 2020, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Templeman, C.; Marshall, S.F.; Ursin, G.; Horn-Ross, P.L.; Clarke, C.A.; Allen, M.; Deapen, D.; Ziogas, A.; Reynolds, P.; Cress, R.; et al. Adenomyosis and Endometriosis in the California Teachers Study. Fertil. Steril. 2008, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Vercellini, P.; Panazza, S.; Chatenoud, L.; Oldani, S. Crosignani PG. Risk factors for adenomyosis. Hum. Reprod. 1997, 12, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Mais, V.; Cipriani, S.; Busacca, M.; Venturini, P. Determinants of Adenomyosis in Women Who Underwent Hysterectomy for Benign Gynecological Conditions: Results from a Prospective Multicentric Study in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 143, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Beyth, Y.; Shapira, J.; Tepper, R.; Fishman, A.; Cordoba, M.; Bernheim, J.; Yigael, D.; Altaras, M.M. High frequency of adenomyosis in postmenopausal breast cancer patients treated with tamoxifen. Gynecol. Obstet. Investig. 1997, 44, 200–205. [Google Scholar] [CrossRef]
- Jin, Z.; Wu, X.; Liu, H.; Xu, C. Celecoxib, a Selective COX2 Inhibitor, Markedly Reduced the Severity of Tamoxifeninduced Adenomyosis in a Murine Model. Exp. Ther. Med. 2020, 19, 3289–3299. [Google Scholar] [CrossRef]
- Shen, M.; Liu, X.; Zhang, H.; Guo, S.W. Transforming Growth Factor Β1 Signaling Coincides with Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Development of Adenomyosis in Mice. Hum. Reprod. 2016, 31, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Rumph, J.T.; Stephens, V.R.; Archibong, A.E.; Osteen, K.G.; Bruner-Tran, K.L. Environmental Endocrine Disruptors and Endometriosis. In Advances in Anatomy Embryology and Cell Biology; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2020; Volume 232, pp. 57–78. [Google Scholar]
- Cho, Y.J.; Yun, J.H.; Kim, S.J.; Kwon, H.Y. Nonpersistent Endocrine Disrupting Chemicals and Reproductive Health of Women. Obstet. Gynecol. Sci. 2020, 63, 1–12. [Google Scholar] [CrossRef]
- Stephens, V.R.; Rumph, J.T.; Ameli, S.; Bruner-Tran, K.L.; Osteen, K.G. The Potential Relationship Between Environmental Endocrine Disruptor Exposure and the Development of Endometriosis and Adenomyosis. Front. Physiol. 2022, 28, 807685. [Google Scholar] [CrossRef]
- Schug, T.T.; Johnson, A.F.; Birnbaum, L.S.; Colborn, T.; Guillette, L.J.; Crews, D.P.; Collins, T.; Soto, A.M.; Vom Saal, F.S.; McLachlan, J.A.; et al. Minireview: Endocrine Disruptors: Past Lessons and Future Directions. Mol. Endocrinol. 2016, 30, 833–847. [Google Scholar] [CrossRef]
- Signorile, P.G.; Spugnini, E.P.; Mita, L.; Mellone, P.; D’Avino, A.; Bianco, M.; Diano, N.; Caputo, L.; Rea, F.; Viceconte, R.; et al. Pre-Natal Exposure of Mice to Bisphenol A Elicits an Endometriosis-like Phenotype in Female Offspring. Gen. Comp. Endocrinol. 2010, 168, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Karimi Kinyamu, H.; Wang, T.; Miranda, A.X.; Padilla-Banks, E.; Suen, A.A.; Williams, C.J. Widespread Enhancer Activation via ERα Mediates Estrogen Response in Vivo during Uterine Development. Nucleic Acids Res. 2018, 46, 5487–5503. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, D.; Shen, M.; Yan, D.; Guo, S.W. Shorter Anogenital Distance in Women with Ovarian Endometriomas and Adenomyosis, but Not Uterine Leiomyomas. Biomedicines 2023, 11, 2618. [Google Scholar] [CrossRef] [PubMed]
- Louis, G.M.; Peterson, C.M.; Chen, Z.; Hediger, M.L.; Croughan, M.S.; Sundaram, R.; Stanford, J.B.; Fujimoto, V.Y.; Varner, M.W.; Giudice, L.C.; et al. Perfluorochemicals and endometriosis: The ENDO study. Epidemiology 2012, 23, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Matta, K.; Lefebvre, T.; Vigneau, E.; Cariou, V.; Marchand, P.; Guitton, Y.; Royer, A.L.; Ploteau, S.; Le Bizec, B.; Antignac, J.P.; et al. Associations between Persistent Organic Pollutants and Endometriosis: A Multiblock Approach Integrating Metabolic and Cytokine Profiling. Environ. Int. 2022, 158, 106926. [Google Scholar] [CrossRef] [PubMed]
- Rier, S.E.; Martin, D.C.; Bowman, R.E.; Dmowski, W.P.; Becker, J.L. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1993, 21, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Szántó, M.; Gupte, R.; Kraus, W.L.; Pacher, P.; Bai, P. PARPs in lipid metabolism and related diseases. Prog. Lipid Res. 2021, 84, 101117. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Gnecco, J.; Ding, T.; Glore, D.R.; Pensabene, V.; Osteen, K.G. Exposure to the Environmental Endocrine Disruptor TCDD and Human Reproductive Dysfunction: Translating Lessons from Murine Models. Reprod. Toxicol. 2017, 68, 59–71. [Google Scholar] [CrossRef]
- Huang, P.C.; Tsai, E.M.; Li, W.F.; Liao, P.C.; Chung, M.C.; Wang, Y.H.; Wang, S.L. Association between Phthalate Exposure and Glutathione S-Transferase M1 Polymorphism in Adenomyosis. Hum. Reprod. 2010, 25, 986–994. [Google Scholar] [CrossRef]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic Transgenerational Actions of Environmental Factors in Disease Etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef]
- Alavian-Ghavanini, A.; Rüegg, J. Understanding Epigenetic Effects of Endocrine Disrupting Chemicals: From Mechanisms to Novel Test Methods. Basic. Clin. Pharmacol. Toxicol. 2018, 122, 38–45. [Google Scholar] [CrossRef]
- Czernych, R.; Chraniuk, M.; Zagożdżon, P.; Wolska, L. Characterization of Estrogenic and Androgenic Activity of Phthalates by the XenoScreen YES/YAS in Vitro Assay. Environ. Toxicol. Pharmacol. 2017, 53, 95–104. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rozati, R.; Reddy, S.; Kodampur, S.; Reddy, P.; Reddy, R. High Plasma Concentrations of Polychlorinated Biphenyls and Phthalate Esters in Women with Endometriosis: A Prospective Case Control Study. Fertil. Steril. 2006, 85, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Rozati, R.; Reddy, B.V.R.; Raman, N.V.V.S.S. Association of Phthalate Esters with Endometriosis in Indian Women. BJOG 2006, 113, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Sathyanarayana, S.; De Roos, A.J.; Thompson, M.L.; Scholes, D.; Dills, R.; Holt, V.L. Phthalates and Risk of Endometriosis. Environ. Res. 2013, 126, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Li, W.F.; Liao, P.C.; Sun, C.W.; Tsai, E.M.; Wang, S.L. Risk for Estrogen-Dependent Diseases in Relation to Phthalate Exposure and Polymorphisms of CYP17A1 and Estrogen Receptor Genes. Environ. Sci. Pollut. Res. 2014, 21, 13964–13973. [Google Scholar] [CrossRef] [PubMed]
- Aldad, T.S.; Rahmani, N.; Leranth, C.; Taylor, H.S. Bisphenol-A Exposure Alters Endometrial Progesterone Receptor Expression in the Nonhuman Primate. Fertil. Steril. 2011, 96, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Long-Term Adverse Effects of Neonatal Exposure to Bisphenol A on the Murine Female Reproductive Tract. Reprod. Toxicol. 2007, 24, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Giusti, R.M.; Iwamoto, K.; Hatch, E.E. Diethylstilbestrol revisited: A review of the long-term health effects. Ann. Intern. Med. 1995, 122, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Sathyanarayana, S.; Scholes, D.; Holt, V.L. Early-Life Factors and Endometriosis Risk. Fertil. Steril. 2015, 104, 964–971.e5. [Google Scholar] [CrossRef]
- Huseby, R.A.; Thurlow, S. Effects of Prenatal Exposure of Mice to “Low-Dose” Diethylstilbestrol and the Development of Adenomyosis Associated with Evidence of Hyperprolactinemia. Am. J. Obstet. Gynecol. 1982, 144, 939–949. [Google Scholar] [CrossRef]
- McLachlan, J.A.; Newbold, R.R.; Bullock, B.C. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980, 40, 3988–3999. [Google Scholar]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]
- Youseflu, S.; Sadatmahalleh, S.J.; Mottaghi, A.; Kazemnejad, A. Dietary Phytoestrogen Intake and the Risk of Endometriosis in Iranian Women: A Case-Control Study. Int. J. Fertil. Steril. 2020, 13, 296–300. [Google Scholar] [CrossRef]
- Kouzmenko, A.; Ohtake, F.; Fujiki, R.; Kato, S. Hormonal Gene Regulation through DNA Methylation and Demethylation. Epigenomics 2010, 2, 765–774. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Resuehr, D.; Ding, T.; Lucas, J.A.; Osteen, K.G. The Role of Endocrine Disruptors in the Epigenetics of Reproductive Disease and Dysfunction: Potential Relevance to Humans. Curr. Obstet. Gynecol. Rep. 2012, 1, 116–123. [Google Scholar] [CrossRef]
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging Role of Micro-RNA in the Effect of the Endocrine Disruptors. Front. Neurosci. 2016, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Winterroth, L.C.; Garcia, M.; Weiman, S.; Wong, J.W.; Sunwoo, J.B.; Nadeau, K.C. Epigenetically Mediated Pathogenic Effects of Phenanthrene on Regulatory T Cells. J. Toxicol. 2013, 2013, 967029. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Tao, S.; Guan, Y.; Zhang, T.; Wang, Z. Global DNA Methylation in Gonads of Adult Zebrafish Danio Rerio under Bisphenol A Exposure. Ecotoxicol. Environ. Saf. 2016, 130, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Chevalier, D.M.; Phelps, J.Y.; Cantor, A.M.; Padilla-Banks, E.; Newbold, R.R.; Archer, T.K.; Karimi Kinyamu, H.; Williams, C.J. Persistently Altered Epigenetic Marks in the Mouse Uterus after Neonatal Estrogen Exposure. Mol. Endocrinol. 2013, 27, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, T.; Bruner-Tran, K.L.; Piestrzeniewicz-Ulanska, D.; Osteen, K.G. Developmental Exposure of Mice to TCDD Elicits a Similar Uterine Phenotype in Adult Animals as Observed in Women with Endometriosis. Reprod. Toxicol. 2007, 23, 326–336. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Routledge, E.J.; White, R.; Parker, M.G.; Sumpter, J.P. Differential Effects of Xenoestrogens on Coactivator Recruitment by Estrogen Receptor (ER) α and ERβ. J. Biol. Chem. 2000, 275, 35986–35993. [Google Scholar] [CrossRef]
- Clinical Trial: Adenomyosis and ART (ADENOFERT). Available online: https://clinicaltrials.gov/study/NCT05937490 (accessed on 18 January 2024).
- Moawad, G.; Kheil, M.H.; Ayoubi, J.M.; Klebanoff, J.S.; Rahman, S.; Sharara, F.I. Adenomyosis and Infertility. J. Assist. Reprod. Genet. 2022, 39, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Moriarty, S.; Taskin, O.; Allaire, C.; Williams, C.; Yong, P.; Bedaiwy, M.A. Reproductive Outcomes after Fertility-Sparing Surgery for Focal and Diffuse Adenomyosis: A Systematic Review. J. Minim. Invasive Gyneco. 2018, 25, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Bandini, V.; Buggio, L.; Berlanda, N.; Somigliana, E. Association of Endometriosis and Adenomyosis with Pregnancy and Infertility. Fertil. Steril. 2023, 119, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Szubert, M.; Koziróg, E.; Olszak, O.; Krygier-Kurz, K.; Kazmierczak, J.; Wilczynski, J. Adenomyosis and Infertility—Review of Medical and Surgical Approaches. Int. J. Environ. Res. Public Health 2021, 18, 1235. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. What We Know about Primary Dysmenorrhea Today: A Critical Review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.Y. Primary dysmenorrhea: Advances in pathogenesis and management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.; Yacobson, I.; Grimes, D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1999, 181 Pt 1, 1263–1269. [Google Scholar] [CrossRef]
- Osayande, A.S.; Mehulic, S. Diagnosis and initial management of dysmenorrhea. Am. Fam. Physician 2014, 89, 341–346. [Google Scholar] [PubMed]
- Wong, C.L.; Farquhar, C.; Roberts, H.; Proctor, M. Oral Contraceptive Pill as Treatment for Primary Dysmenorrhoea. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Hassanin, A.I.; Youssef, A.A.; Yousef, A.M.; Ali, M.K. Comparison of Dienogest versus Combined Oral Contraceptive Pills in the Treatment of Women with Adenomyosis: A Randomized Clinical Trial. Int. J. Gynaecol. Obstet. 2021, 154, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, O.M.; Ali, M.K.; Sabra, A.M.A.; Abd El Aal, D.E.M. Levonorgestrel-Releasing Intrauterine System versus a Low-Dose Combined Oral Contraceptive for Treatment of Adenomyotic Uteri: A Randomized Clinical Trial. Contraception 2015, 92, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Samy, A.; Atwa, K.; Ghoneim, H.M.; Lotfy, M.; Saber Mohammed, H.; Abdellah, A.M.; El Bahie, A.M.; Aboelroose, A.A.; El Gedawy, A.M.; et al. The role of levonorgestrel intra-uterine system in the management of adenomyosis: A systematic review and meta-analysis of prospective studies. Acta Obstet. Gynecol. Scand. 2020, 99, 571–581. [Google Scholar] [CrossRef]
- Beatty, M.N.; Blumenthal, P.D. The levonorgestrel-releasing intrauterine system: Safety, efficacy, and patient acceptability. Ther. Clin. Risk Manag. 2009, 5, 561–574. [Google Scholar] [CrossRef][Green Version]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.M. Conservative Management of Uterine Adenomyosis: Medical vs. Surgical Approach. J. Clin. Med. 2021, 10, 4878. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GNRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef]
- Nelson, J.R.; Corson, S.L. Long-Term Management of Adenomyosis with a Gonadotropin-Releasing Hormone Agonist: A Case Report. Fertil. Steril. 1993, 59, 441–443. [Google Scholar] [CrossRef]
- Imaoka, I.; Ascher, S.M.; Sugimura, K.; Takahashi, K.; Li, H.; Cuomo, F.; Simon, J.; Arnold, L.L. MR Imaging of Diffuse Adenomyosis Changes after GnRH Analog Therapy. J. Magn. Reson. Imaging 2002, 15, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrun-Cutler, M.T.; Alvero, R. Short- and Long-Term Impact of Gonadotropin-Releasing Hormone Analogue Treatment on Bone Loss and Fracture. Fertil. Steril. 2019, 112, 799–803. [Google Scholar] [CrossRef]
- Donnez, J.; Donnez, O.; Dolmans, M.M. Evolution of Uterine Adenomyosis Volume during and after GnRH Antagonist (Linzagolix) Treatment: Lessons for Further Clinical Trials. Fertil. Steril. 2023, 120, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Lee, W.L.; Chen, C.Y.; Sheu, B.C.; Yen, M.S.; Chang, T.C.; Wang, P.H. Medical Treatment for Adenomyosis and/or Adenomyoma. Taiwan. J. Obstet. Gynecol. 2014, 53, 459–465. [Google Scholar] [CrossRef]
- Guo, S.W.; Groothuis, P.G. Is It Time for a Paradigm Shift in Drug Research and Development in Endometriosis/Adenomyosis? Hum. Reprod. Update 2018, 24, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Matsuda, M.; Mori, T.; Sakamoto, S.; Mitamura, T. Effects of mifepristone (RU486) treatment on the development of uterine adenomyosis induced by pituitary grafting in mice. Life Sci. 2000, 67, 2713–2720. [Google Scholar] [CrossRef]
- Che, X.; Wang, J.; He, J.; Yu, Q.; Sun, W.; Chen, S.; Zou, G.; Li, T.; Guo, X.; Zhang, X. A New Trick for an Old Dog: The Application of Mifepristone in the Treatment of Adenomyosis. J. Cell. Mol. Med. 2020, 24, 1724–1737. [Google Scholar] [CrossRef]
- Che, X.; Wang, J.; Sun, W.; He, J.; Wang, Q.; Zhu, D.; Zhu, W.; Zhang, J.; Dong, J.; Xu, J.; et al. Effect of Mifepristone vs Placebo for Treatment of Adenomyosis With Pain Symptoms: A Randomized Clinical Trial. JAMA Netw. Open. 2023, 6, e2317860. [Google Scholar] [CrossRef]
- Rosato, E.; Farris, M.; Bastianelli, C. Mechanism of Action of Ulipristal Acetate for Emergency Contraception: A Systematic Review. Front. Pharmacol. 2016, 6, 315. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, L.H.R.; Middleton, L.J.; Daniels, J.P.; Williams, A.R.W.; Priest, L.; Odedra, S.; Cheed, V.; Stubbs, C.E.; Clark, T.J.; Lumsden, M.A.; et al. Ulipristal acetate versus levonorgestrel-releasing intrauterine system for heavy menstrual bleeding (UCON): A randomised controlled phase III trial. EClinicalMedicine 2023, 60, 101995. [Google Scholar] [CrossRef]
- Ferrero, S.; Scala, C.; Racca, A.; Tafi, E.; Venturini, P.; Leone Roberti Maggiore, U. Changes in Adenomyosis after Treatment with Ulipristal Acetate. Fertil. Steril. 2016, 106, e281–e282. [Google Scholar] [CrossRef]
- Gonçalves-Henriques, M.; de Pinho, A.; Freixo, M.; Liz-Coelho, M.; Castro, F.; Ceschin, N.; Brandão, P. Ulipristal Acetate in Adenomyosis. Gynecol. Minim. Invasive Ther. 2022, 11, 198. [Google Scholar] [CrossRef]
- Kimura, F.; Takahashi, K.; Takebayashi, K.; Fujiwara, M.; Kita, N.; Noda, Y.; Harada, N. Concomitant Treatment of Severe Uterine Adenomyosis in a Premenopausal Woman with an Aromatase Inhibitor and a Gonadotropin-Releasing Hormone Agonist. Fertil. Steril. 2007, 87, 1468.e9–1468.e12. [Google Scholar] [CrossRef]
- Badawy, A.M.; Elnashar, A.M.; Mosbah, A.A. Aromatase Inhibitors or Gonadotropin-Releasing Hormone Agonists for the Management of Uterine Adenomyosis: A Randomized Controlled Trial. Acta Obstet. Gynecol. Scand. 2012, 91, 489–495. [Google Scholar] [CrossRef]
- Rižner, T.L. The Important Roles of Steroid Sulfatase and Sulfotransferases in Gynecological Diseases. Front. Pharmacol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Messinger, J.; Husen, B.; Koskimies, P.; Hirvelä, L.; Kallio, L.; Saarenketo, P.; Thole, H. Estrone C15 Derivatives-A New Class of 17β-Hydroxysteroid Dehydrogenase Type 1 Inhibitors. Mol. Cell. Endocrinol. 2009, 301, 216–224. [Google Scholar] [CrossRef][Green Version]
- Clinical Trial: A Study to Investigate Efficacy and Safety of OG-6219 BID in 3 Dose Levels Compared with Placebo in Participants Aged 18 to 49 with Moderate to Severe Endometriosis-Related Pain (ELENA). Available online: https://clinicaltrials.gov/study/NCT05560646 (accessed on 18 January 2024).
- Poirier, D.; Nyachieo, A.; Romano, A.; Roy, J.; Maltais, R.; Chai, D.; Delvoux, B.; Tomassetti, C.; Vanhie, A. An Irreversible Inhibitor of 17β-Hydroxysteroid Dehydrogenase Type 1 Inhibits Estradiol Synthesis in Human Endometriosis Lesions and Induces Regression of the Non-Human Primate Endometriosis. J. Steroid Biochem. Mol. Biol. 2022, 222, 106136. [Google Scholar] [CrossRef]
- Poirier, D.; Roy, J.; Maltais, R. A Targeted-Covalent Inhibitor of 17β-HSD1 Blocks Two Estrogen-Biosynthesis Pathways: In Vitro (Metabolism) and In Vivo (Xenograft) Studies in T-47D Breast Cancer Models. Cancers 2021, 13, 1841. [Google Scholar] [CrossRef]
- Sahin, C.; Mamillapalli, R.; Yi, K.W.; Taylor, H.S. MicroRNA Let-7b: A Novel Treatment for Endometriosis. J. Cell. Mol. Med. 2018, 22, 5346–5353. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.K.; Pozzi Mucelli, R.; Epstein, E.; Stewart, E.A.; Gemzell-Danielsson, K. Vaginal Bromocriptine for Treatment of Adenomyosis: Impact on Magnetic Resonance Imaging and Transvaginal Ultrasound. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, R.S.; Del Vecchio, G.; Scairati, R.; Pirchio, R.; Liccardi, A.; Verde, N.; de Angelis, C.; Menafra, D.; Pivonello, C.; Conforti, A.; et al. The Interplay Between Prolactin and Reproductive System: Focus on Uterine Pathophysiology. Front. Endocrinol. 2020, 11, 594370. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ponandai-srinivasan, S.; Frisendahl, C.; Andersson, J.K.; Pavone, D.; Stewart, E.A.; Lalitkumar, P.G.L.; Korsching, E.; Bogavarappu, N.R.; Gemzell-Danielsson, K. Bromocriptine Inhibits Proliferation in the Endometrium from Women with Adenomyosis. Front. Endocrinol. 2023, 14, 1026168. [Google Scholar] [CrossRef]
- Mahar, K.M.; Enslin, M.B.; Gress, A.; Amrine-Madsen, H.; Cooper, M. Single- and Multiple-Day Dosing Studies to Investigate High-Dose Pharmacokinetics of Epelsiban and Its Metabolite, GSK2395448, in Healthy Female Volunteers. Clin. Pharmacol. Drug Dev. 2018, 7, 33–43. [Google Scholar] [CrossRef]
- Clinical Trial: Placebo-Controlled Proof of Concept Study of Epelsiban in Women with Adenomyosis. Available online: https://clinicaltrials.gov/study/NCT02794467 (accessed on 18 January 2024).
- Barberis, C. L372662; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Xue, J.; Zhang, H.; Liu, W.; Liu, M.; Shi, M.; Wen, Z.; Li, C. Metformin Inhibits Growth of Eutopic Stromal Cells from Adenomyotic Endometrium via AMPK Activation and Subsequent Inhibition of AKT Phosphorylation: A Possible Role in the Treatment of Adenomyosis. Reproduction 2013, 146, 397–406. [Google Scholar] [CrossRef]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.K.; Greaves, E. Macrophage-Derived Insulin-like Growth Factor-1 Is a Key Neurotrophic and Nerve-Sensitizing Factor in Pain Associated with Endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef] [PubMed]
- Yalçın Bahat, P.; Ayhan, I.; Üreyen Özdemir, E.; İnceboz, Ü.; Oral, E. Dietary supplements for treatment of endometriosis: A review. Acta Biomed. 2022, 93, e2022159. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, B.; Zhang, H.; Liu, X.; Guo, S.W. Epigallocatechin-3-Gallate Reduces Myometrial Infiltration, Uterine Hyperactivity, and Stress Levels and Alleviates Generalized Hyperalgesia in Mice Induced with Adenomyosis. Reprod. Sci. 2013, 20, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, B.; Zhang, H.; Ding, D.; Liu, X.; Guo, S.W. Possible Loss of GABAergic Inhibition in Mice with Induced Adenomyosis and Treatment with Epigallocatechin-3-Gallate Attenuates the Loss with Improved Hyperalgesia. Reprod. Sci. 2014, 21, 869–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moldassarina, R.S.; Manabayeva, G.K.; Akylzhanova, Z.Y.; Rashidova, A.M. The Importance of Vitamin D in the Diagnosis and Treatment of Adenomyosis. Mol. Cell. Biochem. 2023, 478, 571–579. [Google Scholar] [CrossRef]
- Singh, A.; Dasgupta, S.; Bhattacharya, A.; Mukherjee, G.; Chaudhury, K. Therapeutic Potential of Curcumin in Endometrial Disorders: Current Status and Future Perspectives. Drug Discov. Today 2022, 27, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.X.; Wei, S.B.; Zhou, Y.; Shao, Y.; Li, M.Y. Exosomes: Potential Diagnostic Markers and Drug Carriers for Adenomyosis. Front. Pharmacol. 2023, 14, 1216149. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, H.; Giribabu, N.; Muniandy, S.; Salleh, N. Quercetin induces morphological and proliferative changes of rat’s uteri under estrogen and progesterone influences. Int. J. Clin. Exp. Pathol. 2014, 7, 5484–5494. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).