Abstract
Interstitial expansion is associated with glomerular filtration rate (GFR) loss in many renal diseases, including diabetic nephropathy. The Renin–Angiotensin System Study (RASS) tested whether a 5-year renin–angiotensin system (RAS) blockade with enalapril or losartan versus placebo slowed progression of early diabetic nephropathy lesions in 285 normoalbuminuric, normotensive, normal/high GFR patients with type 1 diabetes. RASS found no benefit to the RAS blockade on diabetic glomerular lesions but observed an unexpected 50% increase in the fractional volume of the renal cortex which is the interstitium. The effects of the RAS blockade on individual interstitial components––striated collagen, interstitial cells, and peritubular capillaries––were not assessed. We evaluated by electron microscopy changes in fractional volume of each component in seven patients from each group between baseline and five years. At baseline, 49% of the interstitium was collagen, 12% cells, 26% peritubular capillaries, 7% space, and 2% artifact. There was no overall change in the interstitial composition during the RASS. There were no statistically significant effects of treatment group on any interstitial components. Renal volume remained stable in all groups. The RAS blockade affected neither the approximately 50% increase in interstitium fractional volume per cortex nor the parallel increase in all interstitial components that occurred over the five years of the RASS.
1. Introduction
Interstitial expansion and fibrosis are important structural contributors to glomerular filtration rate (GFR) loss in a wide variety of chronic renal diseases including diabetic nephropathy [1]. Although glomerular lesions predominate in the structural associations with early-to-moderate GFR reduction in type 1 diabetes, i.e., GFRs between 90 and 45 mL/min/1.73 m2 [2], below this level, tubulointerstitial changes may be the primary drivers of progression towards end-stage kidney disease [3]. The structural composition of the interstitium can be quantitatively dissected using electron microscopic morphometric methods into four components: striated collagen, peritubular capillaries, interstitial cells and amorphous space [4]. Using these methods, we previously showed that biopsies from research participants with type 1 diabetes and very early GFR loss (90 ± 14 mL/min/1.73 m2) had a more than 60% increase in the fraction of the interstitium volume, which is made up of striated collagen [4]. In a small study of patients with type 2 diabetes with microalbuminuria or overt proteinuria, GFR >60 mL/min/1.73 m2, and the interstitium volume fraction of cortex [Vv(Int/cortex)] exceeding 30% at baseline, Cordonnier et al. reported an approximate 30% increase over two years in the morphometrically determined Vv(Int/cortex) in patients randomized to placebo (p = 0.001), while there was no change in this parameter in those randomized to the angiotensin converting enzyme (ACE) inhibitor, perindopril [5]. However, these light microscopic studies did not address the electron microscopic interstitial compositional changes in these cohorts.
The Renin–Angiotensin System Study (RASS) enrolled 285 normoalbuminuric, normotensive patients with type 1 diabetes and normal to high GFR [6]. These study participants volunteered for research kidney biopsies at baseline and after five years to test whether a renin–angiotensin system blockade with the ACE inhibitor enalapril or angiotensin receptor blocker losartan slowed the progression of early diabetic nephropathy lesions compared to placebo. Neither of these renin–angiotensin system (RAS) blockers benefited the progression of the early diabetic nephropathy electron microscopic morphometric glomerular lesions or the light microscopic vascular (arteriolar hyalinosis), tubular (fractional volume of cortical tubules that were atrophic), or interstitial Vv(Int/Cortex) lesions. Surprisingly, given their baseline normal renal functional status, the 172 patients in the RASS with sufficient renal cortical tissue for robust measurements of interstitium had a greater than 50% increase in Vv(Int/cortex) over the five years of the study. Thus, Vv(Int/cortex) increased from 0.11 ± 0.04 to 0.17 ± 0.05 (p = 0.001), a mean value at the study exit above the normal mean values (0.13 ± 0.04, p = 0.04) [7]. This increase was predicted by baseline biomarkers. Thus, higher urinary monocyte chemoattractant protein-1 (MCP-1) was associated with a higher interstitial fractional volume at baseline and after five years in women but not in men [8]. Also, higher plasma bradykinin (1–7) and hyp3-bradykinin (1–7) were associated with lower Vv(Int/cortex) in the RASS [9], which could also have been mediated through influences of this pathway on inflammatory processes [10]. There is a known association of interstitial inflammation with interstitial fibrosis in a variety of renal diseases [11]. We have previously described and quantified the electron microscopic composition of the interstitium [4]: interstitial cells, striated collagen, peritubular capillaries and amorphous space. Although there was no beneficial impact of the RAS blockade on the increase in Vv(Int/cortex), these light microscopic studies did not evaluate the possibility that the blockade could have differentially affected these interstitial components, perhaps by influencing the RAS-associated interstitial inflammatory processes [12], thereby reducing fibrosis as determined by measuring the fraction of the interstitium occupied by striated collagen [Vv(Col/int)]. This important question is addressed here.
2. Material and Methods
2.1. Study Participants
The Renin-Angiotensin System Study was a multicenter, international, double-blinded, placebo controlled, investigator-initiated randomized clinical trial that recruited 285 study participants with type 1 diabetes at 3 sites: the University of Minnesota (Minneapolis, MN, USA), University of Toronto (Toronto, Canada) and McGill University (Montreal, Canada). At the time of enrollment, all participants were normoalbuminuric and normotensive and had normal or elevated GFR. Patients were not eligible if they had hypertension (blood pressure exceeding 135/85 mm Hg or receiving antihypertensive medications), an albumin excretion rate above 20 μg per minute, were pregnant or considering pregnancy during the study period, failed to take at least 85% of the placebo pills during a 2-week run-in period, or had a GFR of less than 90 mL/min/1.73 m2 of body-surface area (<80 mL/min/1.73 m2 if the patient was following a strictly vegan diet).
2.2. Study Design
Study participants were randomized (block randomization per study site and sex) 1:1:1 to receive enalapril 20 mg, losartan 100 mg, or placebo. Patients were followed for 5 years for quarterly measurements of blood pressure, albumin excretion rate, and glycated hemoglobin level. The glomerular filtration rate, determined by the plasma disappearance of iohexol (iGFR), was assessed at baseline every year and at one month and two months after the discontinuation of study drug. Research kidney biopsies were obtained prior to study intervention and five years thereafter.
The study was approved by the appropriate ethical committees at each institution, and written informed consent was obtained from each participant. The study was overseen by a data and safety monitoring board of the National Institutes of Health.
The pre-specified primary RASS endpoint was a change in the fraction of glomerular volume occupied by mesangium (the mesangial fractional volume). Secondary renal endpoints included changes in other glomerular, vascular, tubular, and interstitial variables and changes in the albumin excretion rate and GFR.
2.3. Renal Biopsies
Baseline and 5-year research kidney biopsies from 21 RASS participants (52% male): 7 from each treatment group (placebo, enalapril, losartan), were selected for the current study. Since treatment with either enalapril or losartan had no effect on the changes in the Vv(Int/cortex) as compared with placebo [6], study participants who had a wide range of change in Vv(Int/cortex) were selected for this study to reflect the study cohort as a whole. Participants from each study group (enalapril, losartan, and placebo) were also matched for age and sex without consideration of any other data. The number of participants per group was determined based on our earlier studies indicating that 7 patients per intervention group would provide sufficient power to detect group differences in the key interstitial structural variable, Vv(Col/int)] [4]. Also, given that there were no biopsy differences by site [6], biopsies from a single center using the BioptyTM needle were selected. We had previously shown that this needle caused less renal tissue trauma in these RASS research biopsies [13], and given the small number of subjects per group for these very time-consuming electron microscopic morphometric measurements, we aimed to reduce tissue artifacts, which could add technical variability to the measurements of the primary endpoint.
2.4. Light Microscopic Measurements
The fraction of the renal cortex that is interstitium Vv(Int/cortex) was estimated by point counting on Zenker’s fixed paraffin embedded periodic acid-Schiff (PAS) stained slides as previously described [4]. Images were projected onto a white surface with a projection microscope, and a double-lattice grid with four fine points for each coarse point was used. The coarse points were 25,000 µm apart. The number of fine points falling on the interstitium—defined as points falling on the cortex outside the glomeruli, tubules, tubular basement membranes—and vessels larger than the average tubular diameter were counted. Course points falling on the renal cortex also were counted. Because there were four fine points for each coarse point,
where FP is the number of fine points hitting the interstitium, and CP is the number of coarse points hitting the cortex. In research kidney biopsy tissues from normal individuals in the age range of the study participants with diabetes selected for this study, Vv(Int/cortex) did not correlate with age (n = 22, r = 0.11, p = 0.6).
Vv(Int/cortex) = FP/(CP × 4)(mm3/mm3)
2.5. Electron Microscopic Measurements
Tissues were processed for the electron microscope as described elsewhere [4]. Quantitation was performed on micrographs from sections where cortical tissue was verified by the identification of glomeruli or proximal tubular profiles on 1 µm thick, toluidine blue-stained sections. At least two ultrathin sections from two different blocks were assessed for each patient along with a calibration grid for each block at each level of magnification.
Unbiased random sampling of the renal cortex was performed as follows: Digital images of the renal cortical interstitium were obtained at a magnification of 11,000× using a Joel CX1010 electron microscope through predetermined movements of its stage controls. The only fields not photographed were glomeruli. These images were then transferred to a Macintosh computer and visualized on a 17-inch monitor using Photoshop version 10. Digital cortical interstitial images were overlain in Photoshop with a double-lattice grid grid where 9 fine points (FP) were equal to 1 coarse point (CP). CP were used to measure the interstitium whereas the FP were used to measure the different components of the interstitium, as detailed below. All measurements were performed by a masked observer with no knowledge of the subject’s identity, treatment group (placebo, enalapril, losartan), biopsy order (baseline or exit), or biopsy pairing. The electron microscope interstitial components, estimated as fractional volumes of the cortical interstitium, included striated collagen, peritubular capillary, interstitial cell and amorphous space. The volume fraction of each interstitial component was calculated as Vv(Component X/int) which equaled Vv(FPx/CP × 9), where FPx is the number of fine points hitting component x and CP is the number of coarse points hitting the interstitium. The number 9 represents the number of fine points to one coarse point on the grid used for these measurements. Thus, as detailed above, the proportion of interstitium that was striated collagen was calculated as Vv(Col/int), peritubular capillaries as Vv(PTC/int), cells as Vv(Cell/int), and amorphous space as Vv(Sp/int). At least 316 CPs hitting the interstitium per biopsy were counted.
2.6. Kidney Volume
Kidney dimensions were measured by ultrasound. Kidney volume was calculated using the length (L), width (W), and anterior–posterior (AP) diameter using the formula (L × W × AP × 0.523) and expressed as cm3.
2.7. Clinical Assessments
The methods used to assess the clinical and laboratory parameters presented in Table 1 have been published elsewhere [2]. Briefly, blood pressure levels were measured using a Dinamap monitor. Study participants who developed persistent hypertension were treated with blood pressure-lowering medications other than RAS blockers. Glycated hemoglobin was measured on a Diamat analyzer (BioRad) until 2002 and using the Tosoh method (Tosoh Medics) afterward. Urinary albumin excretion rate was measured by a solid-phase fluorescent immunoassay [14], and the median value among three samples was reported. Iohexol for iGFR determinations was measured by high-pressure liquid chromatography (HPLC).

Table 1.
Baseline Clinical Characteristics.
2.8. Statistical Methods
Given that baseline demographic, clinical and renal structural parameters—Vv(Int/cortex), Vv(Col/int), Vv(PTC/int), Vv(Cell/int), and Vv(Sp/int)—were not different between the enalapril and losartan groups, they were combined for analysis. Comparisons of variables were done using a paired or unpaired Student’s t-test as appropriate. A p value < 0.05 was deemed statistically significant.
3. Results
There were no statistically significant group differences at baseline in type 1 diabetes duration, body mass index, glycated hemoglobin, blood pressure or iGFR measured between RAS blockade-treated (n = 14; 7 per treatment group) and untreated patients (n = 7). The baseline urinary albumin excretion rate in the normoalbuminuric range in both groups was statistically significantly higher in the blockade-treated patients (Table 1). Study participants were matched for age and sex and thus were not statistically different by design.
Similar to the entire RASS cohort as previously reported, there were no statistically significant changes in HbA1c, systolic or diastolic blood pressure, albumin excretion rate or glomerular filtration rate between baseline and exit in either the placebo or treatment groups (Table 2).

Table 2.
Clinical Characteristics at Study Exit.
There were also no statistically significant group differences in renal structural measurements at baseline (Table 3). Vv(Int/cortex),which was 0.11 ± 0.04 in the treated and 0.11 ± 0.03 in the untreated group at baseline, increased over the 5 years of the study to 0.19 ± 0.05 in the treated and 0.20 ± 0.07 in the untreated group (p = 0.002 and 0.047, respectively). However, the magnitude of increase in Vv(Int/cortex) was virtually identical in the RAS blockade-treated and placebo groups (p = 0.97).

Table 3.
Baseline Renal Morphometric Structural Parameters.
There were no statistically significant changes in Vv(Mes/glom), GBM width or Sv(PGBM/glom) between baseline and exit in either the placebo or treatment groups. As reported for the whole cohort, Vv(Int/cortex) increased significantly in both groups between biopsies (Table 4).

Table 4.
Renal Morphometric Structural Parameters at Study Exit.
Despite the more than 50% increase in Vv(Int/cortex), no statistically significant changes from baseline to 5 years were found in the fractional volumes of any of the interstitial components studied when comparing the treated and untreated groups (Table 5 and Figure 1) or in these groups combined (data not shown).

Table 5.
Interstitial Components at Baseline and 5 years.
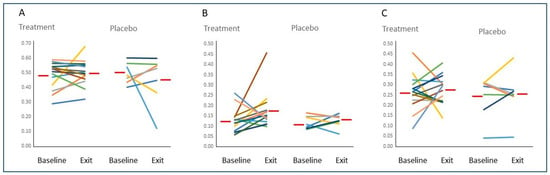
Figure 1.
Figure Legend: Baseline and exit values for Vv(Col/int) (Panel (A)), Vv(Cell/int) (Panel (B)), and Vv(PTC/int) (Panel (C)) for each study participants randomized to Treatment or Placebo. Red bars indicate the average value for each group.
Kidney volume at baseline was also not different between treated (175.8 ± 55.2 cm3) and untreated individuals (162.8 ± 37.6 cm3, (p = 0.582)), and it did not change during the study. Consequently, the increase in the total volume of interstitial collagen per cortex was not significantly statistically different between the RAS blockade and placebo groups and reflected the increase in Vv(Int/cortex) rather than any disproportionate change in Vv(Col/int).
4. Discussion
The Renin-Angiotensin System Study was designed to test whether a renin-angiotensin system blockade could slow development or progression of early renal lesions in persons with type 1 diabetes prior to the emergence of established diabetic nephropathy risk factors such as microalbuminuria, hypertension or early GFR decline [8]. The greater than 50% increase in Vv(Int/cortex) from the sub-normal to normal range at baseline into the abnormal range at the end of the 5 years of the RASS, was unexpected, given the baseline study eligibility criteria of normoalbuminuria, normotension, no antihypertensive medication, and normal-to-high GFR [6].
The initial mean sub-normal values for Vv(Int/cortex) [4] in the baseline biopsies in the study likely indicated that renal hypertrophy in type 1 diabetes largely represents tubular hypertrophy [15], resulting in an initial decrease in Vv(Int/cortex) because of an increase in renal cortical volume. Thus this 50% increase in Vv(Int/cortex) without a significant change in kidney volume during the 5 years of the study was consistent with an increase in the total volume of cortical interstitium. The RAS blockade with either enalapril or losartan had no beneficial effect on this Vv(Int/cortex) increase, but given that this light microscopy parameter cannot determine the structural elements that contribute to this interstitial expansion, systematic unbiased quantitative morphometric electron microscope studies [4] were conducted to determine whether this increase in total cortical interstitium volume was associated with a disproportionate increase in one of the structural components of the interstitium, a phenomenon seen in the glomerular mesangium in persons with type 1 diabetes [16]. We previously showed that mesangial matrix expansion was the major factor in the expansion of the mesangium even though both the matrix and the cellular components of the mesangium increased as fractions of the volume of the glomerulus compared to the same measurements in nondiabetic subjects.
The correct use of the term “interstitial fibrosis” includes the concept of a disproportionate increase in interstitial striated collagen [4]. If, for example, biopsies of the RAS blockade-treated patients over the 5 years had a lower Vv(Col/int) compared to that of the placebo group, the initial conclusion that the blockade provided no benefit to this important renal compartment might, in fact, have been incorrect, i.e., progression in fibrosis would have been reduced. Such a benefit was suggested by Cordonnier et al. in a study of patients with type 2 diabetes, increased albumin excretion and reduced GFR where a 2 year RAS blockade prevented the further interstitial expansion seen in the placebo subjects [5]. However, despite a 5 year blockade, the present study, carried out at a much earlier phase in the development of diabetic kidney disease, found no differences in the composition of the interstitial components or in their change from baseline to 5 years between the RAS blockade and placebo groups.
Higher levels of HbA1C in the RASS were associated with more rapid increases in glomerular basement membrane (GBM) width and mesangial fraction volume Vv(Mes/glom), and Vv(Int/cortex) [8]. However, while the changes over the 5 years in GBM width and Vv(Mes/glom) directly correlated with each other, there were no significant correlations between changes in these two important diabetic nephropathy glomerular structural variables and in Vv(Int/cortex) [8]. Early glomerular changes of diabetic nephropathy were predominantly characterized by the accumulation of extracellular matrix (ECM) in the GBM, mesangium [7], and tubular basement membranes [17], whereas the early interstitial expansion described here represented a parallel and proportionate increase in all interstitial components. Moreover, the interstitial striated collagen [18] was markedly distinct biochemically from the ECM that accumulated in the glomeruli, and in the tubular basement membranes as diabetic nephropathy developed [18,19,20].
The pathogenesis of the early interstitial expansion in type 1 diabetes is poorly understood. The increase in Vv(Int/cortex) in the RASS was weakly but significantly associated with increased baseline urinary monocyte chemoattractant protein-1 (MCP-1) [8], an association primarily found in women. In patients with more advanced diabetic nephropathy, increased urinary MCP-1 was related to the severity of tubulointerstitial injury and inflammation [21] and was an independent predictor of GFR decline in patients with type 1 and type 2 diabetes [22]. Thus, there may be an inflammatory component to this process that operates in the absence of increased numbers of inflammatory cells in the tubulointerstitium. For example, increased tissue kallikrein (KLK1) expression was induced by high glucose in human proximal tubular epithelial cells (PTEC) in culture. Moreover, recombinant KLK1 can stimulate the production of inflammatory cytokines in PTEC through activation of p42/44 and p38 MAPK signaling pathways [23].
The increase in the total quantity of renal cortical striated collagen, estimated from the increase in Vv(Int/cortex) with no change in kidney volume, did not fit the usual concept of “progressive fibrosis”, which connoted the disproportionate accumulation of fibrous connective tissue with disruption of organ architecture, as suggested above [24]. In fact, we showed here that the architecture of the renal cortical interstitium remained remarkably intact despite undergoing a rapid >50% expansion over the 5 years of the RASS. Thus, for patients in the later stages of type 1 diabetic nephropathy, there were higher albuminuria levels, lower GFR, more than double the normal Vv(Int/cortex) and an increase in the fractional volume of interstitial collagen [8]. How this transition from proportional to excessive interstitial collagen accumulation came about is an important question about much too little is known.
Overt proteinuria ushers in the evolution of a new phase in diabetic nephropathy pathology with the emergence of lesions such as glomerular tubular junction injury, atubular glomeruli and tubulointerstitial inflammation [24]. This is consistent with the idea that there may be promoters of diabetic nephropathy progression such as heavy proteinuria, which could induce downstream tubulointerstitial injury [25]. Patients undergoing a pancreas transplantation alone are typically treated with calcineurin inhibitors, which cause interstitial expansion and fibrosis in their native kidneys after 5 years [26]. This correlated with the dose and blood levels of the calcineurin inhibitors, especially in the first post-transplant year [26]. We previously demonstrated that excess renal interstitial-striated collagen accumulation was partially reversible [24] in this setting, and associated with the marked reduction in the dose and blood levels of calcineurin inhibitors between the fifth and the tenth year post-transplant. It is therefore important to understand these processes better since they are potentially amenable to therapeutic manipulation.
In summary, early renal cortical interstitial expansion in type 1 diabetes is characterized by a proportional increase in all interstitial components, including interstitial striated collagen, which is not influenced by 5 years of a renin–angiotensin system blockade.
Author Contributions
Z.K. acquired data, analyzed and interpreted data, revised the manuscript. M.M. designed the study, helped acquire and interpret data, drafted and revised the manuscript. M.L.C. designed the study, analyzed and interpreted data, drafted and revised the manuscript and is responsible for the accuracy or integrity of this work. All authors have read and agreed to the published version of the manuscript.
Funding
The Renin-Angiotensin System Study was funded by the National Institutes of Health (NIH), the National Institute of Diabetes and Digestive and Kidney Diseases (DK51975), Merck (United States), Merck Frosst (Canada), and the Canadian Institutes of Health Research (CIHR) (DCT 14281). The Renin-Angiotensin System Study was supported in part by a grant from the National Center for Research Resources of the NIH, to the University of Minnesota General Clinical Research Center (GCRC) (M01-RR00400). The present work was supported by Dr. Caramori’s Minnesota Medical Foundation grant award and Dr. Mauer’s discretionary research funds. Trial Registration: NCT00143949.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Minnesota. Institutional Review Board-Project identification number—9601M10705.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
Renin-Angiotensin System Study co-investigators, in addition to Mauer in the present study, included Robert Gardiner and Keith Drummond, who directed the Montreal center; Bernie Zinman who directed the Toronto Center, Sandra Donnelly (Toronto Center) and Paul Goodyer (Montreal Center), who were the center nephrologists; Samy Suissa, who was the data center director; Trudy Strand, RN (Minneapolis Center), the lead study coordinator; and Marie Claire Gubler, Paris Light Microscopy Center. Lynette Lohse and Sarah Chapeau assisted in manuscript preparation. The authors are also grateful to the study participants who volunteered for these demanding studies.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Correction Statement
This article has been republished with a minor correction to the Institutional Review Board Statement. This change does not affect the scientific content of the article.
References
- Mackensen-Haen, S.; Bohle, A.; Christensen, J.; Wehrmann, M.; Kendziorra, H.; Kokot, F. The consequences for renal function of widening of the interstitium and changes in the tubular epithelium of the renal cortex and outer medulla in various renal diseases. Clin. Nephrol. 1992, 37, 70–77. [Google Scholar]
- Mauer, M.; Caramori, M.L.; Fioretto, P.; Najafian, B. Glomerular structural-functional relationship models of diabetic nephropathy are robust in type 1 diabetic patients. Nephrol. Dial. Transplant. 2015, 30, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Bohle, A.; Wehrmann, M.; Bogenschütz, O.; Batz, C.; Müller, G.A. The Pathogenesis of Chronic Renal Failure in Diabetic Nephropathy: Investigation of 488 Cases of Diabetic Glomerulosclerosis. Pathol. Res. Pract. 1991, 187, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Caramori, M.L.A.; Sisson-Ross, S.; Groppoli, T.; Basgen, J.M.; Mauer, M. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002, 61, 2058–2065. [Google Scholar] [CrossRef]
- Cordonnier, D.J.; Pinel, N.; Barro, C.; Maynard, M.; Zaoui, P.; Halimi, S.; Hurault de Ligny, B.; Reznic, Y.; Simon, D.; Bilous, R.W. Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. The Diabiopsies Group. J. Am. Soc. Nephrol. 1999, 10, 1253–1263. [Google Scholar] [CrossRef]
- Mauer, M.; Zinman, B.; Gardiner, R.; Suissa, S.; Sinaiko, A.; Strand, T.; Drummond, K.; Donnelly, S.; Goodyer, P.; Gubler, M.C.; et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 2009, 361, 40–51. [Google Scholar] [CrossRef]
- Drummond, K.; Mauer, M.; Group IDNS. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 2002, 51, 1580–1587. [Google Scholar] [CrossRef]
- Fufaa, G.D.; Weil, E.J.; Nelson, R.G.; Hanson, R.L.; Knowler, W.C.; Rovin, B.H.; Wu, H.; Klein, J.B.; Mifflin, T.E.; Feldman, H.I.; et al. Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol. Dial. Transplant. 2015, 30, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, K.M.; Cai, J.; Looker, H.C.; Merchant, M.L.; Nelson, R.G.; Fufaa, G.D.; Weil, E.J.; Feldman, H.I.; Vasan, R.S.; Kimmel, P.L.; et al. Plasma bradykinin and early diabetic nephropathy lesions in type 1 diabetes mellitus. PLoS ONE 2017, 12, e0180964. [Google Scholar] [CrossRef] [PubMed]
- Looker, H.C.; Mauer, M.; Nelson, R.G. Role of Kidney Biopsies for Biomarker Discovery in Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 192–201. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Cao, Z.; Cooper, M.E. Role of angiotensin II in tubulointerstitial injury. Semin. Nephrol. 2001, 21, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.; Goodyer, P.; Mauer, M.; RASS Investigators. Comparing the automated versus manual method of needle biopsy for renal histology artefacts. Nephrol. Dial. Transplant. 2008, 23, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Chavers, B.M.; Simonson, J.; Michael, A.F. A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int. 1984, 25, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.L. Alterations in tubular epithelial cells in diabetic nephropathy. J. Nephrol. 2013, 26, 865–869. [Google Scholar] [CrossRef]
- Steffes, M.W.; Bilous, R.W.; Sutherland, D.E.; Mauer, S.M. Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes 1992, 41, 679–684. [Google Scholar] [CrossRef]
- Kim, Y.; Kleppel, M.M.; Butkowski, R.; Mauer, S.M.; Wieslander, J.; Michael, A.F. Differential expression of basement membrane collagen chains in diabetic nephropathy. Am. J. Pathol. 1991, 138, 413–420. [Google Scholar]
- Stokes, M.B.; Holler, S.; Cui, Y.; Hudkins, K.L.; Eitner, F.; Fogo, A.; Alpers, C.E. Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int. 2000, 57, 487–498. [Google Scholar] [CrossRef]
- Ziyadeh, F.N. Renal tubular basement membrane and collagen type IV in diabetes mellitus. Kidney Int. 1993, 43, 114–120. [Google Scholar] [CrossRef]
- Zhu, D.; Kim, Y.; Steffes, M.W.; Groppoli, T.J.; Butkowski, R.J.; Mauer, S.M. Glomerular distribution of type IV collagen in diabetes by high resolution quantitative immunochemistry. Kidney Int. 1994, 45, 425–433. [Google Scholar] [CrossRef]
- Wada, T.; Furuichi, K.; Sakai, N.; Iwata, Y.; Yoshimoto, K.; Shimizu, M.; Takeda, S.I.; Takasawa, K.; Yoshimura, M.; Kida, H.; et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000, 58, 1492–1499. [Google Scholar] [CrossRef]
- Tam, F.W.; Riser, B.L.; Meeran, K.; Rambow, J.; Pusey, C.D.; Frankel, A.H. Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephrop-athy. Cytokine 2009, 47, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yiu, W.H.; Wong, D.W.L.; Chan, L.Y.Y.; Leung, J.C.K.; Chan, K.W.; Lan, H.Y.; Lai, K.N.; Tang, S.C.W. Tissue Kallikrein Mediates Pro-Inflammatory Pathways and Activation of Protease-Activated Receptor-4 in Proximal Tubular Epithelial Cells. PLoS ONE 2014, 9, e88894. [Google Scholar] [CrossRef]
- Fioretto, P.; Sutherland, D.; Najafian, B.; Mauer, M. Remodeling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int. 2006, 69, 907–912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoja, C.; Abbate, M.; Remuzzi, G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol. Dial. Transplant. 2014, 30, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Steffes, M.W.; Mihatsch, M.J.; Strøm, E.H.; Sutherland, D.E.; Mauer, M. Cyclosporine associated lesions in native kidneys of diabetic pancreas transplant recipients. Kidney Int. 1995, 48, 489–495. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).