Abstract
The communication between brain and peripheral tissues is mediated by neuropeptides that coordinate the functions of each organ with the activities of the entire body in specific environmental conditions. Hypothalamic neuropeptides act as neurotransmitters and hormones to regulate the physiology of food intake, digestion, and metabolism, having a direct or indirect impact on the liver. Investigations on liver pathologies found that dysfunctions of neuropeptides and their receptors are associated with liver disorders such as non-alcoholic fatty liver disease, steatohepatitis, cholestasis, cirrhosis, and liver cancer. In this article, we reviewed neuropeptides that regulate energy homeostasis and lipid and glucose metabolism in the liver and are associated with liver injuries. Firstly, peptides involved in regulatory processes in the brain and liver, such as neuropeptide Y, agouti-related protein, and the galanin family, are related to obesity and its comorbidities, including type 2 diabetes and metabolic syndrome, are presented. Secondly, a comprehensive review of neuropeptides such as secretin, vasoactive intestinal peptide, substance P, and somatostatin, which are involved in liver injuries unrelated to obesity; i.e., cholestasis-induced biliary hyperplasia, cirrhosis, hepatocellular carcinoma, and cholangiocarcinoma, is also presented. The cellular and molecular mechanisms underlining liver injuries related to the dysfunction of these neuropeptides and receptors are also described.
1. Introduction
Neuropeptides are produced in the central and peripheral nervous system and are released into the systemic circulation or locally into specific organs from nerve terminals to regulate physiological functions at the cellular and tissue level [1]. Neuropeptides can act as neurotransmitters, neuromodulators, or hormones via specific receptors which are expressed in all tissues [1]. The hypothalamus regulates adaptive changes of metabolic and physiological functions such as feeding behavior, digestion, energy balance, thermoregulation, immunity, circadian rhythms, and many others according to ever-changing environmental conditions [1,2]. A major signaling pathway mediated by hypothalamic neuropeptides is the hypothalamus–pituitary–adrenal (HPA) axis, functioning as the main controller of the body’s response to stress [3,4,5]. Neuropeptides that are produced mainly in the brain circulate through the blood to modulate hepatic function via specific receptors in hepatic cells [6,7,8]. However, the role of hypothalamic neuropeptides in regulating liver functions is still poorly understood, even though numerous publications have reported significant changes in certain neuropeptides during various liver diseases [9,10,11,12].
In recent years it has been established that several neuropeptides are produced in non-neuronal cells in the liver [13,14], adipose [15], and immune system [16,17]. A diversity of neuropeptides have been detected in the liver, acting on specific receptors in parenchymal cells [14,18], hepatic stellate cells (HSC) [14,19], cholangiocytes [13,14,20], and immune cells [16,17].
Neuropeptides are ligands of G-protein coupled receptors (GPCR) and can modulate a multitude of cellular functions in health and disease [21]. Here, we review recent studies focusing on hypothalamic neuropeptides and their receptors that regulate the physiological processes of the liver. We categorized a multitude of hypothalamic neuropeptides associated with liver diseases into two groups: (i) neuropeptides with roles in feeding behavior, metabolism, and energy homeostasis that are related to liver injuries as comorbidities of obesity, type 2 diabetes (T2D), or metabolic syndrome (MS); these are summarized in Table 1; (ii) neuropeptides associated with liver injuries unrelated to the regulation of food intake or obesity, such as cholestasis, hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and hepatitis due to autoimmune disease. These latter neuropeptides are secreted by sensory nerves or organ-specific cells, acting locally within the liver to control cell proliferation, immune response to injuries, and fibrogenesis.
This review is aimed to cover the most recent reports on hypothalamic neuropeptides that have an impact on the hepatic system. While most of the neuropeptides discussed here have been known and studied for a long time, there are a few, such as spexin and nesfatin-1, which were identified more recently, and have attracted a lot of attention for future research to develop newer, better therapies for liver diseases.
A schematic representation of the hypothalamic regions related to the neuropeptides that influence liver physiological functions, which are discussed in this review, is shown in Figure 1.
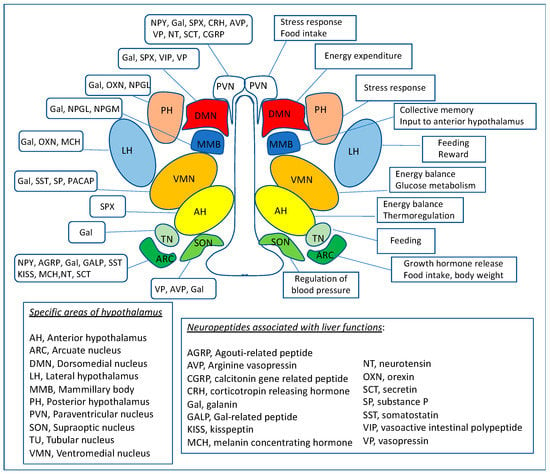
Figure 1.
Map of hypothalamic neural centers which produce or are activated by neuropeptides discussed in this review. The diagram presents the hypothalamic nuclei and their functions (on the right side), including regulation of energy balance, food intake, body weight, glucose, and lipid metabolism that influence the liver. In parallel, on the left side of the map, the neuropeptides involved in numerous aspects of hypothalamus-regulated physiological functions related to the liver are presented.

Table 1.
Summary of hypothalamic neuropeptides associated with liver diseases as comorbidities of obesity, imbalanced food intake, and energy homeostasis.
Table 1.
Summary of hypothalamic neuropeptides associated with liver diseases as comorbidities of obesity, imbalanced food intake, and energy homeostasis.
| Neuropeptide | Disease | Dysregulation/ Symptoms | Signaling Pathways/Therapies | References |
|---|---|---|---|---|
| Neuropeptide Y (NPY) | Obesity and NAFLD | NPY genetic variant rs16147 correlated with obesity metabolic syndrome, insulin resistance | NPY increases proinflammatory response | [22] |
| Liver steatosis | Increased intrahepatic NPY-immuno-reactive fibers. | NPY stimulated fat storage in liver and adipose via macrophage activation | [23] | |
| Agouti-related protein (AGRP) | Obesity and NAFLD | Upregulation of AGRP gene in obese mice | AGRP overexpression increased food intake | [24] |
| Galanin (Gal) | Obesity and hepatic steatosis | Higher than normal levels of serum and liver Gal. | Gal increased feeding rate and body weight. | [25] |
| Gal stimulated fat storage in adipose and liver | [26,27] | |||
| Orexins | NAFLD | Increased orexin in the liver | Orexin stimulated lipogenesis via ERK1/2 (Thr202/Tyr185) | [28] |
| Melanin-concentrating hormone (MCH) | NAFLD, obesity | Overeating and imbalanced energy homeostasis. | Antagonists of MCH receptors in obese mice reduced lipogenesis, inflammation, and fibrosis in liver | [29,30] |
| Neurotensin (NT) | NAFLD, metabolic syndrome | Higher than normal levels of plasma pro-NT in obesity | Increased NT enhanced fat absorption in the gut | [31] |
| Corticotropin releasing factor (CRF) | Hepatic steatosis, inflammation and fibrosis | CRF enhances steatosis and liver inflammation | CRF upregulated SREBP1, TNFα, and IL-1β by stimulation of sympathetic nervous system. | [32] |
| Somatostatin (SST) | Hepatic steatosis and NASH | Decreased SST in obesity-induced fatty liver disease | A synthetic analog of SST, octreotide, was tested as therapeutic strategy for high-fat diet-induced NASH | [33] |
| Neurosecretory peptide GL (NPGL) | Steatosis, NASH, metabolic syndrome | Precursor of NPGL is increased in hypothalamus of sugar-induced obesity | Unknown, still to be investigated | [34,35] |
| Secretin | NAFLD | Secretin (Sct)−/− and Sct-receptor−/− mice had less liver steatosis compared to wild-type mice when fed high-fat diet | Sct/SctReceptor/miR-125b axis promotes hepatic steatosis via Elov1 lipogenic gene upregulation | [36,37] |
2. Neuropeptide Y (NPY)
NPY is a 36 amino acid peptide produced in the brain and autonomic nervous system, being well conserved among different species, with a role in food intake stimulation upon negative energy balance [38]. NPY acts as a neurotransmitter being the most potent appetite stimulant among several neuropeptides secreted by the hypothalamus [39,40]. NPY receptors have been identified in many organs, including neural [41] and gastrointestinal (GI) [42] systems. In healthy individuals, NPY regulates food intake, digestion, and metabolism and has a neuroprotective role during brain activity, sustaining resilience to stress [43]. In patients with metabolic syndrome, obesity, and other diseases related to chronic energy imbalance, enhanced serum NPY levels have been associated with abnormal hormonal and metabolic changes leading to decreased physical activity, increased food intake, and lipid accumulation in the adipose [44].
In addition to being produced in the hypothalamus, NPY was identified in nerve fibers along peripheral organs, especially in the liver and gall bladder [45]. In the gallbladder, NPY-fibers are close to cholangiocytes, smooth muscle cells, and endothelial cells, having roles in the regulation of blood supply and bile flow within the gallbladder [45].
NPY dysfunction in the liver has been related to several injuries, including hepatic cholestasis, nonalcoholic fatty liver disease (NAFLD), and cancer. Thus, in patients suffering from intrahepatic cholestasis of pregnancy (ICP), a marked upregulation of NPY protein and mRNA expression was observed in maternal plasma and placenta, respectively [46]. Furthermore, in a rodent model, ICP offspring exhibited persistent appetite stimulation with upregulated NPY expression from the day of birth to 6 months [47]. These results suggested that the upregulation of NPY could influence fetal health and pregnancy outcomes in ICP patients. In a rodent model of cholestasis-induced liver injury, the expression of NPY and its receptors was increased in the liver after bile duct ligation (BDL) [12]. Furthermore, chronic NPY treatment decreased proliferating cellular nuclear antigen (PCNA) expression in cholangiocytes of intrahepatic bile ductal mass (IBDM) in BDL rats, suggesting that targeting NPY-signaling may be beneficial for the treatment of biliary hyperplasia [12].
A possible role of NPY in obesity-related NAFLD was studied using NPY-knockout (NPY−/−) versus wild-type (WT) mice that were fed a high-fat diet (HFD) [23]. These data demonstrated that NPY−/− mice did not gain as much weight as the WT-mice even though both groups of mice consumed the same amount of food. This was consistent with the upregulation of several thermogenic genes in brown adipose tissue of NPY−/− mice. Furthermore, NPY deletion attenuated liver steatosis induced by HFD and reduced adipose inflammation, suggesting that a strategy of NPY antagonism could be an efficient therapy to treat NAFLD and obesity [23].
Research on the possible role of NPY in the impaired metabolism of lipids in NAFLD and steatohepatitis indicated that NPY could be indirectly involved, acting via its receptors in the hypothalamus and adipose. Thus, hormones such as ghrelin, leptin, and insulin can modulate NPY transcription in the hypothalamus via specific signaling pathways, as described [48]. Insulin downregulates NPY transcription in neurons under normal conditions; however, NAFLD is usually associated with insulin resistance [49]. This implies that in NAFLD, the insulin-mediated inhibition of NPY transcription via the InsR/IRS/PI3K/AKT/FOXO1 pathway is weakened, increasing NPY-mediated appetite and food consumption [48]. Overproduction of hypothalamic NPY results in increased fat tissue since NPY stimulates adipocyte proliferation and differentiation, acting via Y2R receptors in adipocytes [50]. It was demonstrated that adipocytes express NPY as well as its receptors YR1 and YR2 which are highly produced in obesity [51]. Normally, NPY inhibits lipolysis in adipocytes via YR1 [51]. However, insulin resistance counteracts the inhibitory effect of NPY and causes persistent lipolysis, resulting in an increased concentration of free fatty acids in the systemic circulation and, subsequently, lipid accumulation in hepatocytes [49]. These combined effects of insulin-resistance on NPY neurons and adipocytes increased circulating fatty acids and lipid accumulation in the liver. An additional factor that contributes to the indirect influence of NPY on NAFLD is the adipocyte-macrophage crosstalk which is stimulated by NPY [23]. Thus, WT mice, when fed HFD, had increased expression of NPY mRNA in adipose macrophages, which was positively correlated with liver mass/body weight ratio [23]. In contrast, NPY-null mice with HFD had lower free fatty acids in macrophages from adipose and less liver steatosis, suggesting that NPY is critical for NAFLD pathogenesis [23]. These studies that were focused on fatty acid metabolism and circulation identified the significant impact of insulin resistance on NPY expression in the hypothalamus and on NPY functions in the adipose, leading to liver steatosis.
Chen et al. tested the hypothesis that NAFLD may be partially attributed to increased cholesterol in the liver due to enhanced sympathetic NPY [52]. It was determined that genes of cholesterol biosynthesis encoding for 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and sterol regulatory element binding protein 2 (SREBP2) were upregulated upon intraportal vein injection with NPY [52]. It was also demonstrated that NPY activated cholesterol biosynthesis via Y1 and Y5 receptors in a rat fibroblast cell line (BRL-3A) [52]. These data suggest that NPY contributes to hepatic steatosis by enhancing cholesterol biosynthesis in the liver.
In regard to the influence of NPY on liver fibrosis, a recent report by Ortiz et al. described an intricate process in which NPY is involved and contributes to HSC activation, hepatic fibrosis, and portal hypertension [53]. Two factors, in addition to NPY, are important in the signaling of progressive liver fibrosis, i.e., (i) neprisyl (NEP), which is an endopeptidase that acts on NPY in HSC, producing fragments that are the actual activators of genes directing HSC to change the phenotype toward fibroblast-like cells; (ii) angiotensin-converting enzyme (ACE) that cleaves angiotensin II (AngII); both ACE and AngII are upregulated in activated HSC and in fibrosis [53]. Using NEP-null mice in vivo and NEP inhibitors in HSC in vitro, this study demonstrated that NPY acted via the Y1R receptor to induce HSC activation only when NEP was present to produce cleavage-fragments of NPY. However, NEP downregulation did not reduce portal hypertension in spite of alleviating liver fibrosis. The study proposes two signaling pathways initiated by full-length NPY (fl-NPY) and cleaved NPY (c-NPY), which have opposite effects via two G-proteins coupled to Y1R, i.e., Gαi and Gq. Thus, fl-NPY action results in weak signaling via Gαi (to adenylate cyclase/cAMP/ERK→p-ERK) to stimulate fibrosis and strong activity via Gq (ROCK→contraction) to increase contraction. In contrast, cNPY has the opposite effect, acting strongly via Gαi and weakly via Gq protein, causing increased fibrosis but less contraction. Finally, administration of Entresto (sacubril/valsartan) medical drug, which is a combination of NEP inhibitor with angiotensin I receptor (ATIR1) antagonist, achieved a significant reduction of both fibrosis and portal hypertension when applied to models of liver fibrosis [53]. This study emphasizes the ability of Y1R to modulate different signaling pathways at the same time via Gαi or Gq, depending on small structural differences of its ligands, such as the differences between full-length vs. cleaved NPY peptides. Thus, it can be concluded that NPY and its various receptors in the hypothalamus, adipose, and liver play crucial roles in obesity, insulin resistance, NAFLD, and NASH.
In regard to the effects of NPY on various forms of liver cancers, the data are somewhat different depending on the cell type involved. Thus, a study on the influence of NPY on the proliferation rate of several cell lines of CCA indicated that NPY inhibited CCA cell proliferation via elevation of inositol triphosphate (IP3) secretion and protein kinase C-alpha (PKC-α) activation [11]. This was consistent with elevated NPY levels in tumor samples from CCA patients [11] and with decreased tumor volume in Mz-ChA-1 xenograft mice induced by NPY treatments, indicating promising anti-cancer effects against CCA. In contrast, a study on NPY and its receptors in HCC showed that the NPY5 receptor (Y5R) was increased in HCC and correlated with tumor growth and survival [54]. Moreover, NPY was secreted by peritumorous hepatocytes promoting HCC progression via a transforming growth factor-beta (TGF-β/NPY/Y5R pathway) and NPY cleavage by dipeptidylpeptidase 4 enhanced Y5R activation and tumor growth [54]. The differential effects of NPY in CCA and HCC indicate that NPY signaling pathways are very different and have opposite outcomes in tumoral cholangiocytes of CCA compared to tumoral hepatocytes of HCC. These findings emphasize the fact that each type of cancer has cell-specific anomalies even when occurring in the same organ, in this case, the liver.
While it is clear that NPY signaling plays an important role in liver pathology, more investigations are needed to understand the role of NPY in regulating liver functions, as well as the influence of NPY on various types of liver injuries. So far, clinical studies on strategies to antagonize NPY receptors in order to reduce metabolic syndrome were focused on lipid metabolism in the adipose tissue but not in the liver [48]. However, given the increasing frequency of non-obese NAFLD, it is expected that future research to test the possibility of using Y1-Y5 antagonists as drugs to reduce hepatic steatosis in fatty liver disease in non-obese patients.
3. Agouti-Related Protein (AGRP) and Melanocortin Receptors
AGRP, also known as agouti signaling protein (ASIP) or agouti-related transcript (ART)-peptide, is encoded by the AGRP gene. This gene was discovered as being associated with the agouti locus in mutant mice that were prone to obesity, diabetes, and tumor formation [55]. Human AGRP was isolated in two forms, full size, and a cleaved form, both acting as competitive antagonists of α-melanocyte stimulating hormone (α-MSH) at melanocortin-3 and -4 receptors (MC3R, MC4R) [55,56]. Furthermore, overexpression of human AGRP in transgenic mice caused obesity, indicating that it is an orexigenic peptide and it was expressed predominantly in the ventromedial arcuate nucleus (VAN) of the hypothalamus. Together with NPY, AGRP acts to increase food intake and decrease metabolism, being a very potent and long-lasting appetite stimulator [24,57]. However, AGRP-deficient mice were not only viable, but they had normal responses to starvation or HFD-induced obesity [58]. In addition, a double knockout of AGRP and NPY genes did not produce major phenotypic changes in feeding behavior or energy homeostasis compared to wild-type (WT) mice [58]. These data suggest that there are compensatory mechanisms in place to maintain feeding and survival even when one or multiple neuropeptides with roles in feeding are dysfunctional [59]. Interestingly, in contrast to AGRP, NPY, and other peptides of the proopiomelanocortin family (POMC), mutations in melanocortin receptors are associated with obesity and its comorbidities, including liver steatosis [59]. Thus, the knockout of the MC4R gene in mice caused severe hepatic steatosis at an early age, suggesting that the MC4R receptor could have a critical role in the regulation of fatty acid metabolism in the liver [60]. Data from experiments with both MC3R−/− and MC4R−/− mice indicated that even on a normal diet, these mice developed obesity, hepatic insulin resistance, and steatosis. POMC overexpression induced in the caudal brain stem of rats resulted in a significant reduction in food intake, body weight, serum-free fatty acids, and triglycerides (TG), as well as a 26% decrease in hepatic TG [61]. This shows that POMC overproduction could sustain an anorectic response without upregulation of orexigenic peptides since the mRNA expression of AGRP and NPY did not change. However, similar studies on POMC overexpression in the hypothalamus in lean rats via adenoviral delivery concluded that excessive hypothalamic POMC resulted in the downregulation of MC3R and MC4R and increased AGRP expression that exacerbated obesity in these transgenic rats [62]. Several studies showed that natural and synthetic agonists of MC4R decreased hepatic lipogenic gene expression, including sterol regulatory element-binding protein 1 (SREBP-1), peroxisome proliferator-activated receptor γ2 (PPARγ2), stearoyl-CoA desaturase 1 (SCD1), and acyl-CoA/diacylglycerol acyltransferase 1-2 (DGAT1-2), significantly reducing hepatic steatosis in overweight and diabetic mice [63,64]. Therefore, MC4R-deficient mice have been proposed to be used as a model for NAFLD [65]. Moreover, experiments with MC4R-null mice fed on a Western diet revealed strong inflammation of the liver described as “hepatic crown-like structures” whereby CD11c-positive macrophages surround hepatocytes with large lipid droplets, as found in the mouse model for human NASH [66]. Interestingly, in this particular model of NASH, depletion of CD11c macrophages abolished the “crown-like structure” together with most of the hepatic fibrosis, suggesting that CD11c+ cells have a critical role in aggravating liver injury in steatosis [67].
Overall, it can be concluded that dysregulation of POMC-derived neuropeptides, and especially dysfunctions in MC3R and MC4R, influence obesity-related pathology of the liver and could be targets for new therapies to treat obesity-related NAFLD.
4. Galanin Family (Galanin, Galanin-like Peptide, Spexin, Kisspeptin)
Several neuropeptides, including galanin (Gal), Gal-like peptide (GALP), spexin (SPX), and kisspeptin (KISS), have roles in the regulation of food intake, digestion, and metabolism and are members of the same protein family [2,68,69]. Gal, GALP, and SPX act as neurotransmitters and hormones through specific receptors known as GalR1-3, which have very diverse pharmacological profiles [70,71]. GalR1-3 are seven-transmembrane G protein-coupled receptors (GPCR) signaling through different types of G proteins [68,69]. Even though the KISS peptide acts through a different receptor, the homology of the KISS receptor with GalRs is significant.
4.1. Galanin (Gal)
Gal is a 29/30 amino acid bioactive peptide highly expressed in the central and peripheral nervous system [72,73,74]. One of the main functions of Gal is to regulate neuroendocrine signaling pathways that modulate fat intake and metabolism [25,75]. The liver can be negatively affected by lipid metabolism disorders such as obesity and metabolic syndrome which are often associated with NAFLD and non-alcoholic steatohepatitis (NASH) [76,77]. Numerous clinical studies indicate that the serum level of Gal is significantly increased in patients with obesity, dyslipidemia, and metabolic syndrome [26,27,78].
Recently, increasing evidence has emerged regarding the expression of Gal by hepatocytes, HSC, and cholangiocytes in response to liver injuries [14]. Specifically, systemic and hepatic Gal was increased in BDL-induced cholestasis in rodents [79] and in patients suffering from cholestatic liver diseases [80,81]. Gal stimulated cholangiocyte proliferation in BDL rats via GalR1, which was highly expressed in cholangiocytes [13]. A study on Gal effects on the liver in the Mdr2−/− mouse model of cholestasis indicated that Gal contributed to hepatic fibrogenesis via coordinated activation of GalR1 in cholangiocytes and GalR2 in HSC [14]. Based on data showing that cholestasis in Mdr2−/− mice was correlated with increased Gal in serum and liver, it was demonstrated that Gal enhanced ductular reaction via GalR1 in cholangiocytes while stimulating activation of HSC via GalR2 (Figure 2). Experiments in vivo and in vitro using M871 antagonists specific to GalR2 and M40 pan-antagonist of GalR1 and GalR2 confirmed that Gal affected cholangiocytes and HSC, thus enhancing cell proliferation and hepatic fibrosis [14]. These data suggested that GalR1 and GalR2 could be targeted for developing pharmacological therapies for biliary hyperplasia and liver fibrosis in cholestasis-induced liver injury.
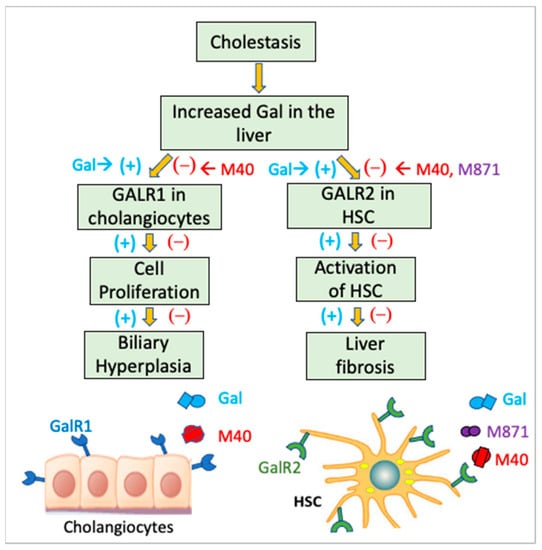
Figure 2.
Role of Gal in ductular reaction-induced activation of HSC in hepatic cholestasis. It has been reported that in a rodent model of cholestasis, Gal was upregulated and induced excessive proliferation of cholangiocytes via GalR1 and activation of HSC via GalR2. Using GalR antagonists such as M871, which specifically inhibits GalR2, and M40, a pan-antagonist of both GalR1 and GalR2, this mechanism was confirmed.
4.2. Galanin-like Peptide (GALP)
GALP is a peptide that shares a partial sequence with Gal, being produced mainly in the hypothalamic arcuate nucleus (ARC) to regulate feeding behavior, energy homeostasis, and reproduction [82]. GALP has an anorexigenic effect and acts through GalR2 [82] and GalR3 [83].
Following reports on the weight loss effects of GALP, studies on its influence on lipid metabolism in the liver and adipose have been carried out, demonstrating that mice administered intracranioventricular (icv) GALP exhibited lower respiratory exchange ratio and increased mitochondrial fatty acid oxidation (FAO) in the liver, as well as enhanced lipolysis in the adipose [84]. Additional studies confirmed the beneficial use of intranasal administration of GALP, reporting that GALP reduced lipid droplet levels in hepatocytes and enhanced FAO in the liver of rodents with obesity [85]. Even though GALP has been recognized as a peptide that attenuates obesity and related fatty liver diseases, the molecular mechanism underlining these effects is not completely understood and warrants further investigation.
4.3. Spexin (SPX)
A newly discovered member of the Gal family is spexin (SPX) or neuropeptide Q (NPQ), which is highly produced in the hypothalamus and has roles in energy homeostasis [86,87]. Unlike Gal, SPX binds preferentially to GalR2/GalR3 and initiates signaling pathways that counteract the orexigenic effects of Gal [88]. Extensive studies on SPX’s impact on obesity [89], metabolic syndrome [90], and T2DM [91,92] have been reported, focusing on the beneficial effects of SPX on reducing body weight and improving insulin sensitivity. In regard to SPX’s impact on liver function, exogenous SPX improved hepatic transaminase levels and lowered steatosis and inflammation while inhibiting long-chain fatty acid uptake into hepatocytes isolated from diet-induced obese mice [93]. A study on the influence of SPX on the liver in patients with NAFLD compared to normal controls indicated that NAFLD patients had significantly less serum SPX [94].
Given the negative correlation of systemic SPX with obesity and dyslipidemia in children and adult patients, several in vitro studies focused on the effect of lipids on SPX expression in different types of cells, including hepatocytes. Thus, it was demonstrated that SPX inhibited gluconeogenesis in a dose- and time-dependent manner in an insulin-resistant cell model [95]. In a study using hepatocytes isolated from diet-induced obesity mice, SPX decreased long-chain fatty acid uptake by 70% [93].
Interestingly, SPX was described as a regulator of BA synthesis, reducing systemic and hepatic total bile acids (BA) in experimental animals [96]. SPX also reduced gall bladder weights and decreased hepatic cholesterol 7α-hydroxylase 1 (Cyp7A1), the rate-limiting enzyme in the BA biosynthesis pathway [97]. Future studies are warranted to investigate the cell and molecular mechanisms underlying these positive effects of SPX on the liver in the context of hepatic cholestasis.
4.4. Kisspeptin (KISS)
KISS is another hypothalamic neuropeptide from the Gal family and acts via the GPR54 receptor, which has significant homology to GalR1-R3 and is expressed in the central and peripheral nervous system as well as in nonneuronal cells in the liver [98]. However, GPR54 has no affinity for Gal; instead, it binds three peptides formed by enzymatic cleavage of the KISS-1 precursor protein [99]. An alternative receptor of KISS is human AXOR12, a GPCR with 81% homology to rat GPR54, expressed predominately in the brain, pituitary, and placenta [100]. Another KISS-1 receptor, GPR49, has been associated with many forms of cancer, especially HCC, even though the molecular mechanisms are still being investigated [101,102].
The effects of KISS peptides and GPR54 receptor, also known as KISS1R, on lipid metabolism in the liver and adipose were also investigated, showing that their expression positively correlates to enhanced lipid biogenesis and accumulation in these organs [103]. A study on KISS-10 from the same family showed that it stimulated lipid synthesis in primary hepatocytes in culture [104]. Recently it has been reported that plasma KISS levels were significantly reduced as a result of gastric bypass surgery in obese patients with T2DM [105]. In contrast, experiments using HFD-fed mice with liver-specific deletion of the KISS1R gene indicated that KISS1R has a role in preventing NAFLD and NASH [106]. The functions of KISS and its receptors in lipid metabolism and energy homeostasis are increasingly recognized [107].
In conclusion, the neuropeptides of the Gal family play different roles in various regulatory pathways of liver physiology, acting either on the same types of receptors or on different ones. Thus, ongoing and future studies are warranted to test multiple strategies to target Gal and KISS receptors for developing drugs against liver injuries from cirrhosis to NASH and liver cancer.
5. Orexins
Orexins A and B, also known as hypocretins, have substantial amino acid homology with the gut hormone secretin. However, they are highly expressed in neurons within and around the posterior and lateral hypothalamus, having a role in the regulation of appetite [108,109]. The orexin receptors OX1R and OX2R were also identified [108] and characterized as a GPCR coupled to Gq and Gi, respectively [108]. Structurally, the orexin receptors are somewhat similar to the Y2 NPY receptor and localized mostly within neurons of the lateral hypothalamus, which receive terminal appositions from NPY-, AGRP-, and α-MSH-peptidergic fibers [110].
It has been demonstrated that the orexin receptors are expressed in the liver, mainly in hepatocytes, modulating metabolic pathways of energy homeostasis. Thus, OX1R is expressed in rat hepatocytes, and it is activated and upregulated by orexin A in a dose-dependent manner, inducing cell proliferation and reducing apoptosis via OX1R/PI3K/AKT signaling to transactivate Foxo1 and mTORC1 [111]. Recently, the orexin system was identified in the liver of birds as having a role in the regulation of lipogenesis without changing the rate of food intake [28]. Thus, orexin upregulated fatty acid synthase, acetyl-CoA carboxylase, and other lipogenic enzymes via phosphorylation of ERK1/2Thr202/Tyr204, but not p38Thr180/Tyr182, nor JNKThr183/Tyr185, in chicken hepatocytes [28].
The effects of orexin A on liver cancer cells were also investigated. It was demonstrated that OX1R was predominantly located within HepG2 cell nuclei, suggesting a possible influence on transcription factors [112]. Indeed, orexin treatment of HepG2 resulted in oxygen-independent upregulation of HIF-1α via activation of PI3K/AKT/mTOR pathway and also increased GLUT1, glucose uptake, and ATP synthesis, suggesting a significant role of orexin in sustaining glucose-based energy production in HCC cells [112].
Interestingly, orexin A had beneficial effects on the liver in the context of the hepatitis B virus (HBV), which is one of the main causes of HCC [113]. HBx is a protein with a role in HBV pathogenicity, and it downregulates OX1R [113]. Orexin A treatment of hepatocytes can attenuate the negative effects of HBV, reversing the oxidative stress caused by HBx that is due to the abnormal polarization of mitochondrial membranes and the excessive production of proinflammatory cytokines [113].
In summary, the dysfunction of orexin or its receptors is very different from one form of liver disease to another, and therefore, specific strategies for developing pharmacological therapies have to be applied for each type of injury. Thus, based on reported data, antagonists of OX1R should be tested as drugs for steatosis, NAFLD, and also for certain forms of liver cancer. In contrast, orexin or synthetic agonists of OX1R could have antiviral benefits in the case of HBV.
6. Melanin Concentrating Hormone (MCH)
MCH is an orexigenic neuropeptide produced in the hypothalamus, acting through two receptors, MCHR1 and MCHR2, to regulate energy balance by influencing hepatic and adipose lipid metabolism. While MCHR1 is associated with diet-induced obesity and NAFLD in WT mice that express only MCHR1, transgenic mice that express both MCHR2 and MCHR1 consumed less food, resisted diet-induced obesity, and had reduced steatosis, counteracting the effect of MCH via MCHR1 [114,115]. MCH promotes lipid accumulation in the liver via the MCHR1 from the lateral hypothalamic area (LH) and the parasympathetic nervous system, while it increases fat storage in the adipose through activation of MCHR1 in the arcuate nucleus (ARC) and by suppressing the sympathetic traffic [29]. Efforts were made to develop drugs based on MCHR1 antagonists. However, these therapeutics have reported hepatobiliary toxicity limiting their use [116].
Interestingly, the MCH function in LH is modulated by elements of the opioid system, such as kappa opioid receptors (kOR) from the same area of the brain [117]. Thus, kOR colocalized with MCHR1 in LH, and kOR gene knockout caused a significant reduction in MCH-induced liver steatosis. Moreover, silencing kOR in LH resulted in the decreased endoplasmic reticulum (ER) stress, inflammation, and fibrosis of the liver in both obesity-related NAFLD and in the methionine and choline-depleted (MCD) diet model of lean body steatohepatitis in mice [117].
Taking into account the interesting reports on the role of MCH and its receptors in hepatic steatosis, it can be concluded that future investigations are going to find new, safer strategies to use MCH, MCHR1, and MCHR2 as targets for treating NAFLD.
7. Corticotropin Releasing Hormone (CRH)
CRH, also known as corticotropin-releasing factor (CRF), is a hypothalamic neuropeptide produced predominantly within PVN and also in the amygdala, having roles in stress responses and circadian cycle function via behavioral, autonomic, and hormonal pathways [118]. Stress-induced CRH stimulates the anterior pituitary gland to produce POMC, the precursor of ACTH, α-MSH, and other neuropeptides with a role in the regulation of feeding behavior and energy balance [119,120]. The CRH/ACTH mediated neuroendocrine signaling to the adrenal glands, also known as the hypothalamic-pituitary-adrenal (HPA) axis, is a stress response pathway, resulting in increased systemic levels of glucocorticoids that act on vital organs, including the liver, to maintain continuous energy balance as well as metabolic and immunological homeostasis [4].
A recent study on the effect of CRH on hepatic lipid metabolism and inflammation was performed by injecting CRH into the central brain of rats, followed by the analysis of changes in the expression of liver genes induced by CRH [32]. The results indicated that central CRF modulated lipogenesis and proinflammatory genes through the sympathetic and noradrenergic nervous systems [32].
An alternative signaling pathway of CRH-induced regulation of liver functions was found to be mediated by fibroblast growth factor 21 (FGF21) [121]. It was demonstrated that hepatocyte-secreted FGF21 induced gluconeogenesis during fasting via the brain-liver axis. Fasting caused PPARα activation in the liver and subsequent upregulation of FGF21, which was released into the systemic circulation, reached the brain, and activated the HPA axis. As a result, FGF21 acted on hypothalamic neurons to activate ERK1/2 and cAMP-signaling pathways, upregulating CRH secretion and stimulating corticosterone-mediated glucogenesis in the liver [121].
There is two-way communication via the HPA axis, so liver injuries can cause dysregulation of the HPA axis. For example, during hepatic cholestasis, the systemic levels of BA become abnormally high, penetrate the blood–brain barrier and activate glucocorticoid receptors in hypothalamic neurons, causing a significant reduction in CRH secretion and suppression of HPA activity [122,123,124].
Three other neuropeptides, i.e., urocortin 1,2 and 3 (UCN1-3), have significant sequence homology to CRH [125] and act through specific receptor proteins, which are GPCR, i.e., CRHR1 and CRHR2 [126,127]. An important role of UCN2 acting via CRHR2, was found in relation to pathways that are dysregulated in metabolic syndrome. Thus, in a metabolomics study, delivery of UCN2 into HFD-fed mice restored liver metabolites to normal levels, increased glucose disposal, and reduced insulin resistance [128]. Similarly, strategies to increase UCN2 in WT and CRHR2−/− mice, when fed an HFD diet, indicated that UCN2 reduced lipid accumulation in the liver while increasing insulin sensitivity via CRHR2 [129]. An investigation on the metabolic response to nutritional stress, such as HFD in CRFR2−/− mice, found that systemic cholesterol and hepatic steatosis were increased in male but not female CRFR2−/−, pointing to a sexually dimorphic risk factor and a possible differential response to UCN2-based therapy of patients with fatty liver disease [130]. Further studies on the possible beneficial effects of UCN2 on the liver in humans are still warranted.
In summary, CRH is critical for a complex regulatory process that involves the liver, among other organs, to ensure the coordination of hepatic metabolism rate with the energy level required by the entire body according to many factors, including nutrition, digestion, and environmental stressors.
8. Somatostatin
Somatostatin (SST) is a 14/28 amino acid neuropeptide highly expressed in the hypothalamus as well as in the peripheral nervous system and in nonneuronal cells in the liver [131]. An important role of SST was revealed in relation to an autofeedback loop in the process of growth hormone (GH) regulation at the level of the hypothalamus [132]. Thus, GH secretion by the pituitary gland is controlled by SST and GH-releasing factor (GHRF) in the hypothalamic PVN and ARC areas. Systemic GH acts on NPY neurons in ARC and also on SST neurons in PVN via GH receptors, increasing SST secretion and inhibiting GH production [132].
The effects of SST are mediated through five specific GPCRs (SSTR1-5), which have nanomolar affinities for SST and are widely expressed in various normal and neoplastic tissues [131]. SSTR1-5 share common signaling pathways, including adenylate cyclase inhibition, modulation of mitogen-activated protein kinases (MAPK’s), and activation of phosphotyrosine phosphatases (PTP’s) having an overall inhibitory effect of cell proliferation via cell cycle arrest while stimulating pro-apoptotic mechanisms [131].
In the liver, GH acts through insulin-like growth factor (IGF1,2) and its receptors IGFR1,2 [133]. IGFs are produced mostly by hepatocytes and are controlled by insulin signaling. A pathophysiological association between IGF2 and cancer cell proliferation was demonstrated using human hepatoma cell lines [133]. Since it was established that the obvious function of SST is counteracting GH activity, SST and synthetic SST analogs were tested for the ability to stop excess cell growth in hepatic diseases such as HCC, CCA, cirrhosis, and polycystic liver disease (PLD). Experiments with rodent models indicated that SST and its analogs protected the liver from injuries caused by several types of stress, such as restraint stress [134], TAA-induced cirrhosis [135], and hepatic–ischemia/reperfusion injury [136]. It was reported that a combination of octreotide (SST analog) and celecoxib (COX-2 inhibitor) attenuated TAA-induced liver damage by reducing HSC activation, hepatic inflammation, and fibrosis [137].
Clinical studies followed, indicating positive results in using SST and its analogs to treat PLD [138,139] and hepatic steatosis associated with acromegaly [140]. Numerous studies were also conducted on the effects of SST-like compounds in the treatment of liver cancer [141], and significant improvement was reported for the use of SST and its analogs in HCC therapies [142,143,144]. Fewer studies were published on the effects of SST on CCA [145], and the findings were not consistent in different reports [140].
Interestingly, based on observations of SST treatments of patients with liver steatosis and inflammation in the context of PLD and acromegaly, where GH excess and insulin resistance have a significant impact on the liver [146], recent reports were published on studies of SST’s effect on obesity-induced fatty liver disease. Thus, octreotide was recommended as a novel therapeutic strategy for HFD-induced NASH and obesity-related metabolic syndrome based on data indicating that octreotide reversed HFD-induced steatosis and normalized the rate of glycogen biosynthesis via activation of Akt/GSK3β (glycogen synthase kinase 3β) and upregulation of glycogen synthase mRNA in the liver [33].
A few reports evaluated the possible side effects of using SST and its analogs in different therapies and cautioned about cases of hyperbilirubinemia and gallstones associated with octreotide treatments [147]. Interestingly, it was determined that octreotide is an inhibitor substrate of OATP1B1,2 and MRP-2, which are bile acid and bilirubin transporters from the liver into the bile ducts [148].
Overall, it can be concluded that SST contributes to the regulation of a multitude of systemic and local processes in the brain, liver, and other organs, having the potential to be used in future therapies for fatty liver disease and hepatobiliary dysfunctions.
9. Neurosecretory Protein GL (NPGL) and GM (NPGM)
In 2014, Ukena et al. reported the identification of a novel neuropeptide in the avian brain with regulatory roles in feeding behavior [149]. Based on its amino acid signature, the peptide was named neuropeptide GL, or NPGL, and was characterized as being produced in the medial mammillary (MM) and infundibular nucleus (IN) of the hypothalamus of chicks during certain periods of development [149]. Subcutaneous infusion of NPGL caused chicks to gain weight without changes in food intake. It was then revealed that the NPGL gene is conserved in mice and rats, having roles in the regulation of growth and energy homeostasis [150]. More recently, the same research group investigated the effects of NPLG on obesity using mice that overexpressed the NPGL gene in the hypothalamus and concluded that NPGL peptide in excess could act as an obesogenic factor within a short period (e.g., 8 weeks in mice) causing accumulation of lipids in both adipose and liver [34]. Moreover, a study on sugar consumption in relation to obesity and liver steatosis concluded that mice overexpressing hypothalamic NPGL, and fed a medium fat diet supplemented with either glucose or fructose, resulted in increased body weight and fatty liver with the fructose-rich diet but not with a diet rich in glucose [35]. These data emphasize the specific and very different effects of fructose versus glucose on NPGL-induced signaling in the liver and warrant further research on the effects of specific nutrients on neuropeptides and their signaling in relation to metabolism.
Interestingly, another neuropeptide named NPGM was found to be the product of a paralogous gene of NPGL, i.e., NPGM, also in the hypothalamus of chicken [151]. NPGM is produced in histaminergic neurons in the MM area, and it has a localization similar to histidine decarboxylase, which catalyzes histamine formation [151]. When NPGM is administered via acute icv injection, it reduces food intake, similarly to histamine [151]. However, a following study using a two-week osmotic pump icv infusion of NPGM in six-day-old chicks showed that NPGM increased body mass and water intake but not food appetite while causing liver steatosis via downregulation of PPARα [151]. The regulatory mechanisms of NPGM versus NPGL are still to be investigated and understood.
In summary, NPGL and NPGM are newly discovered hypothalamic neuropeptides that are associated with an increase in body weight and liver steatosis without changes in food appetite. Future work could reveal the receptor molecules through which these peptides act and if they could be targets for treatments of non-obese NAFLD.
10. Nesfatin-1
Nesfatin-1 was first identified in rat hypothalamus as a polypeptide with anorexic effects, formed by enzymatic cleavage of nucleobindin-2 (NUCB2), a protein highly conserved from fish to mammals [152]. Like other neuropeptides with a role in the regulation of feeding behavior, nesfatin-1 is preponderant in ARC and PVN centers of the hypothalamus and also outside CNS, especially in the GI tract having functions related to lipid metabolism, energy homeostasis, and tissue regeneration after injuries [153]. Investigations on the possible role of nesfatin-1 in the liver started when a few clinical studies reported a significant reduction in serum nesfatin-1 levels in patients with NAFLD [154] and obesity [155].
Investigations on molecular mechanisms of action of nesfatin-1 have not found specific receptors so far but have revealed the involvement of G-proteins and protein kinases C and A [152]. Evidence for a GPCR type of nesfatin-1 binding protein in the liver was obtained from experiments with hepatocytes in vitro, whereby nesfatin-1 induced phosphorylation of AMPK, downregulation of lipogenic transcription factors PPARγ and SREBP1 and a significant reduction in lipid synthesis [156]. Moreover, it has been suggested that nesfatin-1 could act as an endogenous inverse agonist of ghrelin receptor GHSR (growth hormone secretagogue receptor) since in HFD-fed mice, nesfatin-1 enhanced AKT level in the liver, which is a GHSR-dependent mechanism [157].
Based on reports about the remarkable antioxidant, anti-inflammatory, and antiapoptotic effects of treatment with nesfatin-1 in models of intestinal ulceration, studies on the influence of nesfatin-1 on the cholestatic injury of the liver followed [158]. Thus, in a rat model of OJ (obstructive jaundice), it was demonstrated that nesfatin-1 treatment alleviated the OJ-caused liver damage, decreasing neutrophile infiltration, bile duct proliferation, edema, and hepatocyte necrosis [158].
In conclusion, nesfatin-1 is a relatively novel neuropeptide, and its characterization and study in relation to a multitude of physiological processes, including liver diseases, has just begun. The data reported so far from investigations of its role in the brain, liver, GI tract, cardiovascular system, and others suggest the following: (i) nesfatin-1 is downregulated in diseases that incur cell apoptosis and necrosis, and its administration as replacement therapy could help cell and organ regeneration; (ii) nesfatin-1 is upregulated above the normal level in diseases such as cancer for which strategies of blocking its effects could result in significant improvement of the present therapies.
Hypothalamic neuropeptides that affect the liver in diseases unrelated to food intake behavior are listed in Table 2. The liver diseases in which these neuropeptides exhibit dysfunctions include cholestasis, cirrhosis, HCC, CCA, and PLD (Table 2). Some of these peptides may have tangency with obesity and fatty liver diseases; however, their main functions are other than regulation of food intake at the central nervous system level.
11. Substance P (SP)
SP is a proteolytic product of tachykinin, a precursor peptide encoded by the Tac1 gene [159].
Even though SP is from the same peptide family as NPY, its functions are very different, being associated with the regulation of cell and molecular mechanisms in liver diseases that are unrelated to obesity, such as cholestasis, hepatitis, HCC or CCA [159]. In the liver, SP is secreted by peripheral terminals of vagal and spinal afferent nerves [160]. SP acts through the neurokinin-1 receptor (NK1R), and it has been shown that NK1R−/− mice that underwent BDL exhibited a reduced ductular reaction [161]. Mechanistic studies on cholestasis-induced fibrosis of the liver indicated that SP/NK1R signaling decreased senescence gene expression in HSC and increased HSC activation via TGF-β1/Smad3 [162]. Antagonists of NK1R were also able to protect hepatocytes from apoptosis in a model of tumor necrosis-alpha (TNF-α)-induced liver damage [163].
A possible role of SP in carcinogenesis was also investigated. Thus, a study of SP influence on human CCA cells and tumor growth in a xenograft model in nu/nu nude mice concluded that inhibition of NK1R signaling and suppression of SP secretion in the liver could help in CCA management [164].
Recent reports indicated functions of SP related to the modulation of the immune response to liver injuries [165]. Thus, using a mouse model of liver damage induced by concanavalin A (ConA), it was demonstrated that the levels of SP and transaminases were increased in ConA-treated mice, and treatment with an NK1R antagonist reversed this trend. It was found that Kupffer cells (KC) express NK1R, and treatment of KC in vitro with SP resulted in increased secretion of proinflammatory cytokines [165].
Thus, it can be concluded that SP is increased in liver injuries and affects cholangiocytes, HSC, hepatocytes, and KC via NK1R, aggravating hepatic inflammation and fibrosis.
12. Calcitonin Gene-Related Peptide (CGRP)
Soon after identification of calcitonin (CT), a hormone with role in autocrine/paracrine regulation of liver metabolism, Bracq et al. found out that calcitonin genes CALCI and CALCII express cell-specific alternative splicing forms in the liver, i.e., CGRPI in hepatocytes and CGRPII in hepatic sensory nerves which are nerve fibers of vagal or dorsal root/spinal origin that do not innervate directly hepatocytes, but the stroma surrounding the hepatic triades consisting of portal vein, hepatic artery and bile duct [166,167]. CGRPI receptors were also identified in the liver, suggesting a paracrine regulation of nonparenchymal cells by hepatocytes via CGRPI signaling [166]. Moreover, clinical observations indicated that CGRPI was elevated in patients suffering from cirrhosis compared to patients with minor hepatic dysregulation [168]. Investigations on the possible role of CGRPI in hepatobiliary diseases showed that CGRPI−/− mice exhibited reduced biliary hyperplasia [169] and also reduced hepatic fibrosis after BDL [170]. Thus, it was concluded that in cholestasis, CGRPI contributes to liver injury promoting fibrosis through changes in the senescence of cholangiocytes and HSCs via p38 and JNK signaling [170].
These data suggest that new therapies for hepatobiliary diseases could target CGRPI.

Table 2.
Summary of neuropeptides with role in liver diseases which are unrelated to obesity, nor regulation of feeding behavior.
Table 2.
Summary of neuropeptides with role in liver diseases which are unrelated to obesity, nor regulation of feeding behavior.
| Neuropeptide | Disease | Dysregulation/Symptoms | Signaling Pathways/ Therapies | References |
|---|---|---|---|---|
| Vasopressin | HRS, Cirrhosis-related hemorrhage | Variceal bleeding in cirrhotic patients | Unknown | [171,172] |
| Arginine Vasopressin (AVP) | Cirrhosis, hepatitis, HCC | Contributes to hepatic inflammation and fibrosis | Blocking AVP receptors may be therapeutic | [173] |
| Substance P | Autoimmune hepatitis | Increased SP-fibers in the liver, close to lymphocytes; | SP colocalization with TNFα and NF-κB in lymphocytes amplifies inflammation. | [174] |
| HCC | Chronic hepatic inflammation and fibrosis, cancer | Blocking SP–HSC–HCC axis causes reduction in HCC development. | [175] | |
| Cholestatic liver injury | Experimental cholestasis by BDL | Systemic application of SP reduced fibrogenic TGFβ and increased anti-inflammatory cytokines; it enhanced regulatory T cells. | [176] | |
| Somatostatin (SST) | Resection/transplantation of liver | Increased portal flow, liver failure | Exogeneous administration regulates portal flow, protects the liver | [177] |
| HCC | Chronic inflammation, fibrosis, carcinogenesis | SST and its analog reduce angiogenesis and hepatoma cell proliferation via apoptosis | [141] | |
| Polycystic liver disease (PLD) | Hepatic inflammation | SST decreases liver volume in PLD | [139] | |
| Pituitary adenylate cyclase-activating peptide (PACAP) | Liver ischemia-reperfusion injury | Acute hepatocyte death in liver transplantation | Promotes hepatocellular protection via CREB and KLF4-enhanced autophagy | [178,179] |
| Vasoactive intestinal peptide (VIP) | Cholestasis | Bile duct injuries and hyperbilirubinemia | Exogenous VIP restores damaged tight junctions in bile ducts | [180] |
| Corticotropin releasing factor (CRF) | Hepatic fibrosis | CRF administration in rats aggravates acute liver injury | CRF enhances TNFα and IL-1β, the latter by stimulation of sympathetic nervous system. | [32] |
| Secretin | Parenteral nutrition (PN)-associated liver disease (PNALD). | Rat model of PNALD: secretin reduced total bilirubin and bile acid | Secretin induces cAMP/PKA activation and increased cholangiocyte proliferation under liver injury | [181] |
| Cholangiocarcinoma | Dysregulation of cAMP/PKA pathway in cholangiocytes | Unknown | [182] |
13. Neurotensin (NT)
Since its discovery as a hypothalamic neuropeptide, NT has been characterized as a brain-gut peptide secreted in the central and peripheral nervous system as well as along the GI tract [183]. Moreover, NT was reported to bind with high affinity to membranes of cells isolated from the digestive system, including the liver [184]. So far, three NT receptors have been identified and are known as NTSR1-3, which are distributed differently in various tissues [185,186]. Two of these are typical GCPR, while NTSR3 is a different type of receptor, having a single transmembrane domain [185].
Several studies investigated the possible functions of NT and its receptors in the liver in health or disease. NT was associated with liver development and regeneration, being increased in the liver during embryogenesis and fetal development and repressed in adult livers [187,188]. Studies on hepatic regeneration using hepatocytes in vitro indicated that NT stimulated DNA synthesis and cell proliferation via EGF- and TGFα-signaling [189]. It was also reported that NT stimulated drug-induced carcinogenesis in rodent models of liver cancer, inducing cell proliferation in preneoplastic lesions [190]. Receptors of NT have been associated with numerous forms of cancer, including HCC [186]. A driving force for tumor invasion is attributed to chronic inflammation caused by various conditions such as viral hepatitis, alcohol addiction, or NAFLD, and NT has been reported to promote proinflammatory cytokines in HCC, particularly via the IL-8, MAPK, and NF-kB pathways [186,191].
Recently, it was revealed that NT could regulate BA metabolism and FXR-dependent BA transporters in the ileum in the context of obesity [192]. However, the possible role of NT in the modulation of FXR activity and BA biosynthesis in the liver is still unknown. Interestingly, NT may have a role in hepatic cholestasis since studies on rats undergoing BDL showed NT-induced improvement of liver injury evidenced by less ductular reaction and reduced hepatic inflammation [193,194].
Since early studies indicated that NT had hyperglycemic and hypercholesterolemic effects, and the liver has a central role in regulating glucose and lipid metabolism, recent investigations have focused on NT’s role in the liver in relation to fatty liver diseases in T2D, metabolic syndrome, and obesity. A bioinformatics analysis of gene expression in the livers of T2D patients versus healthy volunteers revealed NT as one of the eight hub genes involved in this disease [195]. Moreover, NT was proposed to be a prognostic marker for the development of obesity because an increased level of systemic pro-NT (a stable precursor of NT) was correlated to obese and insulin-resistant individuals [196]. Consistent data were obtained in studies on animal models using NT−/− mice, which showed that these mice were protected from HFD-induced obesity, insulin resistance, and hepatic steatosis [196].
A recent study evaluated the impact of two genetic variants, one of NT and the other of NTSR1, on liver damage in patients suffering from metabolic-associated fatty liver disease (MAFLD), showing that both variants were associated with advanced fibrosis and HCC in MAFLD [197]. In spite of some conflicting data [198,199], newer reports confirmed that circulating pro-NT was correlated to NAFLD and should be used as a noninvasive tool for screening for this disease [200].
Given the interesting reports on the involvement of NT in the regulation of energy homeostasis in the liver, new research could be carried out on this topic in the foreseeable future.
14. Secretin, Vasoactive Intestinal Peptide (VIP), and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)
Secretin, VIP, and PACAP belong to a superfamily of peptides that have significant amino acid sequence homology to GHRF, binding with high affinity to specific receptors while being able to act on common receptors for which they exhibit lower affinities [201]. Chronologically, secretin, VIP, and their receptors were discovered and studied since the 1980s, while PACAP was identified a decade later. These peptides are expressed in the central and peripheral nervous system that controls GI physiological functions [201]. The receptors for these neuropeptides are widely distributed in all the vital organs, but they have been studied extensively in the liver and pancreas, being frequently related to the pathologies of these particular organs.
14.1. Secretin (SCT)
SCT was first identified as a hormone peptide released postprandially from duodenal S-cells to stimulate secretions from the pancreas and liver for food digestion in the upper part of the GI system [202]. Later on, it was demonstrated that SCT and its receptor SCTR are expressed in PVN and ARC centers of the hypothalamus that are engaged in controlling body energy homeostasis via appetite regulation [202]. It was reported that SCT could be an anorectic neuropeptide, suppressing food intake via its receptor and melanocortin pathway [202].
SCT was reported to enhance hydrocholeresis [203] and biliary bicarbonate secretion [204] and to have a high affinity for binding sites of membranes isolated from intrahepatic bile duct epithelium, being coupled to G-protein signaling pathways [205]. Moreover, SCT increased cholangiocyte proliferation after liver injuries such as BDL or partial hepatectomy, while it was downregulated when large damage to bile ducts was caused by treatment with hepatotoxic substances such as CCl4 [206]. In Mdr2−/− mice used as a model of hepatic cholestasis, the abnormally high proliferation of cholangiocytes was reversed by the knockout of SCTR [207]. It was demonstrated that SCT was elevated in serum from patients with primary biliary cholangitis (PBC). Studies on models of this dysfunction proposed that the downregulation of TGF-β receptor II (TGFβRII), which occurs in PBC, induces microRNA-125b/TGF-β1/TGF-βR1/VEGF-A signaling contributing to exacerbation of SCT/SCTR, ductular reaction and increased hepatic fibrosis [208]. A multitude of functions of the SCT/SCTR axis in relation to hepatobiliary diseases and detailed mechanisms underlying them have been recently described [36].
In summary, SCT plays an important role in hepatobiliary physiology, and its dysregulation in cholestasis-induced liver injury continues to be studied with great interest.
14.2. VIP
Early studies on the effect of VIP on the liver demonstrated that VIP enhanced glucose output from hepatocytes by increasing the basal cAMP level while stimulating glycogenolysis and gluconeogenesis and counteracting insulin’s effects [209]. Later on, several reports described high affinity binding sites on plasma membranes from hepatocytes [210], followed by the isolation and structural characterization of VIP receptors [211,212].
Another aspect of VIP function was reported to be related to hepatic bile formation and transport. Thus, VIP increased the volume and flow of bile and its output along the biliary tree [213,214]. However, VIP-induced activation of adenylate cyclase was impaired in hepatic cholestasis, probably due to poor interaction between G-proteins and adenylate cyclase [215,216]. The following studies focused on the role of VIP in cholangiocytes and concluded that VIP stimulated the secretion of fluid and bicarbonate via cAMP-independent pathways in these cells [217]. A recent report indicated that VIP administration promoted the restoration of tight junctions between cholangiocytes in bile ducts damaged due to cholestasis [180].
VIP and its receptors were also investigated in human liver cancer HepG2 cells. VIP inhibited HepG2 cell proliferation while increasing cAMP levels and reducing STAT-3 phosphorylation, proving to have an antiproliferative effect in liver cancer [218]. Moreover, VIP counteracted the IL-6-induced proliferation rate of HepG2 cells in vitro [218]. VIP and its receptors, VPAC1/2, were present in resected HCC and in the HCC cell line Huh7, where it was shown to induce apoptosis of cancer cells via the cAMP/Bcl-xL pathway [219].
An anti-inflammatory influence of VIP was reported for immune-mediated liver injury in vivo, taking place mainly via receptor VPAC1 [220]. Moreover, in an ischemia-reperfusion model of liver injury, VIP played an anti-inflammatory and immunomodulatory role, possibly via inhibition of toll-like receptor 4 (TLR4) [221] and activation of cAMP-mediated protein kinase A signaling [222].
In conclusion, VIP has been described as a peptide with beneficial effects on the hepatobiliary system, and it could be a good candidate for clinical trials for treatments of hepatic cholestasis and other liver diseases that involve inflammation, as well as in HCC.
14.3. PACAP
In 1990, PACAP was identified as a new hypothalamic peptide, having 68% homology with VIP and being around 1000 times more potent than VIP in stimulating cAMP in pituitary cells [223]. PACAP was shown to bind receptors in plasma membranes from many peripheral organs, including the liver [223]. Thus, it was suggested that PACAP had a low affinity for VIP binding sites and also exhibited a higher affinity for a specific group of binding sites on hepatocyte membranes [224,225]. Two types of cDNAs encoding for PACAP receptors were identified in the brain, liver, and other organs, having remarkable similarity to receptors of VIP, secretin, glucagon, and GHRH [226,227].
Studies on the function of PACAP in the liver suggested that PACAP stimulated glucose output from the liver more than VIP did, and cAMP and Ca2+ were important secondary messengers for this signaling mechanism [228].
In conclusion, more research is needed to explain the functions of PACAP in relation to liver physiology.
15. Vasopressin and Arginine Vasopressin
Chronic liver diseases, when left untreated, lead to cirrhosis which is conducive to portal hypertension and hepatorenal syndrome, i.e., dysfunctional blood circulation in the GI tract and kidneys [229]. Therefore, in cirrhosis and liver resection surgeries, a common complication is variceal bleeding. For more than 20 years, the recommended therapy for acute variceal bleeding has been the use of vasoactive drugs such as vasopressin (VP, also known as antidiuretic hormone or ADH) and arginine vasopressin (AVP) [229]. These neuropeptides are synthesized in the supraoptic nucleus of the anterior hypothalamus and stored in the posterior lobe of the pituitary gland, having roles in the physiological functions of the HPA axis [230]. Preclinical studies evidenced that VP, AVP, and their synthetic homolog terlipressin (TP), enhanced liver regeneration after partial hepatectomy (PT) in lean and steatotic mice [172]. TP successfully passed clinical trials and has been used as the main therapy for cirrhosis-caused ascites and variceal bleeding for two decades [231]. However, more recent clinical studies concluded that even though TP reverses hepatorenal syndrome in 40% of patients, it has some negative side effects, such as lowering cardiac output [232]. Efforts have been made to improve the established therapies for cirrhosis-related variceal bleeding. Thus, it was proposed that the cardio-suppressive effects of TP could be reversed by using β-adrenoceptors agonists such as dobutamine [232]. Also, synthetic agonists of the AVP receptor were tested versus TP, and it was found that partial agonists of the AVP receptor were more effective and had less negative side effects on the kidneys, as compared to TP [171].
Overall, VP, AVP, and synthetic analogs such as TP have been used with moderate success in the treatment of variceal bleeding associated with hepatorenal syndrome. Recent reports suggest that further investigations are warranted to improve the use of these biomolecules and also to find new synthetic drugs with fewer side effects in the near future.
16. Translational Studies on Hypothalamic Neuropeptides as Targets for Liver Disease Therapies
Generally, biomedical studies are aimed at developing novel therapies based on newly identified biomolecules or procedures with key roles in signaling and metabolic pathways involved in physiological dysfunctions and diseases. Thus, starting with observations from various sources such as in vitro and in vivo experiments or data from medical studies on patients suffering from specific conditions, certain cell and molecular mechanisms have been characterized as targets for appropriate therapies. Thereafter, preclinical studies in vivo were run on potential drugs or procedures, followed by clinical trials on volunteers, and thus, new therapies have been developed.
As evidenced in the previous sections of this review, extensive preclinical research has produced a vast amount of knowledge on the role of hypothalamic neuropeptides in the regulation of various liver physiological functions. However, only a few of these have been considered for translational studies in clinical trials with applications for liver disease therapies. Most hypothalamic neuropeptides have been tested for the ability to improve neurological and psychological dysfunctions such as anxiety, depression, and post-traumatic stress disorder. So far, very few of these peptides have been considered for clinical trials to be used as therapies for liver diseases.
The neuropeptides, analogs, or inhibitors of their respective receptors that were used in clinical trials in relation to liver injuries are shown in Table 3. A few drugs were designed to reduce hyperphagia, acting as antagonists of MC1R or agonists of MC4R, and were tested in clinical trials with the purpose of being used for the treatment of obesity and comorbidities such as fatty liver disease. Thus, the TTP435 inhibitor of AGRP was tested for its efficacy in lowering obesity via hypothalamic suppression of hyperphagia in a study carried out in 2008. During this trial, even though the main goal was to measure the effect of TTP435 on BMI and food appetite, assays for liver function and plasma-free fatty acids were also performed. In a recent clinical trial from 2021, setmelanotide, an agonist of MC4R, was tested for its anti-obesogenic effects based on data from mechanistic studies showing the activity of this particular receptor to reduce food intake [233]. However, the results of these studies were not made public [233]. In a clinical trial using CRH in the form of corticorelin tablets, the effects of CRH on food cravings were measured, and also, liver assays were performed. Similarly, NT, either alone or in combination with GLP-1, was tested as a therapy for obesity and overeating disorders associated with liver damage.

Table 3.
Neuropeptides and analogs tested in clinical trials as therapies for diseases related to liver injury.
SST and its synthetic analog octreotide have been tested in numerous clinical trials for efficacy and safety when used for liver recovery after hepatectomy, polycystic liver disease, and liver cancer (Table 3).
Terlipressin or glycine-vasopressin was introduced to overcome the adverse effects of VP in treatments of cirrhosis and liver failure-related hepatorenal syndrome (HRS) and acute variceal bleeding (AVB) [234]. More recent trials proposed and tested the use of terlipressin in cases of acute-on-chronic liver failure to control variceal bleeding and extend patient survival [234]. Alternative clinical studies assessed the efficacy and safety of terlipressin in combination with octreotide in patients with cirrhosis portal hypertension and variceal bleeding (Table 3). Even though several clinical trials demonstrated that Terlipressin was effective in cirrhosis reversal and had significant survival benefits in patients with HRS and AVB, it has not been approved by the Food and Drug Administration (FDA) [234]. Thus, it has been recognized that terlipressin has secondary effects, such as the risk of respiratory failure and sepsis in patients with acute-on-chronic liver disease and hepatorenal failure type 1 [235,236].
SP was tested as an anti-pruritus medication associated with diseases unrelated to the liver (Table 3). However, future studies could investigate the use of SP or its synthetic analogs to relieve pruritus that is associated with cholestasis and other forms of hepatobiliary dysfunctions.
Interestingly, another hypothalamic peptide that was assessed for a potential drug for fatty liver disease is growth hormone-releasing hormone (GHRH), which acts on the pituitary gland, enhancing GH secretion. Thus, it has been noted that tesamorelin, a commercially available drug based on GHRH, for patients with HIV to reduce visceral fat, alleviated liver steatosis in a clinical study, as shown in Table 3 [237]. Moreover, a report on the effects of tesamorelin on hepatic transcriptomic profiles in HIV patients suffering from NAFLD co-morbidity indicated that tesamorelin upregulated genes of oxidative phosphorylation and downregulated many genes involved in inflammation, cell proliferation, and fibrosis [238]. It would be of interest to assess the effects of GHRH or synthetic analogs on NAFLD in patients without HIV infection. It has been known for a long time that serum GH levels and especially pulsatile secretion of GH were reduced in obese patients and in models of obesity [239]. Preclinical studies indicated that GH increased lipid metabolism by favoring lipolysis and lipid oxidation resulting in reduced body fat and improved insulin sensitivity. However, GH had adverse effects such as hypoglycemia and high systemic levels of IGF-1. New strategies to regulate GH signaling in relation to lipid metabolism are tested using drugs that act upstream GH, such as GHRH, or downstream GH, at the GHSR level [239].
Newer therapies have been developed based on the use of stem cells, gene editing, or immune-related procedures. Even though these newer therapies have been applied for the treatment of several ailments, including osteoarthritis, for example, no such practical applications were made for liver diseases, even though preclinical studies demonstrated promising results. A good example is NPY which was found to regulate vital activities such as the proliferation, differentiation, and migration of stem cells [43]. So far, it was demonstrated that NPY had roles in the regulation of bone marrow and adipose stem cells, with effects on activation versus quiescence, proliferation, and migration. Since NPY and its receptors are expressed and have regulatory functions in the liver, it would be interesting to test the possible effects of NPY on the liver stem and progenitor cells to find new therapies for liver regeneration after hepatectomy or other injuries that require stimulation of hepatocyte proliferation for treatment. A comprehensive review article by Peng et al. describes promising data for the use of NPY in stem cell therapy to treat conditions such as neurodegenerative diseases, retinal degeneration, myocardial infarction, osteoporosis, and obesity [240]. Studies on the potential use of NPY in cell stem therapy for liver injuries that require hepatocyte regeneration are warranted even at the level of preclinical studies.
It can be concluded that translational research on hypothalamic neuropeptides as potential therapies for liver diseases is still to be developed. Based on a large amount of information from preclinical studies indicating a multitude of effects of these peptides on liver functions, new and better therapies are warranted to be applied for the treatment of liver diseases.
17. Conclusions and Future Directions
The research on the role of neuropeptides in controlling the physiological processes of the whole body, from the cellular level to tissues, organs, and functional systems, is expanding, and it reveals intricate networks of communication among various parts of the nervous and peripheral organs through peptides that can act as neurotransmitters, hormones, cytokines or other types of signaling molecules. Based on the information reviewed in this article, we can conclude that many hypothalamic peptides and their specific receptors have important roles in regulating feeding behavior, food digestion, metabolism, and energy homeostasis not only at the level of the nervous system but also in the liver, since most types of hepatic cells express receptors for the hypothalamic peptides known so far. We also reviewed data on dysfunctions of neuropeptides and their receptors that are associated with liver diseases that are affecting millions of people around the world, such as obesity, metabolic syndrome, NAFLD, NASH, cirrhosis, and HCC. It can be concluded that strategies to modulate receptors of hypothalamic neuropeptides can influence the functions of hepatocytes, cholangiocytes, and HSC, thus ameliorating steatosis and steatohepatitis or reducing ductular reactions and hepatic fibrosis in a variety of liver diseases. However, only a few modulators of neuropeptide receptors have been developed as therapies for liver diseases. Based on research results from preclinical studies, more work should be conducted to use these data for promoting new therapies for a large range of diseases that involve liver injury.
An important goal of these studies, for practical reasons, is the development of new therapies for liver diseases based on hypothalamic peptides and their receptors. Drug-based therapies that act on endogenous receptors to change certain functions of the liver have been used for many decades and are continuously improved. However, in addition to drug/receptor types of therapies, newer methods have been recently introduced and can be categorized into cell-, gene-, and immune therapies. However, none of these novel methods of therapy have been applied to the use of hypothalamic peptides for the treatment of liver diseases as yet. During the last two decades, biomedical research made great progress in understanding the functional interaction between brain and peripheral systems as well as the networks of signaling pathways that ensure normal homeostasis of physiological processes. Therefore, the possibilities for developing a new generation of therapies for liver diseases are open. For example, in preclinical studies, much progress was made using adeno-associated virus (AAV)-mediated gene therapy in liver fibrosis, including the use of cell-specific promoters within the AAV genome to limit gene expression in specific cells of the liver [241]. While many genes encoding for transcription factors (e.g., FOXA2), RNAs (e.g., miR-19b, TGF-β1-shRNA), enzymes (ACE2) with anti-fibrotic effects were targeted into hepatocytes or HSC, genes designed to modulate hypothalamic neuropeptides peptides by this method are still warranted [241].
Another new type of therapy involves the use of immune cells that are genetically engineered to suppress and delete specific cells with a critical role in liver pathogenesis. For example, in CCl4-induced liver fibrosis in mice, infusion of T cells designed to clear senescent HSC with a major role in fibrosis resulted in a significant reduction in fibrosis [242]. Frequently, many of these new therapies that are tested in preclinical studies have negative side effects; however, they may be improved by combination therapy, whereby certain negative outcomes could be mitigated by simultaneous or coordinated use in combination with neuropeptides with roles in liver physiology.
In conclusion, hypothalamic neuropeptides and their respective receptors are important regulatory factors in the liver, and their use in newer therapeutical methods could contribute to producing more beneficial and better-performing therapies in the near future.
Author Contributions
Conceptualization, A.D.P., S.Y.A. and S.D.; writing—original draft preparation, A.D.P.; writing—A.D.P., S.Y.A., J.V., M.M. and S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by NIH R01 awards (DK082435 and DK112803) to Dr. DeMorrow.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef]
- Marcos, P.; Covenas, R. Neuropeptidergic Control of Feeding: Focus on the Galanin Family of Peptides. Int. J. Mol. Sci. 2021, 22, 2544. [Google Scholar] [CrossRef]
- Queen, N.J.; Hassan, Q.N., 2nd; Cao, L. Improvements to Healthspan Through Environmental Enrichment and Lifestyle Interventions: Where Are We Now? Front. Neurosci. 2020, 14, 605. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, A.D.; Kain, J.; Liere, V.; Heavener, T.; DeMorrow, S. Hypothalamus-Pituitary-Adrenal Dysfunction in Cholestatic Liver Disease. Front. Endocrinol. 2018, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. The Neuropeptide System and Colorectal Cancer Liver Metastases: Mechanisms and Management. Int. J. Mol. Sci. 2020, 21, 3494. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, J.J.; de Wied, D.; Adan, R.A. Neuropeptides, food intake and body weight regulation: A hypothalamic focus. Peptides 2002, 23, 2283–2306. [Google Scholar] [CrossRef]
- Hokfelt, T.; Bartfai, T.; Bloom, F. Neuropeptides: Opportunities for drug discovery. Lancet Neurol. 2003, 2, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Francis, H.; Zhou, T.; Meng, F.; Kennedy, L.; Ekser, B.; Baiocchi, L.; Onori, P.; Mancinelli, R.; Gaudio, E.; et al. Neuroendocrine Changes in Cholangiocarcinoma Growth. Cells 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, L.; Scrushy, M.; Meng, F.; Lairmore, T.C.; Alpini, G.; Glaser, S. Biliary epithelium: A neuroendocrine compartment in cholestatic liver disease. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 296–305. [Google Scholar] [CrossRef]
- DeMorrow, S.; Onori, P.; Venter, J.; Invernizzi, P.; Frampton, G.; White, M.; Franchitto, A.; Kopriva, S.; Bernuzzi, F.; Francis, H.; et al. Neuropeptide Y inhibits cholangiocarcinoma cell growth and invasion. Am. J. Physiol. Cell. Physiol. 2011, 300, C1078–C1089. [Google Scholar] [CrossRef] [PubMed]
- DeMorrow, S.; Meng, F.; Venter, J.; Leyva-Illades, D.; Francis, H.; Frampton, G.; Pae, H.Y.; Quinn, M.; Onori, P.; Glaser, S.; et al. Neuropeptide Y inhibits biliary hyperplasia of cholestatic rats by paracrine and autocrine mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G250–G257. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Grant, S.; DeMorrow, S. The Neuropeptide Galanin Is Up-Regulated during Cholestasis and Contributes to Cholangiocyte Proliferation. Am. J. Pathol. 2017, 187, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, A.D.; Grant, S.; Williams, E.; Frampton, G.; Parks, N.; Blaney, H.; Davies, M.; John, R.; Reinhart, E.H.; McMillin, M.; et al. Coordinated Targeting of Galanin Receptors on Cholangiocytes and Hepatic Stellate Cells Ameliorates Liver Fibrosis in Multidrug Resistance Protein 2 Knockout Mice. Am. J. Pathol. 2020, 190, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Song, H.D.; Shi, W.J.; Hu, S.M.; Yang, Y.S.; Tang, J.F.; Chen, M.D.; Chen, J.L. Galanin inhibits leptin expression and secretion in rat adipose tissue and 3T3-L1 adipocytes. J. Mol. Endocrinol. 2004, 33, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Brunner, S.M.; Bianchini, R.; Ramspacher, A.; Emberger, M.; Locker, F.; Schlager, S.; Kofler, B. Galanin is a potent modulator of cytokine and chemokine expression in human macrophages. Sci. Rep. 2019, 9, 7237. [Google Scholar] [CrossRef]
- Ramspacher, A.; Neudert, M.; Koller, A.; Schlager, S.; Kofler, B.; Brunner, S.M. Influence of the regulatory peptide galanin on cytokine expression in human monocytes. Ann. N. Y Acad. Sci. 2019, 1455, 185–195. [Google Scholar] [CrossRef]
- Jard, S. Vasopressin isoreceptors in the liver and kidney: Relationship between hormone binding and biological response. J. Physiol. 1981, 77, 621–628. [Google Scholar]
- He, L.; Li, Z.; Zhou, D.; Ding, Y.; Xu, L.; Chen, Y.; Fan, J. Galanin receptor 2 mediates antifibrogenic effects of galanin on hepatic stellate cells. Exp. Ther. Med. 2016, 12, 3375–3380. [Google Scholar] [CrossRef]
- Wu, N.; Meng, F.; Zhou, T.; Venter, J.; Giang, T.K.; Kyritsi, K.; Wu, C.; Alvaro, D.; Onori, P.; Mancinelli, R.; et al. The Secretin/Secretin Receptor Axis Modulates Ductular Reaction and Liver Fibrosis through Changes in Transforming Growth Factor-beta1-Mediated Biliary Senescence. Am. J. Pathol. 2018, 188, 2264–2280. [Google Scholar] [CrossRef]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Neuropeptide G Protein-Coupled Receptors as Oncotargets. Front. Endocrinol. 2018, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Izaola, O.; de la Fuente, B.; Primo, D.; Aller, R. Association of Neuropeptide Y Gene rs16147 Polymorphism with Cardiovascular Risk Factors, Adipokines, and Metabolic Syndrome in Patients with Obesity. J. Nutr. Nutr. 2016, 9, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Komatsu, T.; Hayashi, H.; Mori, R.; Shimokawa, I. The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis. Biomedicines 2021, 9, 1739. [Google Scholar] [CrossRef]
- Shutter, J.R.; Graham, M.; Kinsey, A.C.; Scully, S.; Luthy, R.; Stark, K.L. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997, 11, 593–602. [Google Scholar] [CrossRef]
- Yun, R.; Dourmashkin, J.T.; Hill, J.; Gayles, E.C.; Fried, S.K.; Leibowitz, S.F. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides 2005, 26, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yu, M.; Gu, X.; Shi, M.; Zhu, Y.; Zhang, Z.; Bo, P. Circulating galanin and galanin like peptide concentrations are correlated with increased triglyceride concentration in obese patients. Clin. Chim. Acta 2016, 461, 126–129. [Google Scholar] [CrossRef]
- Poritsanos, N.J.; Mizuno, T.M.; Lautatzis, M.E.; Vrontakis, M. Chronic increase of circulating galanin levels induces obesity and marked alterations in lipid metabolism similar to metabolic syndrome. Int. J. Obes. 2009, 33, 1381–1389. [Google Scholar] [CrossRef]
- Greene, E.S.; Zampiga, M.; Sirri, F.; Ohkubo, T.; Dridi, S. Orexin system is expressed in avian liver and regulates hepatic lipogenesis via ERK1/2 activation. Sci. Rep. 2020, 10, 19191. [Google Scholar] [CrossRef]
- Imbernon, M.; Beiroa, D.; Vazquez, M.J.; Morgan, D.A.; Veyrat-Durebex, C.; Porteiro, B.; Diaz-Arteaga, A.; Senra, A.; Busquets, S.; Velasquez, D.A.; et al. Central melanin-concentrating hormone influences liver and adipose metabolism via specific hypothalamic nuclei and efferent autonomic/JNK1 pathways. Gastroenterology 2013, 144, 636–649 e636. [Google Scholar] [CrossRef]
- Kawata, Y.; Okuda, S.; Hotta, N.; Igawa, H.; Takahashi, M.; Ikoma, M.; Kasai, S.; Ando, A.; Satomi, Y.; Nishida, M.; et al. A novel and selective melanin-concentrating hormone receptor 1 antagonist ameliorates obesity and hepatic steatosis in diet-induced obese rodent models. Eur. J. Pharmacol. 2017, 796, 45–53. [Google Scholar] [CrossRef]
- Barchetta, I.; Baroni, M.G.; Melander, O.; Cavallo, M.G. New Insights in the Control of Fat Homeostasis: The Role of Neurotensin. Int. J. Mol. Sci. 2022, 23, 2209. [Google Scholar] [CrossRef]
- Nakade, Y.; Kitano, R.; Yamauchi, T.; Kimoto, S.; Sakamoto, K.; Inoue, T.; Kobayashi, Y.; Ohashi, T.; Sumida, Y.; Ito, K.; et al. Effect of Central Corticotropin-Releasing Factor on Hepatic Lipid Metabolism and Inflammation-Related Gene Expression in Rats. Int. J. Mol. Sci. 2021, 22, 3940. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Ye, T.; Li, M.; Li, X.; Qiang, O.; Tang, C.W.; Liu, R. Effects of octreotide on hepatic glycogenesis in rats with high fat diet-induced obesity. Mol. Med. Rep. 2017, 16, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, Y.; Iwakoshi-Ukena, E.; Fukumura, K.; Shikano, K.; Furumitsu, M.; Morishita, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Hypothalamic Overexpression of Neurosecretory Protein GL Leads to Obesity in Male C57BL/6J Mice. Neuroendocrinology 2022, 112, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, Y.; Iwakoshi-Ukena, E.; Naito, M.; Moriwaki, S.; Furumitsu, M.; Ukena, K. Neurosecretory Protein GL Accelerates Liver Steatosis in Mice Fed Medium-Fat/Medium-Fructose Diet. Int. J. Mol. Sci. 2022, 23, 2071. [Google Scholar] [CrossRef]
- Wu, N.; Baiocchi, L.; Zhou, T.; Kennedy, L.; Ceci, L.; Meng, F.; Sato, K.; Wu, C.; Ekser, B.; Kyritsi, K.; et al. Functional Role of the Secretin/Secretin Receptor Signaling During Cholestatic Liver Injury. Hepatology 2020, 72, 2219–2227. [Google Scholar] [CrossRef]
- Chen, L.; Wu, N.; Kennedy, L.; Francis, H.; Ceci, L.; Zhou, T.; Samala, N.; Kyritsi, K.; Wu, C.; Sybenga, A.; et al. Inhibition of Secretin/Secretin Receptor Axis Ameliorates NAFLD Phenotypes. Hepatology 2021, 74, 1845–1863. [Google Scholar] [CrossRef]
- Pedragosa-Badia, X.; Stichel, J.; Beck-Sickinger, A.G. Neuropeptide Y receptors: How to get subtype selectivity. Front. Endocrinol. 2013, 4, 5. [Google Scholar] [CrossRef]
- Yi, M.; Li, H.; Wu, Z.; Yan, J.; Liu, Q.; Ou, C.; Chen, M. A Promising Therapeutic Target for Metabolic Diseases: Neuropeptide Y Receptors in Humans. Cell. Physiol. Biochem. 2018, 45, 88–107. [Google Scholar] [CrossRef]
- Chronwall, B.M.; DiMaggio, D.A.; Massari, V.J.; Pickel, V.M.; Ruggiero, D.A.; O’Donohue, T.L. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience 1985, 15, 1159–1181. [Google Scholar] [CrossRef]
- Durkin, M.M.; Walker, M.W.; Smith, K.E.; Gustafson, E.L.; Gerald, C.; Branchek, T.A. Expression of a novel neuropeptide Y receptor subtype involved in food intake: An in situ hybridization study of Y5 mRNA distribution in rat brain. Exp. Neurol. 2000, 165, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, L.; Segain, J.P.; Bonnet, C.; Cherbut, C.; Lehur, P.A.; Jarry, A.; Galmiche, J.P.; Blottiere, H.M. Functional mapping of NPY/PYY receptors in rat and human gastro-intestinal tract. Peptides 2002, 23, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.Y.; Chen, W.C.; Shi, Y.C.; Wang, C.M.; Lin, S.; He, H.F. Regulation of neuropeptide Y in body microenvironments and its potential application in therapies: A review. Cell Biosci. 2021, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, H.; Cheng, Z.; Bai, Y.; Ma, X. The Role of Neuropeptide Y and Peptide YY in the Development of Obesity via Gut-brain Axis. Curr. Protein Pept. Sci. 2019, 20, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.G.; Fujimura, M.; Mori, A.; Tooyama, I.; Kimura, H. Light and electron microscopy of neuropeptide Y-containing nerves in human liver, gallbladder, and pancreas. Gastroenterology 1991, 101, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Xu, D.; Wang, Y.; Wang, X.; Xia, F. Effect of inducible nitric oxide synthase and neuropeptide Y in plasma and placentas from intrahepatic cholestasis of pregnancy. J. Obstet. Gynaecol. Res. 2018, 44, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, J.; Yan, S.; Zhao, J.; Li, H. Expression of neuropeptide Y and pro-opiomelanocortin in hypothalamic arcuate nucleus in 17alpha-ethinyl estradiol-induced intrahepatic cholestasis pregnant rat offspring. J. Obstet. Gynaecol. Res. 2014, 40, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, X.; Lin, S. Neuropeptide Y and Metabolism Syndrome: An Update on Perspectives of Clinical Therapeutic Intervention Strategies. Front. Cell Dev. Biol. 2021, 9, 695623. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Kuo, L.E.; Kitlinska, J.B.; Tilan, J.U.; Li, L.; Baker, S.B.; Johnson, M.D.; Lee, E.W.; Burnett, M.S.; Fricke, S.T.; Kvetnansky, R.; et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007, 13, 803–811. [Google Scholar] [CrossRef]
- Zhang, W.; Cline, M.A.; Gilbert, E.R. Hypothalamus-adipose tissue crosstalk: Neuropeptide Y and the regulation of energy metabolism. Nutr. Metab. 2014, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, Y.; Yang, K.; Shen, M.; Wang, Y. NPY stimulates cholesterol synthesis acutely by activating the SREBP2-HMGCR pathway through the Y1 and Y5 receptors in murine hepatocytes. Life Sci. 2020, 262, 118478. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.; Klein, S.; Reul, W.H.; Magdaleno, F.; Groschl, S.; Dietrich, P.; Schierwagen, R.; Uschner, F.E.; Torres, S.; Hieber, C.; et al. Neprilysin-dependent neuropeptide Y cleavage in the liver promotes fibrosis by blocking NPY-receptor 1. Cell Rep. 2023, 42, 112059. [Google Scholar] [CrossRef]
- Dietrich, P.; Wormser, L.; Fritz, V.; Seitz, T.; De Maria, M.; Schambony, A.; Kremer, A.E.; Gunther, C.; Itzel, T.; Thasler, W.E.; et al. Molecular crosstalk between Y5 receptor and neuropeptide Y drives liver cancer. J. Clin. Investig. 2020, 130, 2509–2526. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.D.; Zeni, L.; Welcher, A.A.; Narhi, L.O.; Hale, C.; Marasco, J.; Delaney, J.; Gleason, T.; Philo, J.S.; Katta, V.; et al. Biochemical, biophysical, and pharmacological characterization of bacterially expressed human agouti-related protein. Biochemistry 1998, 37, 16041–16052. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ollmann, M.M.; Wilson, B.D.; Dickinson, C.; Yamada, T.; Barsh, G.S.; Gantz, I. Effects of recombinant agouti-signaling protein on melanocortin action. Mol. Endocrinol. 1997, 11, 274–280. [Google Scholar] [CrossRef]
- Ollmann, M.M.; Wilson, B.D.; Yang, Y.K.; Kerns, J.A.; Chen, Y.; Gantz, I.; Barsh, G.S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 1997, 278, 135–138. [Google Scholar] [CrossRef]
- Qian, S.; Chen, H.; Weingarth, D.; Trumbauer, M.E.; Novi, D.E.; Guan, X.; Yu, H.; Shen, Z.; Feng, Y.; Frazier, E.; et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 2002, 22, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A. The melanocortin system and energy balance. Peptides 2006, 27, 281–290. [Google Scholar] [CrossRef]
- Albarado, D.C.; McClaine, J.; Stephens, J.M.; Mynatt, R.L.; Ye, J.; Bannon, A.W.; Richards, W.G.; Butler, A.A. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology 2004, 145, 243–252. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Rodrigues, E.; Zheng, D.; Matheny, M.; Cheng, K.Y.; Scarpace, P.J. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E252–E258. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Cheng, K.Y.; Scarpace, P.J. Lean rats with hypothalamic pro-opiomelanocortin overexpression exhibit greater diet-induced obesity and impaired central melanocortin responsiveness. Diabetologia 2007, 50, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Leckstrom, A.; Lew, P.S.; Poritsanos, N.J.; Mizuno, T.M. Central melanocortin receptor agonist reduces hepatic lipogenic gene expression in streptozotocin-induced diabetic mice. Life Sci. 2011, 88, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Leckstrom, A.; Lew, P.S.; Poritsanos, N.J.; Mizuno, T.M. Treatment with a melanocortin agonist improves abnormal lipid metabolism in streptozotocin-induced diabetic mice. Neuropeptides 2011, 45, 123–129. [Google Scholar] [CrossRef]
- Itoh, M.; Suganami, T.; Nakagawa, N.; Tanaka, M.; Yamamoto, Y.; Kamei, Y.; Terai, S.; Sakaida, I.; Ogawa, Y. Melanocortin 4 receptor-deficient mice as a novel mouse model of nonalcoholic steatohepatitis. Am. J. Pathol. 2011, 179, 2454–2463. [Google Scholar] [CrossRef]
- Itoh, M.; Kato, H.; Suganami, T.; Konuma, K.; Marumoto, Y.; Terai, S.; Sakugawa, H.; Kanai, S.; Hamaguchi, M.; Fukaishi, T.; et al. Hepatic crown-like structure: A unique histological feature in non-alcoholic steatohepatitis in mice and humans. PLoS ONE 2013, 8, e82163. [Google Scholar] [CrossRef]
- Itoh, M.; Suganami, T.; Kato, H.; Kanai, S.; Shirakawa, I.; Sakai, T.; Goto, T.; Asakawa, M.; Hidaka, I.; Sakugawa, H.; et al. CD11c+ resident macrophages drive hepatocyte death-triggered liver fibrosis in a murine model of nonalcoholic steatohepatitis. JCI Insight 2017, 2, e92902. [Google Scholar] [CrossRef]
- Langel, U. Galanin receptor ligands. Springerplus 2015, 4, L18. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.L.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hokfelt, T.; Kofler, B. Physiology, signaling, and pharmacology of galanin peptides and receptors: Three decades of emerging diversity. Pharmacol. Rev. 2015, 67, 118–175. [Google Scholar] [CrossRef]
- Amiranoff, B.; Lorinet, A.M.; Laburthe, M. A clonal rat pancreatic delta cell line (Rin14B) expresses a high number of galanin receptors negatively coupled to a pertussis-toxin-sensitive cAMP-production pathway. Eur. J. Biochem. 1991, 195, 459–463. [Google Scholar] [CrossRef]
- Parker, E.M.; Izzarelli, D.G.; Nowak, H.P.; Mahle, C.D.; Iben, L.G.; Wang, J.; Goldstein, M.E. Cloning and characterization of the rat GALR1 galanin receptor from Rin14B insulinoma cells. Brain Res. Mol. Brain Res. 1995, 34, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Webling, K.E.; Runesson, J.; Bartfai, T.; Langel, U. Galanin receptors and ligands. Front. Endocrinol. 2012, 3, 146. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Rokaeus, A.; Jornvall, H.; McDonald, T.J.; Mutt, V. Galanin-a novel biologically active peptide from porcine intestine. FEBS Lett. 1983, 164, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Gundlach, A.L.; Kofler, B. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 2007, 115, 177–207. [Google Scholar] [CrossRef] [PubMed]
- Kyrkouli, S.E.; Stanley, B.G.; Seirafi, R.D.; Leibowitz, S.F. Stimulation of feeding by galanin: Anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides 1990, 11, 995–1001. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef]
- Acar, S.; Paketci, A.; Kume, T.; Demir, K.; Gursoy Calan, O.; Bober, E.; Abaci, A. Positive correlation of galanin with insulin resistance and triglyceride levels in obese children. Turk. J. Med. Sci. 2018, 48, 560–568. [Google Scholar] [CrossRef]
- Alpini, G.; Lenzi, R.; Sarkozi, L.; Tavoloni, N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J. Clin. Investig. 1988, 81, 569–578. [Google Scholar] [CrossRef]
- Gao, J.; Qiao, L.; Wang, B. Primary biliary cirrhosis is a generalized autoimmune epithelitis. Int. J. Mol. Sci. 2015, 16, 6432–6446. [Google Scholar] [CrossRef]
- Dong, M.; Li, J.; Tang, R.; Zhu, P.; Qiu, F.; Wang, C.; Qiu, J.; Wang, L.; Dai, Y.; Xu, P.; et al. Multiple genetic variants associated with primary biliary cirrhosis in a Han Chinese population. Clin. Rev. Allergy Immunol. 2015, 48, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, T.; Kumano, S.; Ishibashi, Y.; Ogi, K.; Matsui, H.; Harada, M.; Kitada, C.; Kurokawa, T.; Onda, H.; Fujino, M. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J. Biol. Chem. 1999, 274, 37041–37045. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Berger, A.; Santic, R.; Geisberger, R.; Hermann, A.; Herzog, H.; Kofler, B. Pharmacological and functional characterization of galanin-like peptide fragments as potent galanin receptor agonists. Neuropeptides 2005, 39, 179–184. [Google Scholar] [CrossRef]
- Hirako, S.; Wada, N.; Kageyama, H.; Takenoya, F.; Izumida, Y.; Kim, H.; Iizuka, Y.; Matsumoto, A.; Okabe, M.; Kimura, A.; et al. Autonomic nervous system-mediated effects of galanin-like peptide on lipid metabolism in liver and adipose tissue. Sci. Rep. 2016, 6, 21481. [Google Scholar] [CrossRef] [PubMed]
- Takenoya, F.; Hirako, S.; Wada, N.; Nonaka, N.; Hirabayashi, T.; Kageyama, H.; Shioda, S. Regulation of Feeding Behavior and Energy Metabolism by Galanin-like Peptide (GALP): A Novel Strategy to Fight Against Obesity. Curr. Pharm. Des. 2018, 24, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Bai, J.; He, M.; Wong, A.O.L. Spexin as a neuroendocrine signal with emerging functions. Gen. Comp. Endocrinol. 2018, 265, 90–96. [Google Scholar] [CrossRef]
- Zheng, B.; Li, S.; Liu, Y.; Li, Y.; Chen, H.; Tang, H.; Liu, X.; Lin, H.; Zhang, Y.; Cheng, C.H.K. Spexin Suppress Food Intake in Zebrafish: Evidence from Gene Knockout Study. Sci. Rep. 2017, 7, 14643. [Google Scholar] [CrossRef]
- Kim, D.K.; Yun, S.; Son, G.H.; Hwang, J.I.; Park, C.R.; Kim, J.I.; Kim, K.; Vaudry, H.; Seong, J.Y. Coevolution of the spexin/galanin/kisspeptin family: Spexin activates galanin receptor type II and III. Endocrinology 2014, 155, 1864–1873. [Google Scholar] [CrossRef]
- Walewski, J.L.; Ge, F.; Lobdell, H.t.; Levin, N.; Schwartz, G.J.; Vasselli, J.R.; Pomp, A.; Dakin, G.; Berk, P.D. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity 2014, 22, 1643–1652. [Google Scholar] [CrossRef]
- Kumar, S.; Hossain, J.; Nader, N.; Aguirre, R.; Sriram, S.; Balagopal, P.B. Decreased Circulating Levels of Spexin in Obese Children. J. Clin. Endocrinol. Metab. 2016, 101, 2931–2936. [Google Scholar] [CrossRef]
- Gu, L.; Ma, Y.; Gu, M.; Zhang, Y.; Yan, S.; Li, N.; Wang, Y.; Ding, X.; Yin, J.; Fan, N.; et al. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides 2015, 71, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.; Bakar-Ates, F.; Ersoz-Gulcelik, N. Decreased Spexin Levels in Patients with Type 1 and Type 2 Diabetes. Med. Princ. Pract. 2018, 27, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Walewski, J.L.; Anglade, D.; Berk, P.D. Regulation of Hepatocellular Fatty Acid Uptake in Mouse Models of Fatty Liver Disease with and without Functional Leptin Signaling: Roles of NfKB and SREBP-1C and the Effects of Spexin. Semin. Liver Dis. 2016, 36, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; She, Y.; Zhang, Z. Low levels of spexin and adiponectin may predict insulin resistance in patients with non-alcoholic fatty liver. Pract. Lab. Med. 2021, 24, e00207. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Ding, X.; Wang, Y.; Gu, M.; Zhang, J.; Yan, S.; Li, N.; Song, Z.; Yin, J.; Lu, L.; et al. Spexin alleviates insulin resistance and inhibits hepatic gluconeogenesis via the FoxO1/PGC-1alpha pathway in high-fat-diet-induced rats and insulin resistant cells. Int. J. Biol. Sci. 2019, 15, 2815–2829. [Google Scholar] [CrossRef]
- Lin, C.Y.; Zhao, L.; Huang, T.; Lu, L.; Khan, M.; Liu, J.; Zhong, L.L.D.; Cai, Z.W.; Fan, B.M.; Wong, A.O.L.; et al. Spexin Acts as Novel Regulator for Bile Acid Synthesis. Front. Physiol. 2018, 9, 378. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, T.; Zhao, L.; Zhong, L.L.D.; Lam, W.C.; Fan, B.M.; Bian, Z.X. Circulating Spexin Levels Negatively Correlate With Age, BMI, Fasting Glucose, and Triglycerides in Healthy Adult Women. J. Endocr. Soc. 2018, 2, 409–419. [Google Scholar] [CrossRef]
- Lee, D.K.; Nguyen, T.; O’Neill, G.P.; Cheng, R.; Liu, Y.; Howard, A.D.; Coulombe, N.; Tan, C.P.; Tang-Nguyen, A.T.; George, S.R.; et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999, 446, 103–107. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brezillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef]
- Muir, A.I.; Chamberlain, L.; Elshourbagy, N.A.; Michalovich, D.; Moore, D.J.; Calamari, A.; Szekeres, P.G.; Sarau, H.M.; Chambers, J.K.; Murdock, P.; et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 2001, 276, 28969–28975. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sakamoto, M.; Fujii, G.; Tsuiji, H.; Kenetaka, K.; Asaka, M.; Hirohashi, S. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology 2003, 37, 528–533. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Hirooka, Y.; Kaibara, N. Quantitative reverse transcriptase polymerase chain reaction analysis for KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2003, 129, 531–535. [Google Scholar] [CrossRef]
- Wang, T.; Cui, X.; Xie, L.; Xing, R.; You, P.; Zhao, Y.; Yang, Y.; Xu, Y.; Zeng, L.; Chen, H.; et al. Kisspeptin Receptor GPR54 Promotes Adipocyte Differentiation and Fat Accumulation in Mice. Front. Physiol. 2018, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, W.; Huang, Y.; Ni, Y. Effects of kisspeptin-10 on lipid metabolism in cultured chicken hepatocytes. Asian-Australas. J. Anim. Sci. 2012, 25, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Flynn, C.R.; Albaugh, V.L.; Tamboli, R.A.; Gregory, J.M.; Bosompem, A.; Sidani, R.M.; Winnick, J.J. Roux-en-Y gastric bypass surgery improves hepatic glucose metabolism and reduces plasma kisspeptin levels in morbidly obese patients with type 2 diabetes. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G370–G374. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.; Dragan, M.; Kwon, H.; de Oliveira, V.; Rao, S.; Bhatt, V.; Kalemba, K.M.; Shah, A.; Rustgi, V.K.; Wang, H.; et al. Targeting hepatic kisspeptin receptor ameliorates non-alcoholic fatty liver disease in a mouse model. J. Clin. Investig. 2022, 132, e145889. [Google Scholar] [CrossRef]
- Hudson, A.D.; Kauffman, A.S. Metabolic actions of kisspeptin signaling: Effects on body weight, energy expenditure, and feeding. Pharmacol. Ther. 2022, 231, 107974. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.; Gautvik, V.T.; Bartlett, F.S., 2nd; et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef]
- Sakurai, T. Orexins and orexin receptors: Implication in feeding behavior. Regul. Pept. 1999, 85, 25–30. [Google Scholar] [CrossRef]
- Ju, S.J.; Zhao, Y.; Chang, X.; Guo, L. Orexin A protects cells from apoptosis by regulating FoxO1 and mTORC1 through the OX1R/PI3K/AKT signaling pathway in hepatocytes. Int. J. Mol. Med. 2014, 34, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, Y.; Zhao, Y.; Sun, X.; Fan, D.; Guo, L. Orexin A affects HepG2 human hepatocellular carcinoma cells glucose metabolism via HIF-1alpha-dependent and -independent mechanism. PLoS ONE 2017, 12, e0184213. [Google Scholar] [CrossRef]
- Wang, L.; He, T.; Wan, B.; Wang, X.; Zhang, L. Orexin A ameliorates HBV X protein-induced cytotoxicity and inflammatory response in human hepatocytes. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Gomori, A.; Ishihara, A.; Ito, M.; Matsushita, H.; Ito, M.; Mashiko, S.; Iwaasa, H.; Matsuda, M.; Bednarek, M.A.; Qian, S.; et al. Blockade of MCH1 receptor signalling ameliorates obesity and related hepatic steatosis in ovariectomized mice. Br. J. Pharmacol. 2007, 151, 900–908. [Google Scholar] [CrossRef]
- Chee, M.J.; Pissios, P.; Prasad, D.; Maratos-Flier, E. Expression of melanin-concentrating hormone receptor 2 protects against diet-induced obesity in male mice. Endocrinology 2014, 155, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Otieno, M.A.; Bhaskaran, V.; Janovitz, E.; Callejas, Y.; Foster, W.B.; Washburn, W.; Megill, J.R.; Lehman-McKeeman, L.; Gemzik, B. Mechanisms for Hepatobiliary Toxicity in Rats Treated with an Antagonist of Melanin Concentrating Hormone Receptor 1 (MCHR1). Toxicol. Sci. 2017, 155, 379–388. [Google Scholar] [CrossRef]
- Imbernon, M.; Sanchez-Rebordelo, E.; Romero-Pico, A.; Kallo, I.; Chee, M.J.; Porteiro, B.; Al-Massadi, O.; Contreras, C.; Ferno, J.; Senra, A.; et al. Hypothalamic kappa opioid receptor mediates both diet-induced and melanin concentrating hormone-induced liver damage through inflammation and endoplasmic reticulum stress. Hepatology 2016, 64, 1086–1104. [Google Scholar] [CrossRef]
- Caruso, A.; Gaetano, A.; Scaccianoce, S. Corticotropin-Releasing Hormone: Biology and Therapeutic Opportunities. Biology 2022, 11, 1785. [Google Scholar] [CrossRef]
- Autelitano, D.J.; Lundblad, J.R.; Blum, M.; Roberts, J.L. Hormonal regulation of POMC gene expression. Annu. Rev. Physiol. 1989, 51, 715–726. [Google Scholar] [CrossRef]
- Wardlaw, S.L. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur. J. Pharmacol. 2011, 660, 213–219. [Google Scholar] [CrossRef]
- Liang, Q.; Zhong, L.; Zhang, J.; Wang, Y.; Bornstein, S.R.; Triggle, C.R.; Ding, H.; Lam, K.S.; Xu, A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 2014, 63, 4064–4075. [Google Scholar] [CrossRef]
- McMillin, M.; DeMorrow, S. Effects of bile acids on neurological function and disease. FASEB J. 2016, 30, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Quinn, M.; Divan, A.; Grant, S.; Patel, N.; Newell-Rogers, K.; DeMorrow, S. Suppression of the HPA Axis During Cholestasis Can Be Attributed to Hypothalamic Bile Acid Signaling. Mol. Endocrinol. 2015, 29, 1720–1730. [Google Scholar] [CrossRef]
- Dautzenberg, F.M.; Hauger, R.L. The CRF peptide family and their receptors: Yet more partners discovered. Trends Pharmacol. Sci. 2002, 23, 71–77. [Google Scholar] [CrossRef]
- Grammatopoulos, D.K. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br. J. Pharmacol. 2012, 166, 85–97. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Zoumakis, E. Milestones in CRH Research. Curr. Mol. Pharmacol. 2017, 10, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Truax, A.D.; Giamouridis, D.; Lai, N.C.; Guo, T.; Hammond, H.K.; Gao, M.H. Significant alteration of liver metabolites by AAV8.Urocortin 2 gene transfer in mice with insulin resistance. PLoS ONE 2019, 14, e0224428. [Google Scholar] [CrossRef]
- Gao, M.H.; Giamouridis, D.; Lai, N.C.; Walenta, E.; Paschoal, V.A.; Kim, Y.C.; Miyanohara, A.; Guo, T.; Liao, M.; Liu, L.; et al. One-time injection of AAV8 encoding urocortin 2 provides long-term resolution of insulin resistance. JCI Insight 2016, 1, e88322. [Google Scholar] [CrossRef]
- Paruthiyil, S.; Hagiwara, S.I.; Kundassery, K.; Bhargava, A. Sexually dimorphic metabolic responses mediated by CRF(2) receptor during nutritional stress in mice. Biol. Sex. Differ. 2018, 9, 49. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Minami, S.; Kamegai, J.; Sugihara, H.; Suzuki, N.; Wakabayashi, I. Growth hormone inhibits its own secretion by acting on the hypothalamus through its receptors on neuropeptide Y neurons in the arcuate nucleus and somatostatin neurons in the periventricular nucleus. Endocr. J. 1998, 45, S19–S26. [Google Scholar] [CrossRef]
- Scharf, J.G.; Dombrowski, F.; Ramadori, G. The IGF axis and hepatocarcinogenesis. Mol. Pathol. 2001, 54, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.M.; Ragy, M.M.; Ahmed, S.M. Somatostatin analogue, Octreotide, improves restraint stress-induced liver injury by ameliorating oxidative stress, inflammatory response, and activation of hepatic stellate cells. Cell Stress. Chaperones 2018, 23, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Gao, J.H.; Zhao, C.; Wen, S.L.; Tang, C.W.; Wang, Y.F. Cyclooxygenase-2 up-regulates hepatic somatostatin receptor 2 expression. Sci. Rep. 2018, 8, 11033. [Google Scholar] [CrossRef]
- El-Sisi, A.E.E.; Sokar, S.S.; Shebl, A.M.; Mohamed, D.Z.; Abu-Risha, S.E. Octreotide and melatonin alleviate inflammasome-induced pyroptosis through inhibition of TLR4-NF-kappaB-NLRP3 pathway in hepatic ischemia/reperfusion injury. Toxicol. Appl. Pharmacol. 2021, 410, 115340. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Tong, H.; Gao, J.H.; Tang, S.H.; Yang, W.J.; Wang, G.M.; Zhou, H.Y.; Wen, S.L. Anti-inflammation treatment for protection of hepatocytes and amelioration of hepatic fibrosis in rats. Exp. Ther. Med. 2021, 22, 1213. [Google Scholar] [CrossRef]
- Griffiths, J.; Mills, M.T.; Ong, A.C. Long-acting somatostatin analogue treatments in autosomal dominant polycystic kidney disease and polycystic liver disease: A systematic review and meta-analysis. BMJ Open 2020, 10, e032620. [Google Scholar] [CrossRef]
- Garofalo, C.; Capuano, I.; Pennino, L.; De Gregorio, I.; Riccio, E.; Provenzano, M.; Crocetto, F.; Buonanno, P.; Pandolfo, S.D.; Andreucci, M.; et al. The effects of somatostatin analogues on liver volume and quality of life in polycystic liver disease: A meta-analysis of randomized controlled trials. Sci. Rep. 2021, 11, 23500. [Google Scholar] [CrossRef]
- Fiebiger, W.C.; Scheithauer, W.; Traub, T.; Kurtaran, A.; Gedlicka, C.; Kornek, G.V.; Virgolini, I.; Raderer, M. Absence of therapeutic efficacy of the somatostatin analogue lanreotide in advanced primary hepatic cholangiocellular cancer and adenocarcinoma of the gallbladder despite in vivo somatostatin-receptor expression. Scand. J. Gastroenterol. 2002, 37, 222–225. [Google Scholar] [CrossRef]
- Periferakis, A.; Tsigas, G.; Periferakis, A.T.; Badarau, I.A.; Scheau, A.E.; Tampa, M.; Georgescu, S.R.; Didilescu, A.C.; Scheau, C.; Caruntu, C. Antitumoral and Anti-inflammatory Roles of Somatostatin and Its Analogs in Hepatocellular Carcinoma. Anal. Cell. Pathol. 2021, 2021, 1840069. [Google Scholar] [CrossRef]
- Huang, C.Z.; Huang, A.M.; Liu, J.F.; Wang, B.; Lin, K.C.; Ye, Y.B. Somatostatin Octapeptide Inhibits Cell Invasion and Metastasis in Hepatocellular Carcinoma Through PEBP1. Cell. Physiol. Biochem. 2018, 47, 2340–2349. [Google Scholar] [CrossRef]
- Raderer, M.; Hejna, M.H.; Kurtaran, A.; Kornek, G.V.; Valencak, J.B.; Oberhuber, G.; Vorbeck, F.; Virgolini, I.; Scheithauer, W. Successful treatment of an advanced hepatocellular carcinoma with the long-acting somatostatin analog lanreotide. Am. J. Gastroenterol. 1999, 94, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Lasfer, M.; Vadrot, N.; Schally, A.V.; Nagy, A.; Halmos, G.; Pessayre, D.; Feldmann, G.; Reyl-Desmars, F.J. Potent induction of apoptosis in human hepatoma cell lines by targeted cytotoxic somatostatin analogue AN-238. J. Hepatol. 2005, 42, 230–237. [Google Scholar] [CrossRef]
- Tan, C.K.; Podila, P.V.; Taylor, J.E.; Nagorney, D.M.; Wiseman, G.A.; Gores, G.J.; LaRusso, N.F. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology 1995, 108, 1908–1916. [Google Scholar] [CrossRef]
- Ciresi, A.; Guarnotta, V.; Campo, D.; Giordano, C. Hepatic Steatosis Index in Acromegaly: Correlation with Insulin Resistance Regardless of the Disease Control. Int. J. Endocrinol. 2018, 2018, 5421961. [Google Scholar] [CrossRef]
- Brighi, N.; Lamberti, G.; Maggio, I.; Manuzzi, L.; Ricci, C.; Casadei, R.; Santini, D.; Mosconi, C.; Lisotti, A.; Ambrosini, V.; et al. Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig. Liver Dis. 2019, 51, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Stieger, B.; Merz, M.; Kullak-Ublick, G.A. Octreotide inhibits the bilirubin carriers organic anion transporting polypeptides 1B1 and 1B3 and the multidrug resistance-associated protein 2. J. Pharmacol. Exp. Ther. 2015, 355, 145–151. [Google Scholar] [CrossRef]
- Ukena, K.; Iwakoshi-Ukena, E.; Taniuchi, S.; Bessho, Y.; Maejima, S.; Masuda, K.; Shikano, K.; Kondo, K.; Furumitsu, M.; Tachibana, T. Identification of a cDNA encoding a novel small secretory protein, neurosecretory protein GL, in the chicken hypothalamic infundibulum. Biochem. Biophys. Res. Commun. 2014, 446, 298–303. [Google Scholar] [CrossRef]
- Ukena, K. Avian and murine neurosecretory protein GL participates in the regulation of feeding and energy metabolism. Gen. Comp. Endocrinol. 2018, 260, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Shikano, K.; Bessho, Y.; Kato, M.; Iwakoshi-Ukena, E.; Taniuchi, S.; Furumitsu, M.; Tachibana, T.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Localization and function of neurosecretory protein GM, a novel small secretory protein, in the chicken hypothalamus. Sci. Rep. 2018, 8, 704. [Google Scholar] [CrossRef]
- Rupp, S.K.; Wolk, E.; Stengel, A. Nesfatin-1 Receptor: Distribution, Signaling and Increasing Evidence for a G Protein-Coupled Receptor-A Systematic Review. Front. Endocrinol. 2021, 12, 740174. [Google Scholar] [CrossRef]
- Kras, K.; Muszynski, S.; Tomaszewska, E.; Arciszewski, M.B. Minireview: Peripheral Nesfatin-1 in Regulation of the Gut Activity-15 Years since the Discovery. Animals 2022, 12, 101. [Google Scholar] [CrossRef]
- Basar, O.; Akbal, E.; Koklu, S.; Kocak, E.; Tuna, Y.; Ekiz, F.; Gultuna, S.; Yiotalmaz, F.M.; Aydogan, T. A novel appetite peptide, nesfatin-1 in patients with non-alcoholic fatty liver disease. Scand. J. Clin. Lab. Investig. 2012, 72, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Hallschmid, M.; Kern, W.; Lehnert, H.; Randeva, H.S. Decreased cerebrospinal fluid/plasma ratio of the novel satiety molecule, nesfatin-1/NUCB-2, in obese humans: Evidence of nesfatin-1/NUCB-2 resistance and implications for obesity treatment. J. Clin. Endocrinol. Metab. 2011, 96, E669–E673. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.; Gao, L.; Li, Y.; Zhao, J.; Zhang, W. AMPK-dependent modulation of hepatic lipid metabolism by nesfatin-1. Mol. Cell. Endocrinol. 2015, 417, 20–26. [Google Scholar] [CrossRef]
- Fan, X.T.; Tian, Z.; Li, S.Z.; Zhai, T.; Liu, J.L.; Wang, R.; Zhang, C.S.; Wang, L.X.; Yuan, J.H.; Zhou, Y.; et al. Ghrelin Receptor Is Required for the Effect of Nesfatin-1 on Glucose Metabolism. Front. Endocrinol. 2018, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, A.; Gulcicek, O.B.; Ercetin, C.; Yigitbas, H.; Yavuz, E.; Arici, S.; Erzik, C.; Zengi, O.; Demirturk, P.; Celik, A.; et al. Nesfatin-1 alleviates extrahepatic cholestatic damage of liver in rats. Bosn. J. Basic. Med. Sci. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Palma, C. Tachykinins and their receptors in human malignancies. Curr. Drug. Targets 2006, 7, 1043–1052. [Google Scholar] [CrossRef]
- Uyama, N.; Geerts, A.; Reynaert, H. Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 280, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.; Gaudio, E.; Renzi, A.; Mancinelli, R.; Ueno, Y.; Venter, J.; White, M.; Kopriva, S.; Chiasson, V.; DeMorrow, S.; et al. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G297–G305. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Meng, F.; Wu, N.; Zhou, T.; Venter, J.; Francis, H.; Kennedy, L.; Glaser, T.; Bernuzzi, F.; Invernizzi, P.; et al. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology 2017, 66, 528–541. [Google Scholar] [CrossRef]
- Bang, R.; Biburger, M.; Neuhuber, W.L.; Tiegs, G. Neurokinin-1 receptor antagonists protect mice from CD95− and tumor necrosis factor-alpha-mediated apoptotic liver damage. J. Pharmacol. Exp. Ther. 2004, 308, 1174–1180. [Google Scholar] [CrossRef]
- Meng, F.; DeMorrow, S.; Venter, J.; Frampton, G.; Han, Y.; Francis, H.; Standeford, H.; Avila, S.; McDaniel, K.; McMillin, M.; et al. Overexpression of membrane metalloendopeptidase inhibits substance P stimulation of cholangiocarcinoma growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G759–G768. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, M.; Zhang, H.; Wang, X. Substance P participates in immune-mediated hepatic injury induced by concanavalin A in mice and stimulates cytokine synthesis in Kupffer cells. Exp. Ther. Med. 2013, 6, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Bracq, S.; Clement, B.; Pidoux, E.; Moukhtar, M.S.; Jullienne, A. CGRP is expressed in primary cultures of human hepatocytes and in normal liver. FEBS Lett. 1994, 351, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. Anatomy and function of sensory hepatic nerves. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 280, 827–835. [Google Scholar] [CrossRef]
- Bendtsen, F.; Schifter, S.; Henriksen, J.H. Increased circulating calcitonin gene-related peptide (CGRP) in cirrhosis. J. Hepatol. 1991, 12, 118–123. [Google Scholar] [CrossRef]
- Glaser, S.S.; Ueno, Y.; DeMorrow, S.; Chiasson, V.L.; Katki, K.A.; Venter, J.; Francis, H.L.; Dickerson, I.M.; DiPette, D.J.; Supowit, S.C.; et al. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab. Investig. 2007, 87, 914–926. [Google Scholar] [CrossRef]
- Wan, Y.; Ceci, L.; Wu, N.; Zhou, T.; Chen, L.; Venter, J.; Francis, H.; Bernuzzi, F.; Invernizzi, P.; Kyritsi, K.; et al. Knockout of alpha-calcitonin gene-related peptide attenuates cholestatic liver injury by differentially regulating cellular senescence of hepatic stellate cells and cholangiocytes. Lab. Investig. 2019, 99, 764–776. [Google Scholar] [CrossRef]
- Fernandez-Varo, G.; Oro, D.; Cable, E.E.; Reichenbach, V.; Carvajal, S.; de la Presa, B.G.; Wisniewski, K.; Gines, P.; Harris, G.; Jimenez, W. Vasopressin 1a receptor partial agonism increases sodium excretion and reduces portal hypertension and ascites in cirrhotic rats. Hepatology 2016, 63, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, R.; Patsenker, E.; de Gottardi, A.; Stickel, F.; Montani, M.; Stroka, D.; Candinas, D.; Beldi, G. Elevated liver regeneration in response to pharmacological reduction of elevated portal venous pressure by terlipressin after partial hepatectomy. Transplantation 2014, 97, 892–900. [Google Scholar] [CrossRef]
- Munoz-Ortega, M.; Macias-Segura, N.; Ventura-Juarez, J.; Avila-Blanco, M.E.; Ponce-Damian, L.D.; Gonzalez-Blas, D.; Sanchez-Aleman, E.; Quintanar-Stephano, A. Recovery from Liver Failure and Fibrosis in a Rat Portacaval Anastomosis Model after Neurointermediate Pituitary Lobectomy. J. Immunol. Res. 2021, 2021, 5529784. [Google Scholar] [CrossRef]
- Feher, E.; Pongor, E.; Altdorfer, K.; Kobori, L.; Lengyel, G. Neuroimmunomodulation in human autoimmune liver disease. Cell Tissue Res. 2013, 354, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, F.; Li, Y.; Wang, X.; Lu, Q.; Wang, D.; Qi, C.; Li, C.; Li, Z.; Lian, B.; et al. Combined anti-hepatocellular carcinoma therapy inhibit drug-resistance and metastasis via targeting “substance P-hepatic stellate cells-hepatocellular carcinoma” axis. Biomaterials 2021, 276, 121003. [Google Scholar] [CrossRef]
- Kim, S.; Hong, H.S. Substance-P prevents the cholestatic liver injury by regulating inflammatory responses. Peptides 2021, 137, 170494. [Google Scholar] [CrossRef] [PubMed]
- Hessheimer, A.J.; Martinez de la Maza, L.; Adel Al Shwely, F.; Espinoza, A.S.; Ausania, F.; Fondevila, C. Somatostatin and the “Small-For-Size” Liver. Int. J. Mol. Sci. 2019, 20, 2512. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Liu, Y.; Zhang, C.; Shen, X.D.; Gao, F.; Busuttil, R.W.; Zheng, S.; Kupiec-Weglinski, J.W.; Ji, H. PACAP neuropeptide promotes Hepatocellular Protection via CREB-KLF4 dependent autophagy in mouse liver Ischemia Reperfusion Injury. Theranostics 2020, 10, 4453–4465. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, T.; Zhang, C.; Xue, Z.; Xu, J.; Busuttil, R.W.; Xia, Q.; Xu, N.; Kupiec-Weglinski, J.W.; Ji, H. Pituitary Adenylate Cyclase-activating Polypeptides Prevent Hepatocyte Damage by Promoting Yes-associated Protein in Liver Ischemia-Reperfusion Injury. Transplantation 2019, 103, 1639–1648. [Google Scholar] [CrossRef]
- Sato, A.; Kakinuma, S.; Miyoshi, M.; Kamiya, A.; Tsunoda, T.; Kaneko, S.; Tsuchiya, J.; Shimizu, T.; Takeichi, E.; Nitta, S.; et al. Vasoactive Intestinal Peptide Derived From Liver Mesenchymal Cells Mediates Tight Junction Assembly in Mouse Intrahepatic Bile Ducts. Hepatol. Commun. 2020, 4, 235–254. [Google Scholar] [CrossRef]
- Cao, X.; Feng, F.; Liu, X.; Sun, C.; Yang, X.; Fang, Y.; Li, S. Exogenous Secretin Improves Parenteral Nutrition-associated Liver Disease in Rats. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 430–435. [Google Scholar] [CrossRef]
- Baiocchi, L.; Lenci, I.; Milana, M.; Kennedy, L.; Sato, K.; Zhang, W.; Ekser, B.; Ceci, L.; Meadows, V.; Glaser, S.; et al. Cyclic AMP Signaling in Biliary Proliferation: A Possible Target for Cholangiocarcinoma Treatment? Cells 2021, 10, 1692. [Google Scholar] [CrossRef]
- Muraki, K.; Nishi, Y.; Arai, M.; Kubo, N.; Ueda, K.; Shikata, H.; Nakata, Y.; Segawa, T.; Yanaihara, N.; Yajima, H. Neurotensin receptors on the rat liver plasma membranes. Biochem. Biophys. Res. Commun. 1987, 145, 1071–1079. [Google Scholar] [CrossRef]
- Mitra, S.P.; Carraway, R.E. High affinity binding of 125I-neurotensin to dispersed cells from chicken liver and brain. Peptides 1997, 18, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.P.; Mazella, J.; Kitabgi, P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999, 20, 302–309. [Google Scholar] [CrossRef]
- Nikolaou, S.; Qiu, S.; Fiorentino, F.; Simillis, C.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. The role of Neurotensin and its receptors in non-gastrointestinal cancers: A review. Cell Commun. Signal. 2020, 18, 68. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakao, N.; Yamamoto, I.; Tsushima, N.; Ohta, Y. Changes in expression levels of neurotensin precursor and receptor mRNA in chicken intestinal tissues and liver during late embryonic and early posthatching development. Poult. Sci. 2013, 92, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.M.; Rajaraman, S.; Chung, D.H.; Townsend, C.M.J.; Wang, X.; Graves, K.; Thompson, J.C. Developmental expression of the neurotensin gene in the rat liver. Ann. Surg. 1993, 218, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Carr, B.I. Neurotensin-amplification of DNA synthesis stimulated by EGF or TGF alpha in primary cultures of adult rat hepatocytes. Cell Struct. Funct. 1993, 18, 105–110. [Google Scholar] [CrossRef]
- Nakaizumi, A.; Uehara, H.; Baba, M.; Iishi, H.; Tatsuta, M. Enhancement by neurotensin of hepatocarcinogenesis by N-nitrosomorpholine in Sprague-Dawley rats. Cancer Lett. 1996, 110, 57–61. [Google Scholar] [CrossRef]
- Xiao, P.; Long, X.; Zhang, L.; Ye, Y.; Guo, J.; Liu, P.; Zhang, R.; Ning, J.; Yu, W.; Wei, F.; et al. Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. Oncoimmunology 2018, 7, e1440166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Yan, B.; Weiss, H.L.; Weiss, L.T.; Gao, T.; Evers, B.M. Neurotensin differentially regulates bile acid metabolism and intestinal FXR-bile acid transporter axis in response to nutrient abundance. FASEB J. 2021, 35, e21371. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Vagianos, C.E.; Zervoudakis, G.; Filos, K.S.; Georgiou, C.; Nikolopoulou, V.; Scopa, C.D. Gut regulatory peptides bombesin and neurotensin reduce hepatic oxidative stress and histological alterations in bile duct ligated rats. Regul. Pept. 2004, 120, 185–193. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Tsamandas, A.C.; Georgiou, C.D.; Vagianos, C.E.; Scopa, C.D. Bombesin and neurotensin exert antiproliferative effects on oval cells and augment the regenerative response of the cholestatic rat liver. Peptides 2010, 31, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, W.; Liu, T.; Huang, D.; Xiang, L. Bioinformatics analysis of hepatic gene expression profiles in type 2 diabetes mellitus. Exp. Ther. Med. 2019, 18, 4303–4312. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Zaytseva, Y.Y.; Liu, Y.; Rychahou, P.; Jiang, K.; Starr, M.E.; Kim, J.T.; Harris, J.W.; Yiannikouris, F.B.; et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 2016, 533, 411–415. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Petta, S.; Longo, M.; Alisi, A.; Soardo, G.; Valenti, L.; Miele, L.; Grimaudo, S.; Pennisi, G.; et al. Neurotensin up-regulation is associated with advanced fibrosis and hepatocellular carcinoma in patients with MAFLD. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158765. [Google Scholar] [CrossRef]
- Villar, B.; Bertran, L.; Aguilar, C.; Binetti, J.; Martinez, S.; Sabench, F.; Real, M.; Riesco, D.; Paris, M.; Del Castillo, D.; et al. Circulating Levels of Pro-Neurotensin and Its Relationship with Nonalcoholic Steatohepatitis and Hepatic Lipid Metabolism. Metabolites 2021, 11, 373. [Google Scholar] [CrossRef]
- Auguet, T.; Aragones, G.; Berlanga, A.; Martinez, S.; Sabench, F.; Aguilar, C.; Villar, B.; Sirvent, J.J.; Del Castillo, D.; Richart, C. Low Circulating Levels of Neurotensin in Women with Nonalcoholic Fatty Liver Disease Associated with Severe Obesity. Obesity 2018, 26, 274–278. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abo-Elmatty, D.M.; Ezzat, O.; Mesbah, N.M.; Ali, N.S.; Abd El Fatah, A.S.; Alsayed, E.; Hamada, M.; Hassnine, A.A.; Abd-Elsalam, S.; et al. Pro-Neurotensin as a Potential Novel Diagnostic Biomarker for Detection of Nonalcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2022, 15, 1935–1943. [Google Scholar] [CrossRef]
- Vaudry, D.; Gonzalez, B.J.; Basille, M.; Yon, L.; Fournier, A.; Vaudry, H. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacol. Rev. 2000, 52, 269–324. [Google Scholar] [PubMed]
- Cheng, C.Y.; Chu, J.Y.; Chow, B.K. Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 2011, 36, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Uriz, M.; Saez, E.; Prieto, J.; Medina, J.F.; Banales, J.M. Ursodeoxycholic acid is conjugated with taurine to promote secretin-stimulated biliary hydrocholeresis in the normal rat. PLoS ONE 2011, 6, e28717. [Google Scholar] [CrossRef]
- Banales, J.M.; Saez, E.; Uriz, M.; Sarvide, S.; Urribarri, A.D.; Splinter, P.; Tietz Bogert, P.S.; Bujanda, L.; Prieto, J.; Medina, J.F.; et al. Up-regulation of microRNA 506 leads to decreased Cl−/HCO3− anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 2012, 56, 687–697. [Google Scholar] [CrossRef]
- Farouk, M.; Vigna, S.R.; Haebig, J.E.; Gettys, T.W.; McVey, D.C.; Chari, R.; Pruthi, R.S.; Meyers, W.C. Secretin receptors in a new preparation of plasma membranes from intrahepatic biliary epithelium. J. Surg. Res. 1993, 54, 1–6. [Google Scholar] [CrossRef]
- Guerrier, M.; Attili, F.; Alpini, G.; Glaser, S. Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity. Hepatobiliary Surg. Nutr. 2014, 3, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wu, N.; Meng, F.; Venter, J.; Giang, T.K.; Francis, H.; Kyritsi, K.; Wu, C.; Franchitto, A.; Alvaro, D.; et al. Knockout of secretin receptor reduces biliary damage and liver fibrosis in Mdr2(−/−) mice by diminishing senescence of cholangiocytes. Lab. Investig. 2018, 98, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Francis, H.; Invernizzi, P.; Venter, J.; Wu, N.; Carbone, M.; Gershwin, M.E.; Bernuzzi, F.; Franchitto, A.; Alvaro, D.; et al. Secretin/secretin receptor signaling mediates biliary damage and liver fibrosis in early-stage primary biliary cholangitis. FASEB J. 2019, 33, 10269–10279. [Google Scholar] [CrossRef] [PubMed]
- Feliu, J.E.; Mojena, M.; Silvestre, R.A.; Monge, L.; Marco, J. Stimulatory effect of vasoactive intestinal peptide on glycogenolysis and gluconeogenesis in isolated rat hepatocytes: Antagonism by insulin. Endocrinology 1983, 112, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, P.; Coy, D.H.; De Neef, P.; Camus, J.C.; Waelbroeck, M.; Christophe, J. Specific labelling of high-affinity vasoactive intestinal peptide receptors in rat liver membranes by a growth hormone-releasing factor analog. Neuroendocrinology 1986, 44, 108–111. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Williams, J.A.; Gray, G.M. Vasoactive intestinal peptide receptor on liver plasma membranes: Characterization as a glycoprotein. Biochemistry 1986, 25, 361–368. [Google Scholar] [CrossRef]
- Gagnon, A.W.; Aiyar, N.; Elshourbagy, N.A. Molecular cloning and functional characterization of a human liver vasoactive intestinal peptide receptor. Cell Signal. 1994, 6, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Knodell, R.G.; Steele, N.M.; Stanley, L.N. Hepatic bile formation in the rat. Addition of vasoactive intestinal peptide to the equation. Dig. Dis. Sci. 1987, 32, 1290–1296. [Google Scholar] [CrossRef]
- Nyberg, B.; Einarsson, K.; Sonnenfeld, T. Evidence that vasoactive intestinal peptide induces ductular secretion of bile in humans. Gastroenterology 1989, 96, 920–924. [Google Scholar] [CrossRef]
- Rodriguez-Henche, N.; Guijarro, L.G.; Bajo, A.M.; Arilla, E.; Prieto, J.C. VIP receptor/effector system in liver membranes from cholestatic rats. Peptides 1994, 15, 353–357. [Google Scholar] [CrossRef]
- Rodriguez-Henche, N.; Guijarro, L.G.; Couvineau, A.; Carrero, I.; Arilla, E.; Laburthe, M.; Prieto, J.C. G proteins in rat liver proliferation during cholestasis. Hepatology 1994, 20, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Boyer, J.L. Vasoactive intestinal polypeptide is a potent regulator of bile secretion from rat cholangiocytes. Gastroenterology 1999, 117, 420–428. [Google Scholar] [CrossRef]
- Absood, A.; Hu, B.; Bassily, N.; Colletti, L. VIP inhibits human HepG2 cell proliferation in vitro. Regul. Pept. 2008, 146, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Takeba, Y.; Iiri, T.; Ohta, Y.; Ootaki, M.; Watanabe, M.; Watanabe, D.; Koizumi, S.; Otsubo, T.; Matsumoto, N. Vasoactive intestinal peptide increases apoptosis of hepatocellular carcinoma by inhibiting the cAMP/Bcl-xL pathway. Cancer Sci. 2019, 110, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, Y.; Feng, D.; Xu, Y.; Xu, L. Vasoactive intestinal peptide attenuates concanavalin A-mediated liver injury. Eur. J. Pharmacol. 2009, 607, 226–233. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, W.; Geng, Q.; Xu, X. Inhibition of Toll-like receptor 4 with vasoactive intestinal peptide attenuates liver ischemia-reperfusion injury. Transplant. Proc. 2011, 43, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhang, Y.; Liu, Y.; Shen, X.D.; Gao, F.; Nguyen, T.T.; Busuttil, R.W.; Waschek, J.A.; Kupiec-Weglinski, J.W. Vasoactive intestinal peptide attenuates liver ischemia/reperfusion injury in mice via the cyclic adenosine monophosphate-protein kinase a pathway. Liver Transpl. 2013, 19, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, P.E.; Tatsuno, I.; Miyata, A.; Arimura, A. Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology 1990, 127, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, P.; Gourlet, P.; Cauvin, A.; Buscail, L.; De Neef, P.; Arimura, A.; Christophe, J. PACAP and VIP receptors in rat liver membranes. Am. J. Physiol. 1991, 260, G97–G102. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A. Receptors for pituitary adenylate cyclase-activating polypeptide: Comparison with vasoactive intestinal peptide receptors. Trends Endocrinol. Metab. 1992, 3, 288–294. [Google Scholar] [CrossRef]
- Hosoya, M.; Onda, H.; Ogi, K.; Masuda, Y.; Miyamoto, Y.; Ohtaki, T.; Okazaki, H.; Arimura, A.; Fujino, M. Molecular cloning and functional expression of rat cDNAs encoding the receptor for pituitary adenylate cyclase activating polypeptide (PACAP). Biochem. Biophys. Res. Commun. 1993, 194, 133–143. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Heintz, G.G.; Wolfe, M.S. Structural characterization of PACAP receptors on rat liver plasma membranes. Am. J. Physiol. 1993, 265, G811–G818. [Google Scholar] [CrossRef]
- Yokota, C.; Kawai, K.; Ohashi, S.; Watanabe, Y.; Yamashita, K. PACAP stimulates glucose output from the perfused rat liver. Peptides 1995, 16, 55–60. [Google Scholar] [CrossRef]
- Bosch, J.; Abraldes, J.G.; Groszmann, R. Current management of portal hypertension. J. Hepatol. 2003, 38 (Suppl. S1), S54–S68. [Google Scholar] [CrossRef]
- Yu, M.; Das, J.M. Neuroanatomy, Nucleus Supraoptic. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Papaluca, T.; Gow, P. Terlipressin: Current and emerging indications in chronic liver disease. J. Gastroenterol. Hepatol. 2018, 33, 591–598. [Google Scholar] [CrossRef]
- Israelsen, M.; Dahl, E.K.; Madsen, B.S.; Wiese, S.; Bendtsen, F.; Moller, S.; Fialla, A.D.; Jensen, B.L.; Krag, A. Dobutamine reverses the cardio-suppressive effects of terlipressin without improving renal function in cirrhosis and ascites: A randomized controlled trial. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G313–G321. [Google Scholar] [CrossRef]
- Montero-Melendez, T.; Boesen, T.; Jonassen, T.E.N. Translational advances of melanocortin drugs: Integrating biology, chemistry and genetics. Semin. Immunol. 2022, 59, 101603. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Arab, J.P.; Premkumar, M.; Benitez, C.; Tirumalige Ravikumar, S.; Kumar, P.; Sharma, M.; Reddy, D.N.; Simonetto, D.A.; Rao, P.N. Terlipressin has stood the test of time: Clinical overview in 2020 and future perspectives. Liver Int. 2020, 40, 2888–2905. [Google Scholar] [CrossRef]
- Cavallin, M.; Piano, S.; Romano, A.; Fasolato, S.; Frigo, A.C.; Benetti, G.; Gola, E.; Morando, F.; Stanco, M.; Rosi, S.; et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology 2016, 63, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Pappas, S.C.; Curry, M.P.; Reddy, K.R.; Rubin, R.A.; Porayko, M.K.; Gonzalez, S.A.; Mumtaz, K.; Lim, N.; Simonetto, D.A.; et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N. Engl. J. Med. 2021, 384, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Audsley, J.; Sasadeusz, J.; Lewin, S.R. Tesamorelin, liver fat, and NAFLD in the setting of HIV. Lancet HIV 2019, 6, e808–e809. [Google Scholar] [CrossRef]
- Fourman, L.T.; Billingsley, J.M.; Agyapong, G.; Ho Sui, S.J.; Feldpausch, M.N.; Purdy, J.; Zheng, I.; Pan, C.S.; Corey, K.E.; Torriani, M.; et al. Effects of tesamorelin on hepatic transcriptomic signatures in HIV-associated NAFLD. JCI Insight 2020, 5, e140134. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, X.; Huang, L.; Zhang, C.; Veldhuis, J.D.; Cowley, M.A.; Chen, C. Stimulation of endogenous pulsatile growth hormone secretion by activation of growth hormone secretagogue receptor reduces the fat accumulation and improves the insulin sensitivity in obese mice. FASEB J. 2021, 35, e21269. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, Y.L.; Song, Z.Y.; Lin, S. Effects of Neuropeptide Y on Stem Cells and Their Potential Applications in Disease Therapy. Stem Cells Int. 2017, 2017, 6823917. [Google Scholar] [CrossRef]
- Bu, F.T.; Jia, P.C.; Zhu, Y.; Yang, Y.R.; Meng, H.W.; Bi, Y.H.; Huang, C.; Li, J. Emerging therapeutic potential of adeno-associated virus-mediated gene therapy in liver fibrosis. Mol. Ther. Methods Clin. Dev. 2022, 26, 191–206. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).