Abstract
Background: The differential responses of the myokine irisin, in combination with changes in markers and regulators of bone remodeling to high-intensity interval exercise of high and low impact, were examined in 18 young adult females (22.5 ± 2.7 years). Methods: Participants performed two high-intensity interval exercise trials in random order: running on a treadmill and cycling on a cycle ergometer. Trials consisted of eight 1 min running or cycling intervals at ≥ 90% of maximal heart rate, separated by 1 min passive recovery intervals. Blood samples were collected at rest (pre-exercise) and 5 min, 1 h, and 24 h following each exercise trial. Irisin, osteocalcin, sclerostin, osteoprotegerin (OPG), receptor activator nuclear factor kappa-β ligand (RANKL), and parathyroid hormone (PTH) were analyzed in serum, with post-exercise concentrations being corrected for exercise-induced changes in plasma volume. Results: Irisin was elevated 24 h post-exercise compared to its resting values in both trials (20%, p < 0.05) and was higher after cycling compared to running (exercise mode effect, p < 0.05) with no interaction. Osteocalcin, sclerostin, PTH, and RANKL increased from pre- to 5 min post-exercise (18%, 37%, 83%, and 33%, respectively, p < 0.05), returning to baseline levels in 1 h, with no trial or interaction effects. OPG showed a time effect (p < 0.05), reflecting an overall increase at 5 min and 1 h post-exercise, which was not significant after the Bonferroni adjustment. Conclusions: In young adult females, high-intensity interval exercise induced an immediate response in markers and regulators of bone remodeling and a later response in irisin concentrations, which was independent of the gravitational impact.
1. Introduction
Exercise has a significant impact on bones, and the metabolism of bone cells adjusts quickly to training [1]. The bone response to exercise is based on mechanical stimulation, as well as the integration of biochemical signals generated during and after exercise [2]. Recent evidence indicates that exercise leads to increased secretion of the myokine irisin, which plays a significant role in bone homeostasis and metabolic regulation [3]. In bone, irisin appears to promote bone formation by upregulating osteopontin and β-catenin and downregulating sclerostin synthesis [3], while also inhibiting osteoclast formation and differentiation through the suppression of nuclear factor-B (NF-B) and receptor activator of nuclear factor-kappa-B ligand (RANKL) [4,5]. However, irisin also has the potential to stimulate osteoclastic bone resorption through the activation of the AV/b5 integrins in osteocytes [6]. Thus, the effects of irisin on bone seem to be complex and context-dependent, and further research on the role of irisin in bone metabolism post-exercise is needed.
It is well documented that high-impact, weight-bearing activities, such as running and jumping, are more effective than low-impact, non-weight-bearing activities, such as cycling and swimming, in promoting bone health [7,8,9]. High-impact activities provide a strong mechanical stimulus for osteogenic activity through the combined effects of muscle contraction and ground reaction forces on the skeleton [10]. In contrast, low-impact activities that exert a lesser force on the skeleton may require a longer time to show osteogenic benefits and, in some cases, may even have detrimental effects on bone [11]. Indeed, bone mineral density (BMD) has been found to be lower in younger and older individuals (females and males) who participate in low-impact sports compared to those who participate in high-impact sports [12,13,14]. However, the evidence regarding the effects of cycling on biochemical markers is still inconclusive. Research examining the effects of acute bouts of high-intensity interval cycling on bone markers has shown an overall anabolic effect [15] while longer-term observational studies show that road cycling can have an osteocatabolic effect [11]. Thus, the cycling intensity seems to be a contributing factor to its effect on bone metabolism, with the effects of an acute bout of high-intensity cycling on markers and regulators of bone remodeling being reported as similar to that of high-intensity interval running in young adult males and females [16,17,18].
Osteocalcin is a non-collagenous protein released by osteoblasts that plays an important role in calcium metabolism, bone mineralization, and energy homeostasis [19,20] and has been used as a marker of the overall anabolic effects of exercise on bone metabolism. While some studies have reported an increase in total osteocalcin levels immediately and 3 h after a single bout of high-intensity interval exercise in female participants [21], a recent study of young adult males performing two moderate-intensity exercise trials on a cycle ergometer and on a treadmill found no changes in osteocalcin up to 1 h following either trial [22]. Moreover, the osteoanabolic benefit of exercise has been associated with the upregulation of osteoprotegerin (OPG) and the inhibition of the binding of RANKL to its receptor activator of nuclear factor-kappa-B (OPG/RANKL pathway) [23]. Both OPG and RANKL have been found to increase within minutes following high-intensity acute cycling in young adult males [15], which suggests a potential overall increase in bone turnover, while endurance running has been found to cause a decrease in RANKL and a rise in OPG levels [24], which indicates a net anabolic effect on bone metabolism. However, a recent study reported higher irisin and RANKL but no differences in OPG between male footballers and non-athletic, age-matched controls [25]. Thus, our knowledge about the differential effects of high- and low-impact exercises on this pathway is still limited and requires further research.
Sclerostin is an upstream osteokine secreted by osteocytes to inhibit the canonical Wnt signaling pathway in osteoblast cells, leading to a decrease in bone formation [26,27]. Studies have shown a transient increase in sclerostin following high-intensity interval cycling and running in young adults, regardless of sex [16,17,18]. However, in our study on young adult male participants, we found a larger transient increase in sclerostin following a moderate-intensity running trial compared to an intensity-matched cycling trial, although irisin and parathyroid hormone (PTH) increased similarly following trials [22]. PTH is another biochemical marker that can influence bone metabolism [28]. In vitro, PTH has been shown to prevent the expression of sclerostin [29] and alter the OPG/RANKL ratio, indirectly enhancing osteoclast recruitment and activity [30]. Intermittent PTH administration has also been found to stimulate osteoblastogenesis and decrease osteoblast apoptosis [31]. However, in contrast to these in vitro experiments, PTH was found to increase in concert with sclerostin after a single session of high-impact plyometric exercise in young adult males [32], which supports the need for further research on the effects of different exercise modes on the markers and regulators of bone metabolism.
To date, no human study has compared the acute responses of irisin, in combination with markers and regulators of bone remodeling between high-intensity high-impact and low-impact exercises in females. Thus, the main objective of this study was to examine the differential effect of high-intensity interval running versus cycling, on circulating irisin, osteocalcin, sclerostin, PTH, OPG, and RANKL in young adult females. Examining potential differences in these acute bone-related responses between intense high-impact and low-impact exercise can identify potential mechanisms underlying the short- and long-term bone adaptations to exercise. Furthermore, this may provide more information about the acute response of bone to exercise. Such preliminary research may then play a significant role in designing exercise protocols for women for clinical, research, and recreational goals.
2. Materials and Methods
2.1. Participants
This study involved the further analysis of blood samples collected and analyzed for a previous study examining the exercise-induced response to high-intensity interval running versus cycling of other bone markers and inflammatory cytokines previously reported in both young adult males and females [16,17,18]. The original study included 20 female participants aged 18–28 years. However, the present study includes the analysis of available blood samples from 18 participants (Caucasian, 23.2 ± 3.0 years, 166 ± 6 cm, 61.5 ± 8.3 kg, 26.8 ± 6.5 % fat). Participants were healthy, recreationally active (i.e., exercising 2 to 5 times per week), on birth control, non-smokers, free of injuries or chronic conditions (e.g., ACL or knee/hip/lower back injuries, arthritis, neuromuscular diseases), had no fracture in the last year, and were not taking any medication or dietary supplements affecting bone health (e.g., protein, vitamin D, calcium). All participants agreed to participate in this study by signing a consent form. The study was conducted in accordance with the Declaration of Helsinki and received ethical approval from the Brock University Research Ethics Board (REB #19-131).
2.2. Study Design and Procedures
This study used a crossover, within-subject design, as previously described [16,17,18]. Briefly, each participant performed a high-intensity interval running (HIIR) trial on the treadmill and a high-intensity interval cycling (HIIC) trial on a cycle ergometer. Participants first came to the laboratory prior to the HIIR and HIIC trials for two preliminary visits. During the first visit, participants were informed about the study, signed a consent form, and completed a medical history questionnaire that was used to verify the inclusion criteria. In addition, all participants completed the Godin Shephard Leisure-Time Physical Activity Questionnaire to determine their habitual physical activity levels [33] and a food frequency questionnaire (Block 2014.1_6Mo, Nutrition Quest, Berkeley, CA, USA) to assess habitual nutrient intake. Height was then measured with a stadiometer to the nearest 0.1 cm with no shoes, and body composition was measured via air displacement plethysmography (BodPod; Life Measurement Inc., Concord, CA, USA) to obtain measures of body mass (kg), fat mass (kg), fat-free mass (kg), and percent body fat (%).
In each of the first and second preliminary visits, participants also performed an incremental exercise test to exhaustion either on the treadmill (CYBEX, 515T, Medway, MA, USA) or on the bicycle ergometer (Lode, 911905, Groningen, Netherlands) (random order), which was used to determine the maximal workload. The maximal workload was determined through volitional fatigue when participants could no longer continue running and cycling. The maximal speed and incline for running, and the maximum power output for cycling, as well as the maximum heart rate (Omron, HR-310, Warminster, PA, USA) and perceived exertion (Borg scale) at the point of exhaustion, were recorded.
At least one week after the second preliminary visit, participants returned to the laboratory to perform the HIIR and HIIC trials in random order. The trials were scheduled in the morning between 10.00 and 12.00 h to control for diurnal variation in the biochemical markers. All participants were on birth control; of the 18 participants included in the present study, 16 were on oral contraceptives with downregulated hormonal profiles, and 2 used a hormonal intrauterine device. Thus, for consistency, the trials were scheduled to correspond to the same days of their treatment cycle. Specifically, all the visits took place within three consecutive weeks following the week of menstruation. In addition, as reported by Kouvelioti et al., all participants had similar baseline (i.e., resting) estradiol levels prior to the two high-intensity interval exercise trials (99.8 ± 16.1 vs 93.3 ± 10.7 pg/mL, HIIR vs HIIC, respectively; p = 0.47) [16]. Finally, as we previously showed, there is no effect of the menstrual-cycle-related fluctuations in sex hormones, on either the resting concentrations of sclerostin, PTH, and other bone markers or on their response to intense exercise [34].
Moreover, before every visit to the laboratory, participants were instructed to consume the same standardized breakfast at home, which included one slice of whole-grain bread with butter/margarine or peanut butter, one glass of 2% milk or one cup of 2% fat yogurt, one banana or apple, and one cup of coffee or tea. Likewise, they were instructed not to eat or drink anything (except water) for about 2 h after their breakfast and prior to their laboratory visits.
2.3. Blood Collection and Biochemical Analysis
Approximately 10 mL of whole blood was collected from each participant at each time point (pre-exercise, 5 min, 1 h, and 24 h post-exercise) for a total of 4 blood draws per participant. Samples were collected from the median cubital vein in the antecubital fossa using a standard venipuncture technique. Upon collection, samples sat for 30 min at room temperature. The plasma was then aliquoted into microcentrifuge tubes and stored at −80 °C until analysis. Plasma was used to measure hematocrit. In the present study, serum irisin, osteocalcin, sclerostin, PTH, OPG, and RANKL were measured.
Specifically, after every blood collection, hematocrit was measured in triplicate by the same investigator, using micro-hematocrit tubes with heparin (VWR, Radnor, PA, USA) for each blood sample, and was separated using an international microcapillary centrifuge (model MB, International Equipment Company, Needham, MA, USA). This measurement is important in determining exercise-induced changes in plasma volume, which can affect biomarker serum concentrations after exercise [35]. Relative change in plasma volume (%ΔPV) from pre- to each post-exercise time points for each participant was estimated using the formula of van Beaumon [36]:
where Hct1 is hematocrit at baseline and Hct2 is hematocrit at each post-baseline measurement. The %ΔPV was then used to correct the serum concentrations of irisin, osteocalcin, sclerostin, PTH, OPG, and RANKL at 5 min, 1 h, and 24 h post-exercise in both trials, as previously reported [16,17,18].
Although this was a secondary analysis of serum samples from previous studies, irisin, osteocalcin, PTH, OPG, and RANKL were analyzed for the first time for the purpose of the present study using new commercially available assay kits. Sclerostin concentrations have been previously reported from these samples [18]; however, a new analysis was performed to test the reliability of the serum concentrations after storage. Samples were analyzed in duplicate with a few samples analyzed in triplicate to determine the inter- and intra-assay coefficients of variations (CV). Irisin was measured using an irisin recombinant assay kit (Cat #EK-067-29, Phoenix Pharmaceuticals Inc., Burlingame, CA, USA). The average inter- and intra-assay CV for irisin were 7.3% and 6.7%, respectively. Total osteocalcin, OPG, and PTH were measured using a microbead multiplex kit (cat.# HBNMAG-51K-08, EMD Millipore, Darmstadt, Germany). The inter- and intra-assay CV for osteocalcin were 10.5% and 8.7%, for OPG, they were 10.8% and 8.0%, and for PTH, they were 9.1% and 8.1%, respectively. Sclerostin was analyzed using an ELISA kit (cat.# DSST00; R&D, Minneapolis, MN, USA) with an inter-assay CV of 5.1% and an intra-assay CV of 3.1%. RANKL was measured using a microbead multiplex kit (Human RANKL MAG Bead Single Plex Kit, cat.# HRNKLMAG-51K-01, EMD Millipore, Darmstadt, Germany) with an average intra-assay CV of 6.4% and an inter-assay CV of 5.9%.
2.4. Statistical Analysis
The data were assessed for normality using the Shapiro–Wilk test, z-scores for skewness and kurtosis, and visual screening of histograms for symmetry. The screening found that OC was not normally distributed and was log-transformed for the analysis. There were a few missing values for irisin, PTH, and RANKL due to low serum availability or non-detectable values, so the number of participants is different for these markers. A two-way repeated measures analysis of variance (RM-ANOVA) was used to examine the main effects for trial (running versus cycling) and time, as well as the time-by-trial interactions, for irisin, osteocalcin, sclerostin, PTH, OPG, and RANKL. In the case of a significant time effect or interaction, post hoc pairwise comparisons were performed using paired t-tests with Bonferroni adjustment for multiple comparisons. Effect sizes, including partial eta squared (pη2) for ANOVA and Cohen’s d (mean difference/standard deviation pre-test) for significant pairwise comparisons, were calculated. Effect sizes were then interpreted based on the Cohen criteria: 0.01 = small, 0.06 = moderate, 0.14 = large effect for partial η2, and 0.2 = small, 0.5 = medium, 0.8 = large effect for Cohen’s d [37]. Statistical significance was set at an alpha level of 0.05 and performed using IBM SPSS Statistics 28 (SPSS Inc., Chicago, IL, USA).
3. Results
Serum concentrations of all biomarkers at rest and in response to high-intensity cycling and running are shown in Table 1. Irisin showed no trial-by-time interaction effect (F = 0.87; p = 0.46; pη2 = 0.05). However, there was a significant main effect for trial (F = 9.3; p < 0.004; pη2 = 0.22), as irisin concentrations were higher during the cycling trial compared to running across all time points, and a significant main effect for time (F = 8.7; p < 0.001; pη2 = 0.21), which reflects a higher irisin concentration 24 h post-exercise compared to its resting values (+20%, p = 0.002, d = 0.51) in both trials combined (Figure 1).

Table 1.
Serum concentrations of irisin, osteocalcin, sclerostin, parathyroid hormone (PTH), osteoprotegerin (OPG), and receptor activator of nuclear factor-kappa-B ligand (RANKL) at rest and in response to high-intensity cycling and running in young adult females.
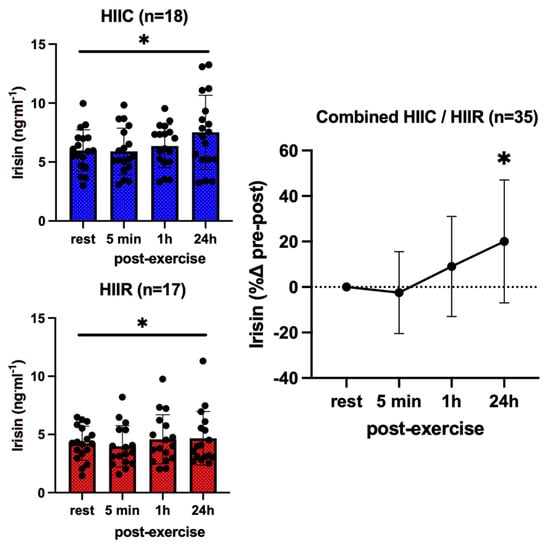
Figure 1.
Serum concentrations (mean ± SD) of irisin before and after exercise in both high-intensity interval cycling (HIIC) and running (HIIR) trials. * denotes significant difference from pre-exercise to 24 h post-exercise in the post hoc pairwise comparisons with groups combined and Bonferroni adjustment for three comparisons (p = 0.015, d = 0.52).
We found no main effect of trial (F = 0.59; p = 0.81; pη2 = 0.02) and no trial-by-time interaction (F = 0.33; p = 0.80; pη2 = 0.01) for osteocalcin, reflecting no differences in concentrations between cycling and running at any time. However, there was a significant main effect for time (F = 9.29; p < 0.001; pη2 = 0.25), according to which osteocalcin was higher than baseline 5 min following both trials (+18%; p = 0.002; d = 0.51) (Figure 2).
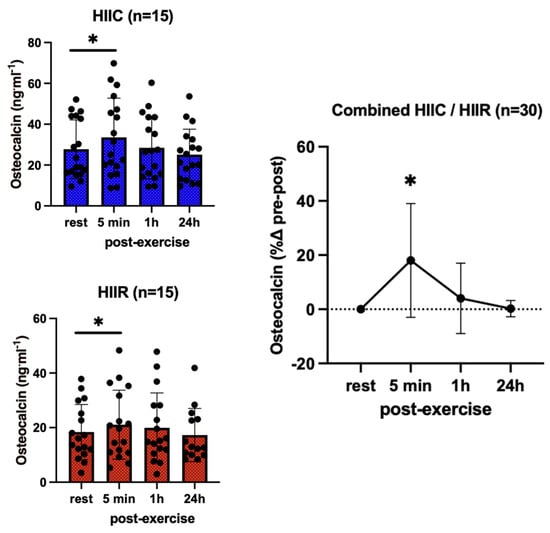
Figure 2.
Serum concentrations (mean ± SD) of total osteocalcin pre- and post-exercise in both high-intensity interval cycling (HIIC) and running (HIIR) trials. * denotes a significant difference from pre-exercise to 5 min post-exercise in post hoc pairwise comparisons with groups combined and Bonferroni adjustment for three comparisons (p = 0.002; d = 0.51).
Sclerostin showed no effect for trial (F = 0.02; p = 0.90; pη2 = 0.001) and no trial-by-time interaction (F = 0.10; p = 0.96; pη2 = 0.004), reflecting that its serum concentrations were not different between cycling and running at any point. However, there was a significant main effect for time (F = 21.2; p < 0.001; pη2 = 0.45), as sclerostin increased immediately post-exercise in both trials (37%, p < 0.001; d = 1.31) then returned to the pre-exercise levels 1 h post-exercise (Figure 3). Likewise, PTH showed no trial-by-time interaction (F = 0.13; p = 0.94; pη2 = 0.006) and no main effect for trial (F = 0.13; p = 0.72; pη2 = 0.006), i.e., PTH was not different between trials, either at rest or post-exercise. There was a significant main effect for time (F = 23.9; p <0.001; pη2 = 0.53), reflecting an overall increase in PTH immediately following high-intensity interval exercise (+83%, p < 0.001; d = 1.32), irrespective of exercise mode (Figure 4).
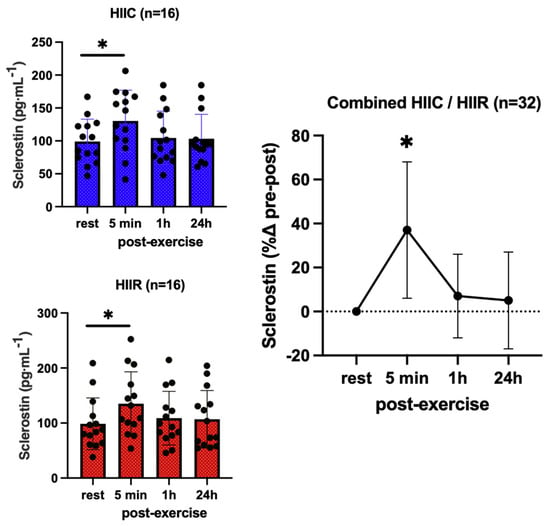
Figure 3.
Serum concentrations (mean ± SD) of sclerostin pre- and post-exercise in both high-intensity interval cycling (HIIC) and running (HIIR) trials. * denotes significant difference from pre-exercise to 5 min post-exercise in post hoc pairwise comparisons with groups combined and Bonferroni adjustment for three comparisons (p < 0.001; d = 1.31).
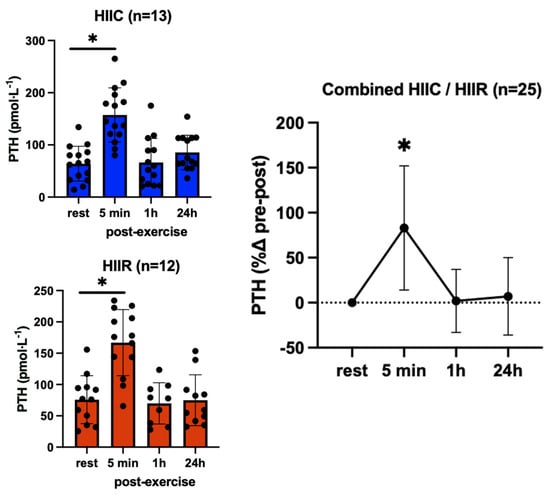
Figure 4.
Serum concentrations (mean ± SD) of parathyroid hormone (PTH) pre- and post-exercise in both high-intensity interval cycling (HIIC) and running (HIIR) trials). * denotes significant difference from pre-exercise to 5 min post-exercise in post hoc pairwise comparisons with groups combined and Bonferroni adjustment for three comparisons (p < 0.001; d = 1.32).
There were no main effects for trial (F = 0.17; p = 0.69; pη2 = 0.005) and no trial-by-time interaction (F = 1.23; p = 0.28; pη2 = 0.04) for OPG, which reflects that its serum concentrations were not different between cycling and running at rest or in response to exercise. However, there was a significant main effect for time (F = 3.93; p = 0.01; pη2 = 1.12), reflecting overall higher OPG concentrations at 5 min (+10%, p = 0.05; d = 0.34) and 1 h (+9%, p = 0.05; d = 0.33) post-exercise compared to pre-exercise, irrespective of exercise mode; however, this increase was not significant after the Bonferroni adjustment (Figure 5). RANKL showed no trial-by-time interaction (F = 0.02; p = 1.00; pη2 = 0.001) and no main effect for trial (F = 0.00; p = 0.99; pη2 = 0.000), in that RANKL concentrations were not different between cycling and running at any point. However, there was a significant main effect for time (F = 3.28; p = 0.02; pη2 = 0.10), reflecting higher RANKL immediately post-exercise compared to pre-exercise, irrespective of exercise mode (+33%, p < 0.02; d = 0.45) (Figure 6).
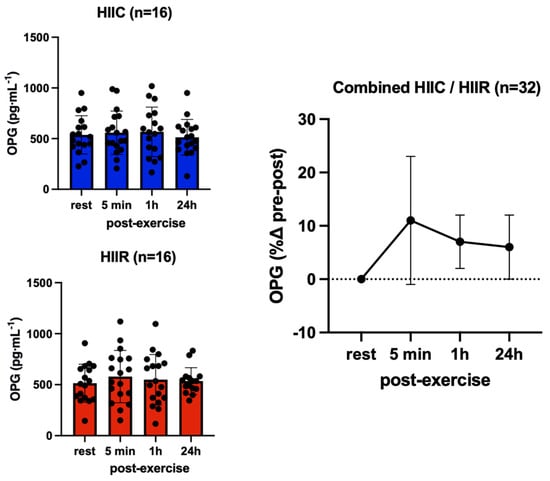
Figure 5.
Serum concentrations (mean ± SD) of osteoprotegerin (OPG) pre- and post-exercise in both high-intensity interval cycling (HIIC) and running (HIIR) trials.
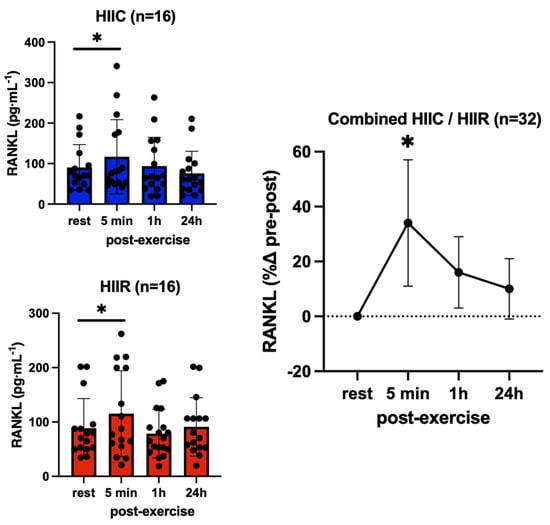
Figure 6.
Serum concentrations (mean ± SD) of receptor activator nuclear factor kappa-β ligand (RANKL) pre- and post-exercise in both high-intensity interval cycling (HIIC) and running (HIIR) trials). * denotes significant difference from pre-exercise to 5 min post-exercise in post hoc pairwise comparisons with groups combined and Bonferroni adjustment for three comparisons (p < 0.02; d = 0.45).
4. Discussion
This study provided novel evidence regarding the acute response of bone turnover markers (osteocalcin), osteokines (sclerostin, OPG, RANKL), as well as bone-modulating factors, including irisin and PTH, to high-intensity impact exercise (running) and high-intensity low-impact exercise (cycling) in the same group of young adult females. After accounting for exercise-induced changes in plasma volume, circulating osteocalcin, sclerostin, PTH, and RANKL were significantly elevated 5 min after exercise and returned to near their pre-exercise levels 1 h post-exercise, with no differences between modes of exercise. This finding suggests that at high intensity, impact does not mediate a post-exercise increase in the regulators of bone metabolism. In addition, irisin was significantly higher than its resting values 24 h post-exercise following both trials, an impact that did not modulate the exercise-induced irisin response. Our OPG results showed a significant main effect for time, reflecting an overall increase in OPG at 5 min and 1 h following high-intensity interval exercise, irrespective of exercise mode, but this response was not significant after the Bonferroni adjustment.
Furthermore, the resting serum irisin levels reported herein were similar to previous publications on young adult females [38]. Also consistent with other human exercise studies, we showed an increase in serum irisin levels following both exercise trials, although later following the exercise (at 24 h) than previously reported [22,39,40]. These results suggest a delayed irisin response to high-intensity interval exercise, irrespective of gravitational impact. This finding contradicts a study reporting no significant changes in irisin levels after aerobic exercise on a treadmill in 20–50-year-old males and females [38], but the divergent findings may be attributed to the difference in exercise intensity and duration. In agreement with our study, others have reported irisin to increase at 6 and 19 h after high-intensity running in adult males [40], as well as 10 min following 50 min of cycling at 80% of VO2max and 10 min post-exhausting running in trained and untrained adults [41]. Likewise, serum irisin concentrations increased immediately following a moderate-intensity protocol of both running and cycling in young adult male participants [22], while a single 90 min treadmill session also resulted in a transitory rise in serum irisin in young adult males and females [42].
Irisin activates the AV/b5 integrins in osteocytes, resulting in increased sclerostin and RANKL expression and osteoclastic bone resorption [6], whereas it has been shown that a loss of bone mineral, which is induced by ovariectomy in mice, can be prevented by irisin gene deletion [43]. Although these studies indicate osteocatabolic effects from irisin, a few studies exist indicating an osteoanabolic effect of irisin. For example, Luo et al. found reduced bone strength and bone mass in mice with global irisin knockout compared to control animals [44]. Additionally, in the irisin-lacking mice, osteoclast number and RANKL cell surface expression were increased [44]. Another study found that FNDC5/irisin deletion within the osteoblast lineage mice (Osx-Cre:FNDC5/irisin KO mice) had reduced irisin mRNA and protein levels in bone, reduced bone density, and delayed bone development and mineralization [45]. Specifically, these irisin knockdown mice had lower cortical bone mineral density and trabecular bone/tissue volume compared to control animals [45]. In addition, the protective effects of exercise, including increased bone strength and body weight loss, were reduced with irisin deficiency and were enhanced with the administration of recombinant irisin during exercise for 14 days [45]. Together, these data suggest that, overall, irisin plays an important role in bone homeostasis.
Based on our data and the available evidence of the osteoanabolic effects of irisin, we speculate that the preceding increase in sclerostin, RANKL, and PTH was meant to induce bone resorption and then irisin increased to compensate, leading to a subsequent increase in bone formation. In other words, resorption typically occurs before bone formation and is required to promote an overall increase in bone turnover [46]. However, the increase in osteocalcin immediately following both high-intensity interval trials may imply a complex feedback-driven timing of catabolic and anabolic events induced by exercise. This post-exercise increase in osteocalcin is in line with a previous study in premenopausal women, reporting an increase in osteocalcin 5 min after acute high-intensity interval exercise [21]. However, a decrease in total osteocalcin levels from baseline to 3 h after high-intensity interval exercise has also been reported in young women [47]. In addition, a previous study comparing moderate-intensity high-impact (jogging) and low-impact exercise (water aerobics) in young females found no significant time effect in osteocalcin up to 24 h following both trials [48]. Nevertheless, the synchronous rise in irisin, osteocalcin, and sclerostin levels after our high-intensity exercise trials may imply a post-exercise bone muscle interaction, which may have significant effects on bone metabolism in young females [30]. In addition, to account for the unexpected difference in the irisin levels between trials, we also examined the percentage changes from pre-exercise values, which confirmed the consistent rise in irisin, osteocalcin, and sclerostin levels after high-intensity exercise.
Furthermore, the present study confirms that sclerostin’s response to high-intensity interval exercise in young females is not mediated by impact. However, since, at moderate intensity, sclerostin levels have been shown to increase only following running but not after cycling in young men [22], exercise intensity may be an important modulator of the response [49,50]. Nevertheless, the immediate transient rise in sclerostin levels following exercise has been consistently reported in other studies and attributed to the release of previously synthesized sclerostin from osteocytes into the bloodstream, rather than increased gene expression and new protein synthesis [32,51]. Another theory is that the positive effect of exercise on blood flow causes the previously synthesized sclerostin to be released more easily during this short time [52]. A decline in waste elimination by the kidneys [53] might play a role in this transient rise in sclerostin following exercise. In any case, sclerostin levels at 1 h and 24 h after exercise were comparable to pre-exercise levels, indicating that the increase that occurred 5 min after exercise was transient, possibly a catabolic step that precedes bone formation. As previously mentioned, there is a temporal response of bone biochemical markers to acute exercise, which might be essential for improving BMD by initiating the resorption of old damaged bone prior to the replacement with new osteoid and, thus, bone through an increase in bone formation. These questions cannot be answered as a result of the present study, but they are intriguing and warrant further study. Alternatively, the increase in circulating sclerostin may be related to the recently suggested endocrine role of osteocytes and osteocyte-derived factors in energy and glucose metabolism [54], beige adipogenesis [55], and white adipose tissue metabolism [56]. Thus, our understanding of the downstream effects of sclerostin’s post-exercise increase in humans is still limited.
Like in our previous study of moderate-intensity exercise in adult males [22], our findings also reflect a transient increase in PTH immediately following high-intensity interval exercise in both modes, which has been suggested to be a response related to calcium homeostasis [57]. Indeed, serum PTH responds quickly to variations in circulating levels of calcium and phosphorus after exercise [58]. It has also been illustrated that exercise intensity affects PTH levels, with a stronger PTH response to higher-intensity exercise in men [59]. Other studies in young adult males have shown both an increase in PTH following running [58,60] and cycling [61] and no change in PTH after a single bout of plyometric exercise [62]. Therefore, the transient rise in PTH, combined with a simultaneous rise in sclerostin, may also be indicative of an immediate catabolic response to exercise that is needed to further stimulate an overall increase in bone turnover, which can eventually lead to an increase in BMD [46,63]. Indeed, it has been demonstrated that intermittent increases in PTH stimulate bone formation [64], while exercise intensity and duration seem to have significant impacts on the fluctuations in PTH concentration [65] that need to be further explored.
Finally, our results showed an overall (time effect) but small (7%, non-significant pairwise comparisons) increase in OPG following high-intensity interval exercise, irrespective of exercise mode. In contrast, RANKL increased considerably from pre- to 5 min post-exercise (33%, p < 0.05), returning to baseline levels in 1 h, with no trial or interaction effects. Together these results reflect an immediate exercise-induced response of the OPG/RANKL/RANK pathway. This finding is consistent with our previous study, where we found an increase in OPG and RANKL levels 5 min following low-impact high-intensity exercise in young males [15]. A similar increase in OPG and RANKL levels was also found following marathon long-distance running in both men and women [25]. Others found no change in either the circulating OPG, RANKL, or OPG mRNA expression following a combined exercise program consisting of 30 min of endurance exercise and 30 min of resistance exercise three times a week for 12 weeks in young women [43].
The main strength of our study is its crossover design, with each participant being their own control, thus controlling for between-subject variability. Additional strengths include the control of the diurnal variation in bone markers by collecting all blood samples in the morning following a consistent and standardized breakfast and accounting for exercise-induced changes in plasma volume. The main limitation of this study is the absence of a non-exercise control trial performed by all participants as part of the original experimental design. However, according to Kouvelioti et al. [16], a non-exercise control trial was subsequently added in a subset of participants, confirming that at least sclerostin resting levels can be considered stable during the morning hours. Another limitation is that, although we consistently advised all participants to continue the same diet, exercise, sleeping habits, and lifestyle, the participants’ habitual diets were not controlled following the trials until the 24 h blood draw. Thus, theoretically, it is possible that differences in the amount of daily energy, protein, and/or calcium could have affected the results of PTH, which is known to be influenced by calcium consumption, albeit this was a very short period. Finally, given its small sample size, caution should be exercised in generalizing the findings of this study to other populations. The original study was designed based on a calculated sample size of n = 35 being required to detect a standardized effect of 0.5, with a power of (1 − β) = 0.9 and a one-tailed probability level of p = 0.05. However, only 18 of the 40 participants enrolled in the original study were included in the present analysis. Thus, although significance was found for most of the markers, the present analysis was underpowered, which may have affected the statistical power to detect additional significant effects or interactions.
5. Conclusions
In young adult females, high-intensity interval exercise resulted in an immediate response in the regulators of bone remodeling and a later response in irisin concentrations, which was independent of the gravitational impact. Specifically, it is shown that both low- and high-impact exercise led to an elevation in bone catabolism factors immediately after the exercise bout and a postponed increase in irisin, which can further induce bone anabolism. As a potent myokine, it is not surprising that irisin increased following exercise to stimulate the post-exercise repair and recovery processes. It is important, however, to note that both low- and high-impact exercise had a similar effect on elevating initial bone catabolism factors, suggesting that the mechanical loading caused by skeletal muscle contraction is the primary factor driving the response. Thus, resistance exercise may be even more effective in promoting bone strength. Therefore, it may be worthwhile to explore the potential benefits of different types of exercise in promoting bone health and preventing osteoporosis in women. Future research should also explore the long-term effects of low- and high-impact exercise on bone metabolism, and whether the initial elevation of bone catabolism has a lasting effect on bone remodelling and mineral density. In addition, the synchronous rise in irisin, osteocalcin, sclerostin, PTH, and RANKL, with an overall small increase also in OPG, after high-intensity interval exercise may suggest that the exercise-induced changes in bone metabolism are the result of a complex interplay between regional and systemic signals and hormones. Further investigation of the mechanisms behind this complex bone response can provide valuable insights into how exercise affects bone integrity. It can help identify specific factors and signaling pathways that promote or hinder bone remodeling and potentially lead to the development of targeted exercise interventions.
Author Contributions
Conceptualization, G.B., R.K. and P.K.; methodology, R.K., W.E.W., E.T. and P.K.; formal analysis, G.B., S.K., M.B. and P.K.; investigation, G.B., S.K., R.K. and P.K.; resources, P.K.; writing—original draft preparation, G.B. and P.K.; writing—review and editing, M.B., W.E.W. and E.T.; supervision, P.K.; project administration, P.K.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), grant number 2020-00014.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Brock University (REB #19-131, 13 November 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the findings of this study are available from the corresponding author (PK) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Banfi, G.; Lombardi, G.; Colombini, A.; Lippi, G. Bone Metabolism Markers in Sports Medicine. J. Sports Med. 2012, 40, 697–714. [Google Scholar] [CrossRef]
- Lombardi, G.; Sanchis-Gomar, F.; Perego, S.; Sansoni, V.; Banfi, G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocr. J. 2015, 54, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef]
- Kawao, N.; Moritake, A.; Tatsumi, K.; Kaji, H. Roles of Irisin in the Linkage from Muscle to Bone During Mechanical Unloading in Mice. Calcif. Tissue Int. 2018, 103, 24–34. [Google Scholar] [CrossRef]
- Li, G.; Cao, N.; Shang, P.; Xu, H. What the discovery of irisin receptor means to bone. Arch. Physiol. Biochem. 2020, 128, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Seo, M.-W.; Jung, H.-C.; Song, J.-K. Effects of High-Impact Weight-Bearing Exercise on Bone Mineral Density and Bone Metabolism in Middle-Aged Premenopausal Women: A Randomized Controlled Trial. Appl. Sci. 2021, 11, 846. [Google Scholar] [CrossRef]
- Morseth, B.; Emaus, N.; Jørgensen, L. Physical activity and bone: The importance of the various mechanical stimuli for bone mineral density. A review. Nor. Epidemiol. 2011, 20, 173–178. [Google Scholar] [CrossRef]
- Lewis, R.D.; Modlesky, C.M. Nutrition, physical activity, and bone health in women. Int. J. Sport Nutr. Exerc. Metab. 1998, 8, 250–284. [Google Scholar] [CrossRef]
- Milgrom, C.; Finestone, A.; Levi, Y.; Simkin, A.; Ekenman, I.; Mendelson, S.; Millgram, M.; Nyska, M.; Benjuya, N.; Burr, D. Do high impact exercises produce higher tibial strains than running? Br. J. Sports Med. 2000, 34, 195–199. [Google Scholar] [CrossRef]
- Olmedillas, H.; González-Agüero, A.; Moreno, L.A.; Casajus, J.A.; Vicente-Rodríguez, G. Cycling and bone health: A systematic review. BMC Med. 2012, 10, 168. [Google Scholar] [CrossRef]
- Duncan, C.S.; Blimkie, C.J.R.; Kemp, A.; Higgs, W.; Cowell, C.T.; Woodhead, H.; Briody, J.N.; Howman-giles, R. Mid-femur geometry and biomechanical properties in 15- to 18-yr-old female athletes. Med. Sci. Sports Exerc. 2002, 34, 673–681. [Google Scholar] [PubMed]
- Torstveit, M.K.; Sundgot-Borgen, J. Low bone mineral density is two to three times more prevalent in non-athletic premenopausal women than in elite athletes: A comprehensive controlled study. Br. J. Sports Med. 2005, 39, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Leigey, D.; Irrgang, J.; Francis, K.; Cohen, P.; Wright, V. Participation in High-Impact Sports Predicts Bone Mineral Density in Senior Olympic Athletes. Sports Health 2009, 1, 508–513. [Google Scholar] [CrossRef]
- Mezil, Y.A.; Allison, D.; Kish, K.; Ditor, D.; Ward, W.; Tsiani, E.; Klentrou, P. Response of Bone Turnover Markers and Cytokines to High-Intensity Low-Impact Exercise. Med. Sci. Sports Exerc. 2015, 47, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Kouvelioti, R.; Kurgan, N.; Falk, B.; Ward, W.; Josse, A.R.; Klentrou, P. Response of Sclerostin and Bone Turnover Markers to High Intensity Interval Exercise in Young Women: Does Impact Matter? Biomed Res. Int. 2018, 2018, 4864952–4864958. [Google Scholar] [CrossRef]
- Kouvelioti, R.; Kurgan, N.; Falk, B.; Ward, W.; Josse, A.R.; Klentrou, P. Cytokine and Sclerostin Response to High-Intensity Interval Running versus Cycling. Med. Sci. Sports Exerc. 2019, 51, 2458–2464. [Google Scholar] [CrossRef]
- Kouvelioti, R.; LeBlanc, P.; Falk, B.; Ward, W.; Josse, A.R.; Klentrou, P. Effects of High-Intensity Interval Running Versus Cycling on Sclerostin, and Markers of Bone Turnover and Oxidative Stress in Young Men. Calcif. Tissue Int. 2019, 104, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Nikel, O.; Poundarik, A.A.; Bailey, S.; Vashishth, D. Structural role of osteocalcin and osteopontin in energy dissipation in bone. J. Biomech. 2018, 80, 45–52. [Google Scholar] [CrossRef]
- Gassel, L.C.; Schneider, S.; Banke, I.J.; Braun, K.F.; Volkering, C.; Zeeb, L.; Burgkart, R.H.H.; von Eisenhart-Rothe, R.; Biberthaler, P.; van Griensven, M.; et al. Dysregulation of Wnt signaling in bone of type 2 diabetes mellitus and diabetic Charcot arthropathy. BMC Musculoskelet. Disord. 2022, 23, 365. [Google Scholar] [CrossRef]
- Hiam, D.; Landen, S.; Jacques, M.; Voisin, S.; Alvarez-Romero, J.; Byrnes, E.; Chubb, P.; Levinger, I.; Eynon, N. Osteocalcin and its forms respond similarly to exercise in males and females. Bone 2012, 144, 115818. [Google Scholar] [CrossRef]
- Dror, N.; Carbone, J.; Haddad, F.; Falk, B.; Klentrou, P.; Radom-Aizik, S. Sclerostin and bone turnover markers response to cycling and running at the same moderate-to-vigorous exercise intensity in healthy men. J. Endocrinol. Investig. 2021, 45, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, X.; Zhang, L.; Wu, J.; Guo, J.; Zou, D.; Chen, B.; Sun, Z.; Shen, C.; Zou, J. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog. Biophys. Mol. Biol. 2016, 122, 122–130. [Google Scholar] [CrossRef]
- Ziegler, S.; Niessner, A.; Richter, B.; Wirth, S.; Billensteiner, E.; Woloszczuk, W.; Slany, J.; Geyer, G. Endurance running acutely raises plasma osteoprotegerin and lowers plasma receptor activator of nuclear factor κ B ligand. Metab. Clin. Exp. 2005, 54, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, A.; Rapisarda, R.; Xourafa, A.; Zanoli, L.; Manfrè, V.; Catalano, A.; Signorelli, S.S.; Castellino, P. Effects of competitive physical activity on serum irisin levels and bone turnover markers. J. Endocrinol. Investig. 2021, 44, 2235–2241. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Holdsworth, G.; Roberts, S.J.; Ke, H.Z. Novel actions of sclerostin on bone. J. Mol. Endocrinol. 2019, 62, R167–R185. [Google Scholar] [CrossRef]
- Cameron, D.A.; Paschall, H.A.; Robinson, R.A. Changes in the Fine Structure of Bone Cells after the Administration of Parathyroid Extract. J. Mol. Cell Biol. 1967, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Saini, V.; Pajevic, P.D. Effects of PTH on osteocyte function. Bone 2012, 54, 250–257. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Kim, S.W.; Pajevic, P.D.; Selig, M.; Barry, K.J.; Yang, J.-Y.; Shin, C.S.; Baek, W.-Y.; Kim, J.-E.; Kronenberg, H.M. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. 2012, 27, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Haddad, F.; Klentrou, P.; Ward, W.; Kish, K.; Mezil, Y.; Radom-Aizik, S. Differential sclerostin and parathyroid hormone response to exercise in boys and men. Osteoporos. Int. 2015, 27, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sci. 1985, 10, 141–146. [Google Scholar]
- Guzman, A.; Kurgan, N.; Moniz, S.C.; McCarthy, S.F.; Sale, C.; Heather Logan-Spenger Elliot-Sale, K.; Hazell, T.J.; Klentrou, P. Menstrual cycle related fluctuations in circulating markers of bone metabolism at rest and in response to running in eumenorrheic females. Calcif. Tissue Int. 2022, 111, 124–136. [Google Scholar] [CrossRef]
- Kargotich, S.; Goodman, C.; Keast, D.; Morton, A.R. The Influence of Exercise-Induced Plasma Volume Changes on the Interpretation of Biochemical Parameters Used for Monitoring Exercise, Training and Sport. J. Sports Med. 1998, 26, 101–117. [Google Scholar] [CrossRef]
- van Beaumont, W. Evaluation of hemoconcentration from hematocrit measurements. J. Appl. Physiol. 1972, 32, 712–713. [Google Scholar] [CrossRef]
- Lachenbruch, P.A.; Cohen, J. Statistical Power Analysis for the Behavioral Sciences. J. Am. Stat. Assoc. 1989, 84, 1096. [Google Scholar] [CrossRef]
- Lagzdina, R.; Rumaka, M.; Gersone, G.; Tretjakovs, P. Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study. Medicina 2020, 56, 274. [Google Scholar] [CrossRef]
- Winn, N.C.; Grunewald, Z.I.; Liu, Y.; Heden, T.D.; Nyhoff, L.M.; Kanaley, J.A. Plasma Irisin Modestly Increases during Moderate and High-Intensity Afternoon Exercise in Obese Females. PLoS ONE 2017, 12, e0170690. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Daisuke, A.; Kazushige, G.; Masataka, K.; Mitsuya, Y. High-Intensity Exercise Causes Greater Irisin Response Compared with Low-Intensity Exercise under Similar Energy Consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef]
- Qiu, S.; Bosnyák, E.; Treff, G.; Steinacker, J.M.; Nieß, A.M.; Krüger, K.; Mooren, F.C.; Zügel, M.; Schumann, U. Acute exercise-induced irisin release in healthy adults: Associations with training status and exercise mode. Eur. J. Sport Sci. 2018, 18, 1226–1233. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Shockett, P.E.; Webb, N.D.; Shah, U.; Castracane, V.D. A Transient Elevated Irisin Blood Concentration in Response to Prolonged, Moderate Aerobic Exercise in Young Men and Women. Med. Sci. Sports Exerc. 2014, 46, 404. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered Metabolism in Mice Lacking Irisin. Sci. Rep. 2020, 10, 17368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, X.; Wang, X.; Chen, T.; Tao, F.; Liu, C.; Tu, Q.; Shen, G.; Chen, J.J. Irisin Deficiency Disturbs Bone Metabolism. J. Cell Physiol. 2021, 236, 664–676. [Google Scholar] [CrossRef]
- Falk, B.; Klentrou, P. Elevation in Sclerostin After Exercise: Is It Affected by Age and Sex? Calcif. Tissue Int. 2017, 102, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Stunes, A.K.; Brobakken, C.L.; Sujan, M.A.J.; Aagård, N.; Brevig, M.S.; Wang, E.; Syversen, U.; Mosti, M.P. Acute Effects of Strength and Endurance Training on Bone Turnover Markers in Young Adults and Elderly Men. Front. Endocrinol. 2022, 13, 915241. [Google Scholar] [CrossRef]
- Morgan, A.L.; Weiss, J.; Kelley, E.T. Bone Turnover Response to Acute Exercise with Varying Impact Levels: A preliminary investigation. Int. J. Exerc. Sci. 2015, 8, 154–163. [Google Scholar]
- Troy, K.L.; Mancuso, M.E.; Butler, T.A.; Johnson, J.E. Exercise Early and Often: Effects of Physical Activity and Exercise on Women’s Bone Health. Int. J. Environ. Res. Public Health 2018, 15, 878. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Kaczmarek, A.; Kaczmarek, M.; Ciałowicz, M.; Arslan, E.; Silva, A.F.; Murawska-Ciałowicz, E. Sclerostin as a biomarker of physical exercise in osteoporosis: A narrative review. Front. Endocrinol. 2022, 13, 954895. [Google Scholar] [CrossRef]
- Pickering, M.-E.; Simon, M.; Sornay-Rendu, E.; Chikh, K.; Carlier, M.-C.; Raby, A.-L.; Szulc, P.; Confavreux, C.B. Serum Sclerostin Increases After Acute Physical Activity. Calcif. Tissue Int. 2017, 101, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.; Kemppainen, J.; Kaskinoro, K.; Langberg, H.; Knuuti, J.; Boushel, R.; Kjaer, M.; Kalliokoski, K.K. Bone blood flow and metabolism in humans: Effect of muscular exercise and other physiological perturbations. J. Bone Miner. 2013, 28, 1068–1074. [Google Scholar] [CrossRef]
- Drüeke, T.B.; Massy, Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016, 89, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Oldknow, K.J.; MacRae, V.E.; Farquharson, C. Endocrine role of bone: Recent and emerging perspectives beyond osteocalcin. J. Endocrinol. 2015, 225, R1–R19. [Google Scholar] [CrossRef]
- Fulzele, K.; Lai, F.; Dedic, C.; Saini, V.; Uda, Y.; Shi, C.; Tuck, P.; Aronson, J.L.; Liu, X.; Spatz, J.M.; et al. Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J. Bone Miner. 2017, 32, 373–384. [Google Scholar] [CrossRef]
- Kurgan, N.; Islam, H.; Matusiak, J.B.L.; Baranowski, B.J.; Stoikos, J.; Fajardo, V.A.; MacPherson, R.E.K.; Gurd, B.J.; Klentrou, P. Subcutaneous adipose tissue sclerostin is reduced and Wnt signaling is enhanced following 4-weeks of sprint interval training in young men with obesity. Physiol. Rep. 2022, 10, e15232. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Wherry, S.J.; Wolfe, P.; Sherk, V.D.; Wellington, T.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Maintenance of Serum Ionized Calcium During Exercise Attenuates Parathyroid Hormone and Bone Resorption Responses. J. Bone Miner. Res. 2018, 33, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.; Elliott-Sale, K.J.; Pinto, A.J.; Thomas, C.; Scott, J.P.R.; Currell, K.; Fraser, W.D.; Sale, C. Parathyroid Hormone Secretion Is Controlled by Both Ionized Calcium and Phosphate During Exercise and Recovery in Men. J. Clin. Endocr. 2016, 101, 3231–3239. [Google Scholar] [CrossRef]
- Scott, J.P.R.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J. Appl. Physiol. 2011, 110, 423–432. [Google Scholar] [CrossRef]
- Śliwicka, E.; Cisoń, T.; Pilaczyńska-Szcześniak, Ł.; Ziemba, A.; Straburzyńska-Lupa, A. Effects of marathon race on selected myokines and sclerostin in middle-aged male amateur runners. Sci. Rep. 2021, 11, 2813. [Google Scholar] [CrossRef]
- Maïmoun, L.; Manetta, J.; Couret, I.; Dupuy, A.M.; Mariano-Goulart, D.; Micallef, J.P.; Peruchon, E.; Rossi, M. The Intensity Level of Physical Exercise and the Bone Metabolism Response. J. Appl. Physiol. 2016, 27, 105–111. [Google Scholar] [CrossRef]
- Guerriere, K.I.; Hughes, J.M.; Gaffney-Stomberg, E.; Staab, J.S.; Matheny, R.W. Circulating sclerostin is not suppressed following a single bout of exercise in young men. Physiol. Rep. 2018, 6, e13695. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Shojaa, M.; Kohl, M.; von Stengel, S. Effects of different types of exercise on bone mineral density in postmenopausal women: A systematic review and meta-analysis. Calc. Tissue Int. 2020, 107, 409–439. [Google Scholar] [CrossRef] [PubMed]
- Onyia, J.E.; Helvering, L.M.; Gelbert, L.; Wei, T.; Huang, S.; Chen, P.; Dow, E.R.; Maran, A.; Zhang, M.; Lotinun, S.; et al. Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: An analysis by DNA microarray. J. Cell. Biochem. 2005, 95, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, A.; Latiri, I.; Bouassida, S.; Zalleg, D.; Zaouali, M.; Feki, Y.; Gharbi, N.; Zbidi, A.; Tabka, Z. Parathyroid hormone and physical exercise: A brief review. J. Sports Sci. Med. 2006, 5, 367–374. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).