Deleting Cellular Retinoic-Acid-Binding Protein-1 (Crabp1) Gene Causes Adult-Onset Primary Hypothyroidism in Mice
Highlights
- CRABP1 knockout (CKO) mice develop primary hypothyroidism with normal HPA-axis regulation.
- CKO thyroid glands exhibit morphological abnormalities with altered expression in genes involved in thyroid hormone synthesis, transport, and metabolism.
- Histological examination reveals smaller follicles and a higher density of non-follicle-forming cells in CKO thyroid glands as compared to the wild-type control.
- CRABP1 plays a critical role in maintaining the health and function of follicular thyrocytes.
Abstract
1. Introduction
2. Materials and Methods
3. Results
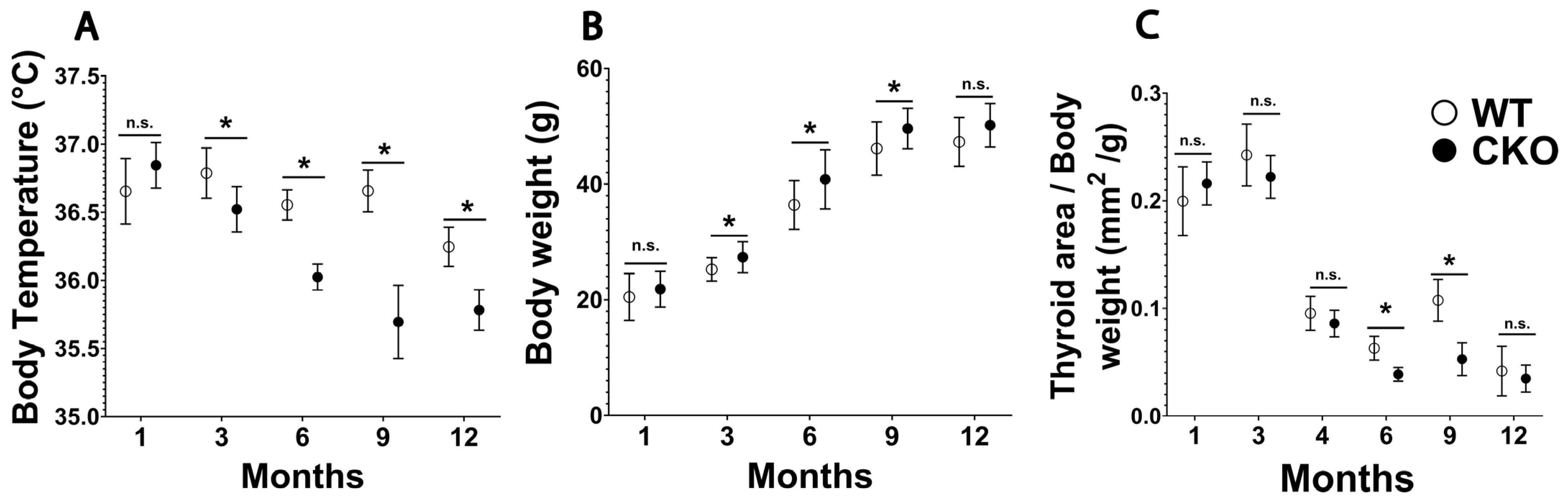
3.1. Male CKO Mouse Phenotype Suggests Adult-Onset Hypothyroidism
3.2. Thyroid Hormone Status of CKO Mouse Suggesting Primary Hypothyroidism in Adults
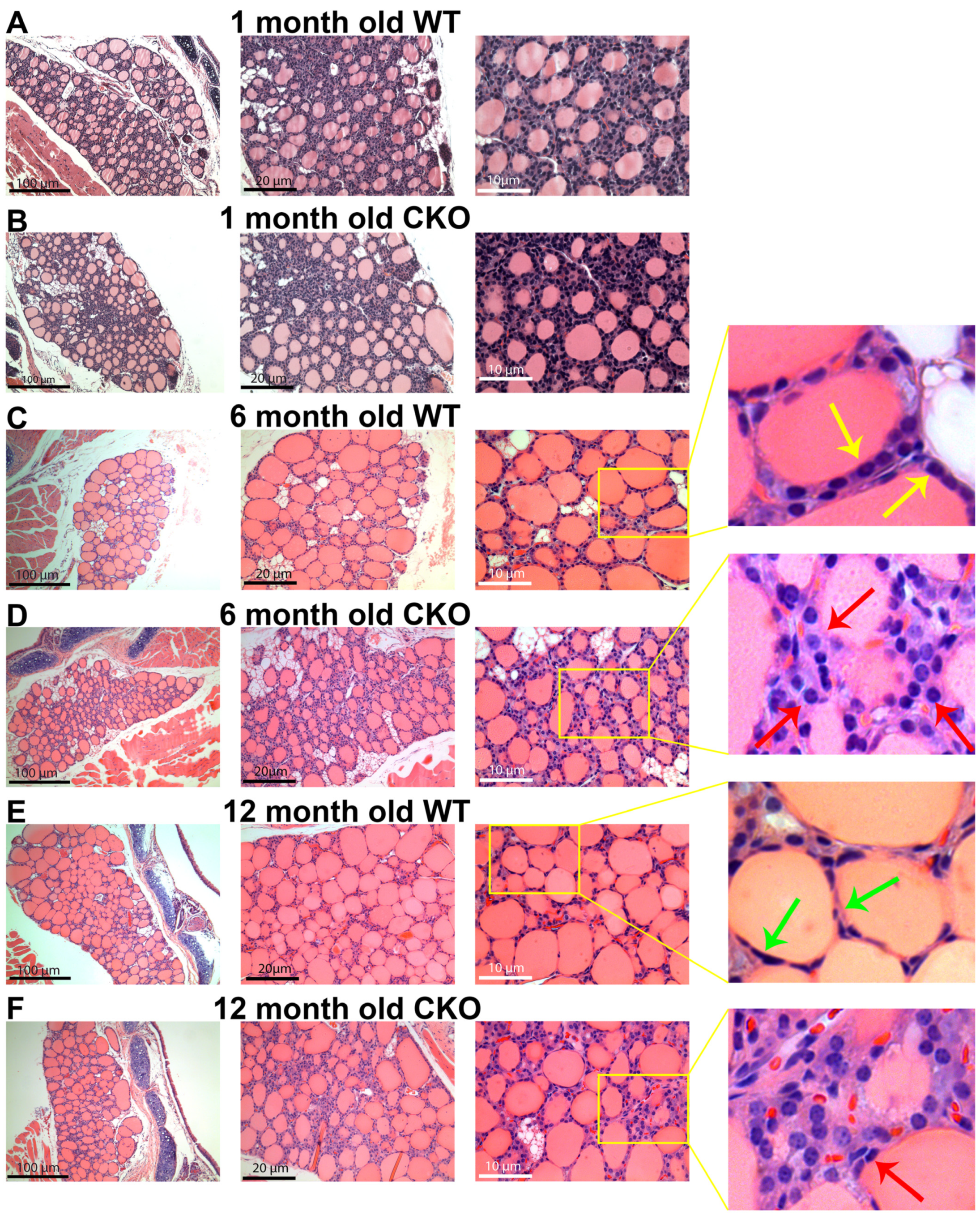
3.3. Histopathology of CKO Thyroid Gland
3.4. Altered Gene Expression in CKO Thyroid Gland Related to Hypothyroidism
3.5. Association between CKO’s Molecular Changes and Human Genetic Defects in Hypothyroidism
4. Discussion
4.1. Physiological and Hormonal Changes in Animal Models of Primary Hypothyroidism or Altered Thyroid Metabolism
4.2. Altered Gene Expression in CKO Mice Recapitulating Genetic Defects in Human Hypothyroidism
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.P.; Dupuy, C. Thyroid hormone biosynthesis and release. Mol. Cell. Endocrinol. 2017, 458, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; da Conceição, R.R. The Deiodinase Trio and Thyroid Hormone Signaling. In Thyroid Hormone Nuclear Receptor; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1801, pp. 67–83. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeöld, A.; Bianco, A.C. Cellular and Molecular Basis of Deiodinase-Regulated Thyroid Hormone Signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef]
- Huang, S.A. Physiology and Pathophysiology of Type 3 Deiodinase in Humans. Thyroid 2005, 15, 875–881. [Google Scholar] [CrossRef]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Grasberger, H.; Refetoff, S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr. Opin. Pediatr. 2011, 23, 421–428. [Google Scholar] [CrossRef]
- Stoupa, A.; Kariyawasam, D.; Polak, M.; Carré, A. Genetics of congenital hypothyroidism: Modern concepts. Pediatr. Investig. 2022, 6, 123–134. [Google Scholar] [CrossRef]
- Kopp, P. Perspective: Genetic Defects in the Etiology of Congenital Hypothyroidism. Endocrinology 2002, 143, 2019–2024. [Google Scholar] [CrossRef]
- Galton, V.A.; Schneider, M.J.; Clark, A.S.; St. Germain, D.L. Life without Thyroxine to 3,5,3′-Triiodothyronine Conversion: Studies in Mice Devoid of the 5′-Deiodinases. Endocrinology 2009, 150, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.; Refetoff, S. Inherited defects of thyroid hormone metabolism. Ann. Endocrinol. 2011, 72, 95–98. [Google Scholar] [CrossRef]
- Visser, W.E.; Van Mullem, A.A.A.; Visser, T.J.; Peeters, R.P. Different causes of Reduced Sensitivity to Thyroid Hormone: Diagnosis and Clinical management. Clin. Endocrinol. 2013, 79, 595–605. [Google Scholar] [CrossRef]
- Nagpal, I.; Wei, L.-N. All-trans Retinoic Acid as a Versatile Cytosolic Signal Modulator Mediated by CRABP. Int. J. Mol. Sci. 2019, 20, 3610. [Google Scholar] [CrossRef] [PubMed]
- Nhieu, J.; Lin, Y.-L.; Wei, L.-N. CRABP1 in Non-Canonical Activities of Retinoic Acid in Health and Diseases. Nutrients 2022, 14, 1528. [Google Scholar] [CrossRef]
- Park, S.W.; Persaud, S.D.; Ogokeh, S.; Meyers, T.A.; Townsend, D.; Wei, L.-N. CRABP1 protects the heart from isoproterenol-induced acute and chronic remodeling. J. Endocrinol. 2018, 236, 151–165. [Google Scholar] [CrossRef]
- Persaud, S.D.; Park, S.W.; Ishigami-Yuasa, M.; Koyano-Nakagawa, N.; Kagechika, H.; Wei, L.-N. All trans-retinoic acid analogs promote cancer cell apoptosis through non-genomic Crabp1 mediating ERK1/2 phosphorylation. Sci. Rep. 2016, 6, 22396. [Google Scholar] [CrossRef]
- Kainov, Y.; Favorskaya, I.; Delektorskaya, V.; Chemeris, G.; Komelkov, A.; Zhuravskaya, A.; Trukhanova, L.; Zueva, E.; Tavitian, B.; Dyakova, N.; et al. CRABP1 provides high malignancy of transformed mesenchymal cells and contributes to the pathogenesis of mesenchymal and neuroendocrine tumors. Cell Cycle 2014, 13, 1530–1539. [Google Scholar] [CrossRef]
- Celestino, R.; Nome, T.; Pestana, A.; Hoff, A.M.; Gonçalves, A.P.; Pereira, L.; Cavadas, B.; Eloy, C.; Bjøro, T.; Sobrinho-Simões, M.; et al. CRABP1, C1QL1 and LCN2 are biomarkers of differentiated thyroid carcinoma, and predict extrathyroidal extension. BMC Cancer 2018, 18, 68. [Google Scholar] [CrossRef]
- Tissue Expression of CRABP1-Staining in Thyroid Gland-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000166426-CRABP1/tissue/thyroid+gland (accessed on 18 January 2023).
- Lin, Y.-W.; Park, S.W.; Lin, Y.-L.; Burton, F.H.; Wei, L.-N. Cellular retinoic acid binding protein 1 protects mice from high-fat diet-induced obesity by decreasing adipocyte hypertrophy. Int. J. Obes. 2019, 44, 466–474. [Google Scholar] [CrossRef]
- Wei, C.-W.; Nhieu, J.; Lin, Y.-L.; Wei, L.-N. Modulation of adipose inflammation by cellular retinoic acid-binding protein. Int. J. Obes. 2022, 46, 1759–1769. [Google Scholar] [CrossRef]
- Gorry, P.; Lufkin, T.; Dierich, A.; Rochette-Egly, C.; Décimo, D.; Dollé, P.; Mark, M.; Durand, B.; Chambon, P. The cellular retinoic acid binding protein I is dispensable. Proc. Natl. Acad. Sci. USA 1994, 91, 9032–9036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kellogg, A.P.; Citterio, C.E.; Zhang, H.; Larkin, D.; Morishita, Y.; Targovnik, H.M.; Balbi, V.A.; Arvan, P. Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death. J. Clin. Investig. 2021, 6, e148496. [Google Scholar] [CrossRef]
- Narumi, S.; Muroya, K.; Abe, Y.; Yasui, M.; Asakura, Y.; Adachi, M.; Hasegawa, T. TSHR Mutations as a Cause of Congenital Hypothyroidism in Japan: A Population-Based Genetic Epidemiology Study. J. Clin. Endocrinol. Metab. 2009, 94, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Cangul, H.; Morgan, N.V.; Forman, J.R.; Saglam, H.; Aycan, Z.; Yakut, T.; Gulten, T.; Tarim, O.; Bober, E.; Cesur, Y.; et al. Novel TSHR mutations in consanguineous families with congenital nongoitrous hypothyroidism. Clin. Endocrinol. 2010, 73, 671–677. [Google Scholar] [CrossRef]
- Biebermann, H.; Schöneberg, T.; Krude, H.; Schultz, G.; Gudermann, T.; Grüters, A. Mutations of the Human Thyrotropin Receptor Gene Causing Thyroid Hypoplasia and Persistent Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 1997, 82, 3471–3480. [Google Scholar] [CrossRef]
- Bakker, B.; Bikker, H.; Vulsma, T.; De Randamie, J.S.E.; Wiedijk, B.M.; De Vijlder, J.J.M. Two Decades of Screening for Congenital Hypothyroidism in the Netherlands: TPO Gene Mutations in Total Iodide Organification Defects (an Update). J. Clin. Endocrinol. Metab. 2000, 85, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Bikker, H.; Hartog, M.T.D.; Baas, F.; Gons, M.H.; Vulsma, T.; De Vijlder, J.J. A 20-basepair duplication in the human thyroid peroxidase gene results in a total iodide organification defect and congenital hypothyroidism. J. Clin. Endocrinol. Metab. 1994, 79, 248–252. [Google Scholar] [CrossRef]
- Rodrigues, C.; Jorge, P.; Soares, J.P.; Santos, I.; Salomão, R.; Madeira, M.; Osorió, R.V.; Santos, R. Mutation screening of the thyroid peroxidase gene in a cohort of 55 Portuguese patients with congenital hypothyroidism. Eur. J. Endocrinol. 2005, 152, 193–198. [Google Scholar] [CrossRef]
- Cangül, H.; Doğan, M.; Sağlam, Y.; Kendall, M.; Boelaert, K.; Barrett, T.G.; Maher, E.R. One Base Deletion (c.2422delT) in the TPO Gene Causes Severe Congenital Hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 169–173. [Google Scholar] [CrossRef]
- Tajima, T.; Tsubaki, J.; Fujieda, K. Two Novel Mutations in the Thyroid Peroxidase Gene with Goitrous Hypothyroidism. Endocr. J. 2005, 52, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Bhayana, S.; Dean, H.J. A Novel Mutation in the Sodium/Iodide Symporter Gene in the Largest Family with Iodide Transport Defect. J. Clin. Endocrinol. Metab. 1999, 84, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Tatsumi, K.-I.; Miki, K.; Harada, T.; Miyai, K.; Takai, S.-I.; Amino, N. Congenital hypothyroidism caused by a mutation in the Na+/l− symporter. Nat. Genet. 1997, 16, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Crushell, E.; Reardon, W. Elevated TSH levels in a mentally retarded boy. Eur. J. Pediatr. 2009, 169, 573–575. [Google Scholar] [CrossRef]
- Liu, S.; Han, W.; Zang, Y.; Zang, H.; Wang, F.; Jiang, P.; Wei, H.; Liu, X.; Wang, Y.; Ma, X.; et al. Identification of Two Missense Mutations in DUOX1 (p.R1307Q) and DUOXA1 (p.R56W) That Can Cause Congenital Hypothyroidism Through Impairing H2O2 Generation. Front. Endocrinol. 2019, 10, 526. [Google Scholar] [CrossRef]
- Dueñas, O.H.R.; Van der Burgh, A.C.; Ittermann, T.; Ligthart, S.; Ikram, M.A.; Peeters, R.; Chaker, L. Thyroid Function and the Risk of Prediabetes and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1789–1798. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, Y.J.; Park, Y.J.; Kim, K.W.; Lee, E.J.; Lim, S.; Park, D.J.; Kim, S.E.; Park, K.S.; Jang, H.C.; et al. Retinol Binding Protein-4 Elevation Is Associated with Serum Thyroid-Stimulating Hormone Level Independently of Obesity in Elderly Subjects with Normal Glucose Tolerance. J. Clin. Endocrinol. Metab. 2008, 93, 2313–2318. [Google Scholar] [CrossRef]
- Udovcic, M.; Pena, R.H.; Patham, B.; Tabatabai, L.; Kansara, A. Hypothyroidism and the Heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 55–59. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Zawalna, N.; Gut, P.; Ruchała, M. Relationship between thyroid hormones and central nervous system metabolism in physiological and pathological conditions. Pharmacol. Rep. 2022, 74, 847–858. [Google Scholar] [CrossRef]
- Aktuna, D.; Buchinger, W.; Langsteger, W.; Meister, E.; Sternad, H.; Lorenz, O.; Eber, O. Beta-carotene, vitamin A and carrier proteins in thyroid diseases. Acta Med. Austriaca 1993, 20, 17–20. [Google Scholar]
- Rashad, N.M.; Sabry, H.M.; Afifi, S.A.; Fathy, M.A.; El-Helaly, A.M.; Mohamed, H.E. Serum retinol-binding protein 4 and the risk of ischemic stroke in Egyptian patients with hypothyroidism. Egypt. J. Intern. Med. 2019, 31, 746–753. [Google Scholar] [CrossRef]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef]
- Yaturu, S.; Prado, S.; Grimes, S.R. Changes in adipocyte hormones leptin, resistin, and adiponectin in thyroid dysfunction. J. Cell. Biochem. 2004, 93, 491–496. [Google Scholar] [CrossRef]
- Beamer, W.G.; Eicher, E.M.; Maltais, L.J.; Southard, J.L. Inherited Primary Hypothyroidism in Mice. Science 1981, 212, 61–63. [Google Scholar] [CrossRef]
- Takabayashi, S.; Umeki, K.; Yamamoto, E.; Suzuki, T.; Okayama, A.; Katoh, H. A Novel Hypothyroid Dwarfism Due to the Missense Mutation Arg479Cys of the Thyroid Peroxidase Gene in the Mouse. Mol. Endocrinol. 2006, 20, 2584–2590. [Google Scholar] [CrossRef]
- Beamer, W.G.; Maltais, L.J.; Debaets, M.H.; Eicher, E.M. Inherited Congenital Goiter in Mice. Endocrinology 1987, 120, 838–840. [Google Scholar] [CrossRef]
- Ferrandino, G.; Kaspari, R.R.; Reyna-Neyra, A.; Boutagy, N.E.; Sinusas, A.J.; Carrasco, N. An extremely high dietary iodide supply forestalls severe hypothyroidism in Na+/I− symporter (NIS) knockout mice. Sci. Rep. 2017, 7, 5329. [Google Scholar] [CrossRef]
- Grasberger, H.; De Deken, X.; Mayo, O.B.; Raad, H.; Weiss, M.; Liao, X.-H.; Refetoff, S. Mice Deficient in Dual Oxidase Maturation Factors Are Severely Hypothyroid. Mol. Endocrinol. 2012, 26, 481–492. [Google Scholar] [CrossRef]
- Cangul, H.; Liao, X.-H.; Schoenmakers, E.; Kero, J.; Barone, S.; Srichomkwun, P.; Iwayama, H.; Serra, E.G.; Saglam, H.; Eren, E.; et al. Homozygous loss-of-function mutations in SLC26A7 cause goitrous congenital hypothyroidism. J. Clin. Investig. 2018, 3, e99631. [Google Scholar] [CrossRef]
- Di Cosmo, C.; Liao, X.-H.; Dumitrescu, A.M.; Philp, N.J.; Weiss, R.E.; Refetoff, S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J. Clin. Investig. 2010, 120, 3377–3388. [Google Scholar] [CrossRef]


| Gene Name | Primer Orientation | Primer Sequences | NCBI Reference |
|---|---|---|---|
| Tshr | Forward | GCTGTCGTTGAGTTTCCTCCAC | NM_011648.1 |
| Reverse | CTGCTCTCATTACACATCAAAGAC | ||
| Thr-α | Forward | CCTGGACAAAGACGAGCAGTGT | NM_178060.4 |
| Reverse | CTGGATTGTGCGGCGAAAGAAG | ||
| Thr-β | Forward | ACCACTATCGCTGCATCACCTG | NM_009380.3 |
| Reverse | ACTGGTTGCGGGTGACTTTGTC | ||
| Dio1 | Forward | GTAGGCAAGGTGCTAATGACGC | NM_007860.4 |
| Reverse | ACTGGATGCTGAAGAAGGTGGG | ||
| Dio2 | Forward | GGTGGTCAACTTTGGTTCAGCC | NM_010050.4 |
| Reverse | AAGTCAGCCACCGAGGAGAACT | ||
| Dio3 | Forward | TGCGTATCAGACGACAACCGTC | NM_172119.2 |
| Reverse | TGGAAGCCATCAGGTCGGACAA | ||
| TPO | Forward | GAGAGGCTCTTCGTGCTGTCTA | NM_009417.1 |
| Reverse | AGGCGTGACAAGCCACAGAACT | ||
| Duox1 | Forward | AGGCGTGACAAGCCACAGAACT | NM_001099297.1 |
| Reverse | AGGCGTGACAAGCCACAGAACT | ||
| Duox2 | Forward | GAGAAAGGCTGTGACCAAGCAG | NM_177610.2 |
| Reverse | TCACGCACTTGCTGGGATGAGT | ||
| Tg | Forward | TTGTAGCCTGGAGAGTCAGCAC | NM_009375.2 |
| Reverse | CACTGCACATCTTTCCTGGTGG | ||
| Slc5a5 (Nis) | Forward | CATGCCATTGCTCGTGTTGGAC | NM_053248.2 |
| Reverse | GCCATAGCGTTGATACTGGTGG | ||
| Slc16a2 | Forward | GTGTATTCCGCCAGCGCACTTA | NM_009197 |
| Reverse | AAGAGCACCCAGGTCTCCTTGA | ||
| Slc16a10 | Forward | GGAGACAACCTATGCAGTGTGG | NM_001114332.1 |
| Reverse | GCCAATGCACATGAAGAGCACC |
| Variables | W.T. Male | CKO Male | |
|---|---|---|---|
| Hormonal changes | |||
| TSH (mIU/L) | 16.9 ± 0.74 | 27.3 ± 0.87 | Increased |
| T4 (ng/mL) | 12.6 ± 0.22 | 7.6 ± 0.39 | Decreased |
| T3 (ng/mL) | 0.19 ± 0.004 | 0.12 ± 0.01 | Decreased |
| rT3 (ng/mL) | 0.014 ± 0.004 | 0.20 ± 0.02 | Increased |
| Physiological parameter changes | |||
| Body temperature (°C) | 36.7 ± 0.05 | 35.9 ± 0.09 | Decreased |
| Body weight (g) | 44 ± 0.54 | 47 ± 0.34 | Increased |
| Normalized thyroid gland area (mm2/g) | 0.06 ± 0.005 | 0.03 ± 0.004 | Decreased |
| Changes in gene expression in the thyroid gland | |||
| TSH-R | 0.007 ± 0.001 | 0.003 ± 0.001 | Decreased |
| DIO-1 | 0.03 ± 0.005 | 0.01 ± 0.001 | Decreased |
| DIO-2 | 0.004 ± 0.001 | 0.001 ± 0.001 | Decreased |
| TPO | 0.09 ± 0.02 | 0.01 ± 0.008 | Decreased |
| DUOX-1 | 0.002 ± 0.001 | 0.004 ± 0.001 | Increased |
| NIS | 0.008 ± 0.002 | 0.003 ± 0.001 | Decreased |
| MCT-8 | 0.007 ± 0.001 | 0.004 ± 0.001 | Decreased |
| Gene Name | Genetic Alterations | Consequence | Reference |
|---|---|---|---|
| TSHR |
| Loss of Function | [26,27,28] |
| TPO |
| Loss of Function | [29,30,31,32,33] |
| NIS |
| Loss of Function | [34,35] |
| MCT-8 |
| Loss of Function | [36] |
| DUOX-1 |
| Loss of Function | [37] |
| Hormone | Observation in Hypothyroidism | Reference |
|---|---|---|
| Beta-carotene |
| [42] |
| Retinol |
| [39,43] |
| Insulin |
| [38,44] |
| Adipokines |
| [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najjar, F.; Nhieu, J.; Wei, C.-W.; Milbauer, L.; Burmeister, L.; Seelig, D.; Wei, L.-N. Deleting Cellular Retinoic-Acid-Binding Protein-1 (Crabp1) Gene Causes Adult-Onset Primary Hypothyroidism in Mice. Endocrines 2023, 4, 138-150. https://doi.org/10.3390/endocrines4010013
Najjar F, Nhieu J, Wei C-W, Milbauer L, Burmeister L, Seelig D, Wei L-N. Deleting Cellular Retinoic-Acid-Binding Protein-1 (Crabp1) Gene Causes Adult-Onset Primary Hypothyroidism in Mice. Endocrines. 2023; 4(1):138-150. https://doi.org/10.3390/endocrines4010013
Chicago/Turabian StyleNajjar, Fatimah, Jennifer Nhieu, Chin-Wen Wei, Liming Milbauer, Lynn Burmeister, Davis Seelig, and Li-Na Wei. 2023. "Deleting Cellular Retinoic-Acid-Binding Protein-1 (Crabp1) Gene Causes Adult-Onset Primary Hypothyroidism in Mice" Endocrines 4, no. 1: 138-150. https://doi.org/10.3390/endocrines4010013
APA StyleNajjar, F., Nhieu, J., Wei, C.-W., Milbauer, L., Burmeister, L., Seelig, D., & Wei, L.-N. (2023). Deleting Cellular Retinoic-Acid-Binding Protein-1 (Crabp1) Gene Causes Adult-Onset Primary Hypothyroidism in Mice. Endocrines, 4(1), 138-150. https://doi.org/10.3390/endocrines4010013






