Neuroendocrine Blockade of the Reproductive Axis in Female Athletes
Abstract
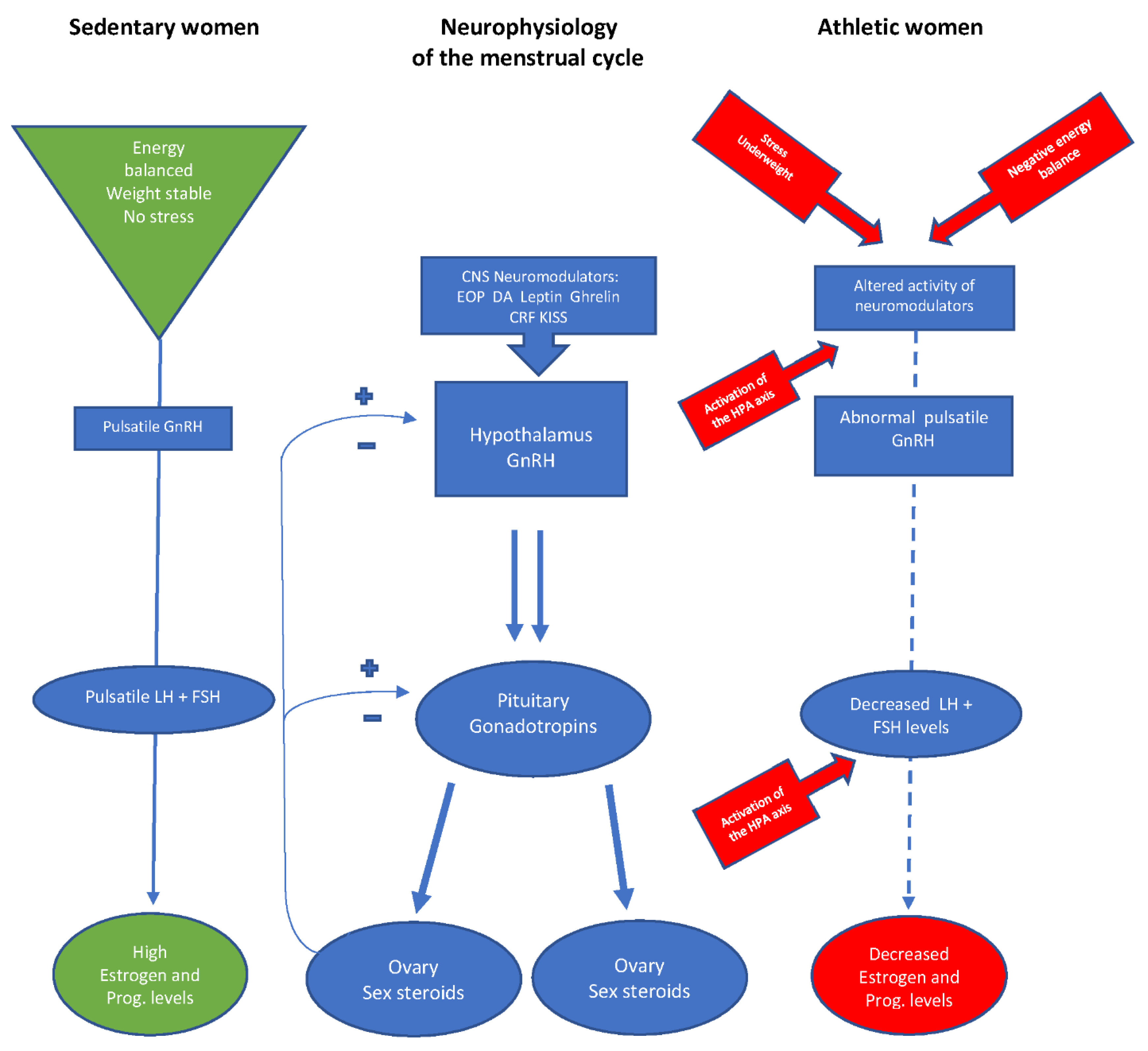
1. Introduction
1.1. Neuroendocrinology of Reproduction
1.2. The Hypothalamic-Pituitary-Ovarian (HPO) Axis in Athletic Women
1.3. Impact of Stress on Reproductive Function
1.4. The Relative Importance of Weight for the Reproductive Axis of Athletic Women
1.5. Energy Availability and the Function of the HPO Axis
2. Conclusions
Funding
Conflicts of Interest
References
- Yen, S.S.C. Chapter Chronic anovulation due to CNS-hypothalamic-pituitary dysfunction. In Yen & Jaffe’s Reproductive Endocrinology: Physiology, Physiopathology and Clinical Management, 4th ed.; Yen, S.S.C., Jaffe, R.B., Barbieri, R.L., Eds.; Saunders: Philadelphia, PA, USA, 1999; pp. 479–561. [Google Scholar]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [PubMed]
- Misra, M. Neuroendocrine mechanism in athletes. Handb. Clin. Neurol. 2014, 124, 373–386. [Google Scholar] [PubMed]
- Hall, J.E. Chapter Neuroendocrine control of the menstrual cycle. In Yen & Jaffe´s Reproductive Endocrinology, 5th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2004; pp. 149–166. [Google Scholar]
- Nass, R.; Helm, K.D.; Evans, W.S. Chapter 21: Physiological and Pathophysiological Alterations of the Neuroendocrine Components of the Reproductive Axis. In Yen & Jaffe´s Reproductive Endocrinology, 7th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014; pp. 439–484. [Google Scholar]
- McCartney, C.R.; Marshall, J.C. Neuroendocrinology of Reproduction. In Yen & Jaffe´s Reproductive Endocrinology, 8th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2019; pp. 1–45. [Google Scholar]
- Wildt, L.; Knobil, E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 1981, 109, 376–385. [Google Scholar] [CrossRef]
- Yen, S.S.C. Chapter Neuroendocrinology of reproduction. In Yen & Jaffe´s Reproductive Endocrinology, 4th ed.; Yen, S.S.S., Jaffe, R.B., Barbieri, R.L., Eds.; Saunders: Philadelphia, PA, USA, 1999; pp. 30–80. [Google Scholar]
- Rossmanith, W.G.; Liu, C.H.; Laughlin, G.A.; Mortola, J.F.; Suh, B.Y.; Yen, S.S.C. Relative changes in LH pulsatility during the menstrual cycle: Using data from hypogonadal women as a reference point. Clin. Endocrinol. 1990, 32, 647–660. [Google Scholar] [CrossRef]
- Rossmanith, W.G.; Yen, S.S.C. Sleep-associated decrease of luteinizing hormone pulse frequency during the early follicular phase of the menstrual cycle: Evidence for an opioidergic mechanism. J. Clin. Endocrinol. Metab. 1987, 65, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.L.; Yen, S.S.S. Chapter Reproductive failure due to central nervous system-hypothalamic-pituitary-dysfunction. In Yen & Jaffe´s Reproductive Endocrinology: Physiology, Physiopathology and Clinical Management, 4th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Saunders: Philadelphia, PA, USA, 1999; pp. 537–596. [Google Scholar]
- De Souza, M.J.; Miller, B.E.; Loucks, A.B.; Luciano, A.A.; Pescatello, L.S.; Campbell, C.G.; Lasley, B.E. High frequency of luteal phase deficiency and anovulation in recreational women runner: Blunted elevation in follicle-stimulating hormone observed during the luteal-follicular transition. J. Clin. Endocrinol. Metab. 1998, 83, 4220–4232. [Google Scholar]
- Nass, R.; Evans, W.S. Chapter 1. Physiologic and Pathophysiologic Alterations of the Neuroendocrine Components of the Reproductive Axis. In Yen & Jaffe´s Reproductive Endocrinology, 8th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2019; pp. 473–519. [Google Scholar]
- Loucks, A.B. Energy availability and infertility. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 470–474. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Yen, S.S.C. Nutritional and endocrine-metabolic aberrations in amenorrheic athletes. J. Clin. Endocrinol. Metab. 1996, 81, 4301–4309. [Google Scholar]
- Loucks, A.B. Low energy availability in the marathon and other endurance sports. Sports Med. 2007, 37, 248–252. [Google Scholar] [CrossRef]
- Loucks, A.B.; Mortola, J.F.; Girton, L.; Yen, S.S.C. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J. Clin. Endocrinol. Metab. 1989, 68, 402–441. [Google Scholar] [CrossRef]
- Loucks, A.B.; Heath, E.M. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J. Clin. Endocrinol. Metab. 1994, 78, 910–915. [Google Scholar] [PubMed]
- Yahiro, J.; Glass, A.R.; Fears, W.B.; Ferguson, E.W.; Vigersky, R.A. Exaggerated gonadotropin response to luteinizing hormone-releasing hormone in amenorrheic runners. Am. J. Obstet. Gynecol. 1987, 156, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Dominguez, C.E.; Yen, S.S.C. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 1998, 83, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Redman, L.M. The effect of stress on menstrual function. Trends Endocrinol. Metab. 2004, 15, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Castellano, J.M.; Fernandez-Fernandez, R.; Tovar, S.; Roa, J.; Mayen, A.; Nogueiras, R.; Vazquez, M.J.; Barreiro, M.L.; Magni, P.; et al. Characterization of the potent luteinizing hormone-releasing activity of KISS-1 peptide the natural ligand of GPR. Endocrinology 2005, 146, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.Y.; Liu, J.H.; Berga, S.L.; Quigley, M.E.; Laughlin, G.A.; Yen, S.S.C. Hypercortisolism in patients with hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 1988, 66, 733–739. [Google Scholar] [CrossRef]
- Rickenlund, A.; Thoren, M.; Carlstrom, K.; von Schultz, B.; Hirschberg, A.L. Diurnal profiles of testosterone and pituitary hormones suggest different mechanisms for menstrual disturbances in endurance athletes. J. Clin. Endocrinol. Metab. 2004, 89, 702–707. [Google Scholar] [CrossRef]
- Breen, K.M.; Davis, T.L.; Doro, L.C. Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 2008, 149, 767–773. [Google Scholar] [CrossRef]
- Herod, S.M.; Pohl, C.R.; Cameron, J.L. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E19–E27. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petraglia, F.; Gastaldi, F.; Volpogni, C.; Gamba, O.; Genazzani, A.R. Naltrexone treatment restores menstrual cycles in patients with weight loss-related amenorrhea. Fertil. Steril. 1995, 64, 951–956. [Google Scholar] [CrossRef]
- Nichols, J.F.; Rauh, M.J.; Barrack, M.T.; Barkai, H.S.; Pernick, Y. Disordered eating and menstrual irregularity in high school athletes in lean-build and non-lean-build sports. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, C.; Martin, B.; Wagner, W.J. Is athletic amenorrhea specific to runners? Am. J. Obstet. Gynecol. 1982, 143, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Fichter, M.M.; Pirke, K.M. Starvation models and eating disorders. In Handbook of Eating Disorders, 1st ed.; Szmukler, G.I., Dare, C., Treasure, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1995; pp. 83–107. [Google Scholar]
- Loucks, A.B. Energy availability, not body fatness, regulates reproductive function in women. Exerc. Sport Sci. Rev. 2003, 31, 144–148. [Google Scholar] [CrossRef]
- Frisch, R.E.; McArthur, J.W. Menstrual cycles: Fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 1974, 185, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Christo, K.; Cord, J.; Mendes, N.; Miller, K.K.; Goldstein, M.A.; Klibanski, A.; Misra, M. Acetylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: A cross-sectional study. Clin. Endocrinol. (Oxf) 2008, 69, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B. The response of luteinizing hormone pulsatility to 5 days of low energy availability disappears by 14 years of gynecological age. J. Clin. Endocrinol. Metab. 2006, 91, 3158–3164. [Google Scholar] [CrossRef]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef]
- Williams, N.I.; Young, J.C.; McArthur, J.W.; Bullen, B.; Skinner, G.S.; Turnbull, B. Strenuous exercise with caloric restriction: Effect on luteinizing hormone secretion. Med. Sci. Sports Exerc. 1995, 27, 1390–1398. [Google Scholar] [CrossRef]
- Clarke, I.; Burger, H. Leptin and reproduction. Rev. Reprod. 1999, 4, 48–55. [Google Scholar] [CrossRef]
- Moschos, S.; Chan, K.L.; Mantzoros, C.S. Leptin, and reproduction: A review. Fertil. Steril. 2002, 77, 433–444. [Google Scholar] [CrossRef]
- De Souza, M.J.; Leidy, H.J.; O´Donnell, E.; Lasley, B.; Williams, N.I. Fasting ghrelin levels in physically active women: Relationship with menstrual disturbances and metabolic hormones. J. Clin. Endocrinol. Metab. 2004, 89, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P.; Voussoughian, F.; Geer, E.B.; Hyle, E.P.; Edberg, C.L.; Ramos, R.H. Functional hypothalamic amenorrhea: Hyperleptinemia and disordered eating. J. Clin. Endocrinol. Metab. 1999, 84, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Welt, C.K.; Chan, J.L.; Bullen, J.; Murphy, R.; Smith, P.; De Paoli, A.M.; Karalis, A.; Mantzoros, C.S. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004, 351, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.E.; Fleming, S.; Levy, M.J. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise, or a combination of these factors. Clin. Endocrinol. 2021, 95, 229–238. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; van Heest, J.; Demers, L.M.; Lasley, B.L. Luteal phase deficiency in recreational runners: Evidence for a hypometabolic state. J. Clin. Endocrinol. Metab. 2003, 88, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Celik, O.; Aydin, S.; Celik, K.; Yilmaz, M. Peptides: Basic determinants of reproductive functions. Peptides 2015, 72, 34–43. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Yano, K.; Mayila, Y.; Yanagihara, R.; Yamamoto, Y.; Kuwahara, A.; Irahara, M. Effects of low energy availability on reproductive functions and their underlying neuroendocrine mechanisms. J. Clin. Med. 2018, 5, 166. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Loucks, A.B.; Verdun, M.; Heath, E.M. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J. Appl. Physiol. 1998, 84, 37–46. [Google Scholar] [CrossRef]
- Lieberman, J.L.; de Souza, M.J.; Wagstaff, D.A.; Williams, N.I. Menstrual disruption with exercise is not linked to an energy availability threshold. Med. Sci. Sports Exerc. 2018, 50, 551–561. [Google Scholar] [CrossRef]
- Gifford, R.M.; O’Leary, T.J.; Wardle, S.L.; Double, R.L.; Homer, N.Z.M.; Howie, A.F.; Greeves, J.P.; Anderson, R.A.; Woods, D.R.; Reynolds, R.M. Reproductive and metabolic adaptation to multistressor training in women. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E281–E291. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.I.; Statuta, S.M.; Austin, A. Female Athlete Triad: Future Directions for Energy Availability and Eating Disorder Research and Practice. Clin. Sports Med. 2017, 36, 671–686. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; West, S.L.; Jamal, S.A.; Hawker, G.A.; Gundberg, C.M.; Williams, N.I. The presence of both an energy deficiency and estrogen deficiency exacerbates alterations of bone metabolism in exercising women. Bone 2008, 43, 140–148. [Google Scholar] [CrossRef]
- Stafford, D.E.J. Altered hypothalamic-pituitary-ovarian axis function in young female athletes: Implications and recommendations for management. Treat. Endocrinol. 2005, 4, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Dipla, K.; Kraemer, R.R.; Constantini, N.W.; Hackney, A.C. Relative energy deficiency in sports (RED-S): Elucidation of endocrine changes affecting the health of males and females. Hormones 2021, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.S.C. Reproductive strategies in women: Neuroendocrine basis for endogenous contraception. In Neuroendocrinology of Reproduction; Roland, R., Ed.; Excerpta Medica Amsterdam: Amsterdam, The Netherlands, 1988; pp. 231–239. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossmanith, W.G. Neuroendocrine Blockade of the Reproductive Axis in Female Athletes. Endocrines 2022, 3, 765-774. https://doi.org/10.3390/endocrines3040063
Rossmanith WG. Neuroendocrine Blockade of the Reproductive Axis in Female Athletes. Endocrines. 2022; 3(4):765-774. https://doi.org/10.3390/endocrines3040063
Chicago/Turabian StyleRossmanith, Winfried G. 2022. "Neuroendocrine Blockade of the Reproductive Axis in Female Athletes" Endocrines 3, no. 4: 765-774. https://doi.org/10.3390/endocrines3040063
APA StyleRossmanith, W. G. (2022). Neuroendocrine Blockade of the Reproductive Axis in Female Athletes. Endocrines, 3(4), 765-774. https://doi.org/10.3390/endocrines3040063





