Cost-Effectiveness of Screening to Identify Pre-Diabetes and Diabetes in the Oral Healthcare Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. The Intervention
2.2. Study Population
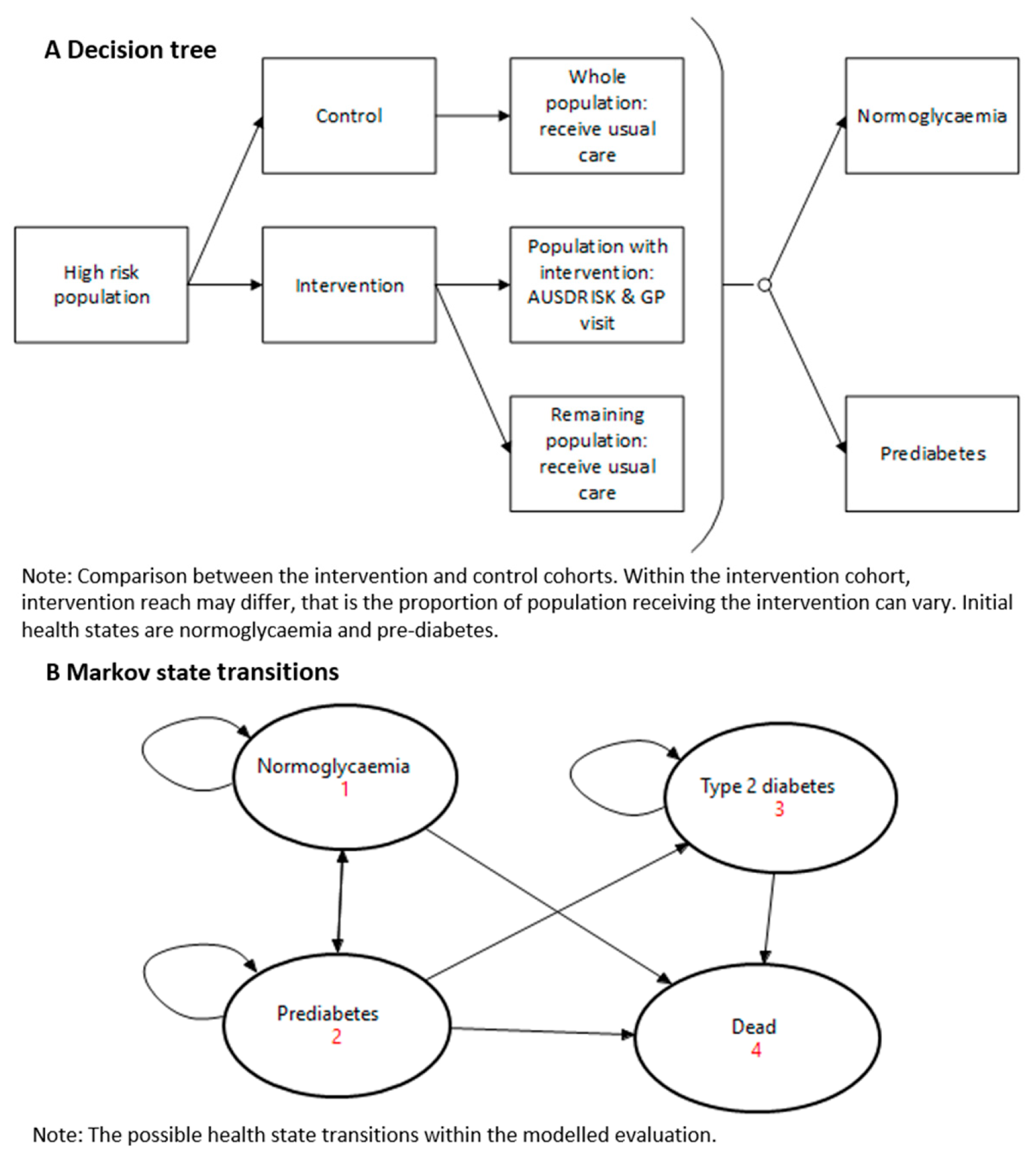
2.3. Structure of the Simulation Model
2.4. Model Inputs
2.4.1. Transition Probabilities
2.4.2. Costs
2.4.3. Utility
2.5. Cost-Effectiveness Analysis
2.6. Sensitivity Analysis
3. Results
3.1. Study Population
3.2. Modelled Cost-Effectiveness Analysis
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preshaw, P.M.; Bissett, S.M. Periodontitis: Oral complication of diabetes. Endocrinol. Metab. Clin. 2013, 42, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Hayes, C.; Taylor, G.W. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent. Oral Epidemiol. 2002, 30, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Curletto, G.; Cipullo, D.; Rigault de la Longrais, R.; Trento, M.; Passera, P.; Taulaigo, A.V.; Di Miceli, S.; Cenci, A.; Dalmasso, P.; et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care 2014, 37, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Estrich, C.G.; Araujo, M.W.B.; Lipman, R.D. Prediabetes and Diabetes Screening in Dental Care Settings: NHANES 2013 to 2016. JDR Clin. Trans. Res. 2019, 4, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Yonel, Z.; Batt, J.; Jane, R.; Cerullo, E.; Gray, L.J.; Dietrich, T.; Chapple, I. The Role of the Oral Healthcare Team in Identification of Type 2 Diabetes Mellitus: A Systematic Review. Current Oral Health Rep. 2020, 7, 87–97. [Google Scholar] [CrossRef]
- Mariño, R.; Priede, A.; King, M.; Adams, G.G.; Sicari, M.; Morgan, M. Oral health professionals screening for undiagnosed type-2 diabetes and prediabetes: The iDENTify study. BMC Endocr. Disord. 2022, 22, 183. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Ageing. The Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK); Australian Government Department of Health and Ageing: Canberra, ACT, Australia, 2010.
- Marino, R.; King, M.; Sicari, M.; Adams, G.; Andre, P. Improving diabetes outcomes by screening for undiagnosed diabetes in dental settings. In Proceedings of the Public Health Prevention Conference, Melbourne, VIC, Australia, 12–14 June 2019. [Google Scholar]
- Wong, K.; Brown, A.; Li, S. AUSDRISK Application in general practice. Aust. Fam. Physician 2011, 40, 524–526. [Google Scholar]
- Chen, L.; Magliano, D.J.; Balkau, B.; Colagiuri, S.; Zimmet, P.Z.; Tonkin, A.M.; Mitchell, P.; Phillips, P.J.; Shaw, J.E. AUSDRISK: An Australian Type 2 Diabetes Risk Assessment Tool based on demographic, lifestyle and simple anthropometric measures. Med. J. Aust. 2010, 192, 197–202. [Google Scholar] [CrossRef]
- Cheung, M.C.; Mittmann, N.; Owen, C.; Abdel-Samad, N.; Fraser, G.A.M.; Lam, S.; Crump, M.; Sperlich, C.; van der Jagt, R.; Prica, A.; et al. A Prospective Economic Analysis of Early Outcome Data From the Alliance A041202/CCTG CLC.2 Randomized Phase III Trial of Bendamustine-Rituximab Compared With Ibrutinib-Based Regimens in Untreated Older Patients With Chronic Lymphocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2021, 21, 766–774. [Google Scholar] [CrossRef]
- Glümer, C.; Yuyun, M.; Griffin, S.; Farewell, D.; Spiegelhalter, D.; Kinmonth, A.L.; Wareham, N.J. What determines the cost-effectiveness of diabetes screening? Diabetologia 2006, 49, 1536–1544. [Google Scholar] [CrossRef]
- Medical Services Advisory Committee. Technical Guidelines for Preparing Assessment Reports for the Medical SERVICES Advisory Committee—Service Type: Investigative; Department of Health and Aged Care: Canberra, ACT, Australia, 2017.
- Dunstan, D.W.; Zimmet, P.Z.; Welborn, T.A.; De Courten, M.P.; Cameron, A.J.; Sicree, R.A.; Dwyer, T.; Colagiuri, S.; Jolley, D.; Knuiman, M.; et al. The rising prevalence of diabetes and impaired glucose tolerance: The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002, 25, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. 3302.0.55.001—Life Tables, States, Territories and Australia, 2016–2018. 2019. Available online: https://www.abs.gov.au/statistics/people/population/life-tables/2016-2018 (accessed on 21 October 2022).
- Stokes, A.; Mehta, N.K. Mortality and excess risk in US adults with pre-diabetes and diabetes: A comparison of two nationally representative cohorts, 1988-2006. Popul. Health Metr. 2013, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Medicare Benefits Schedule. Item 23. Available online: http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&qt=ItemID&q=23 (accessed on 6 October 2022).
- Medicare Benefits Schedule. Item 66542. Available online: http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=66542&qt=item (accessed on 6 October 2022).
- Dunbar, J.A.; Jayawardena, A.; Johnson, G.; Roger, K.; Timoshanko, A.; Versace, V.L.; Shill, J.; Philpot, B.; Vartiainen, E.; Laatikainen, T.; et al. Scaling up diabetes prevention in Victoria, Australia: Policy development, implementation, and evaluation. Diabetes Care 2014, 37, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.Y.; Colagiuri, R.; Magliano, D.J.; Cameron, A.J.; Shaw, J.; Zimmet, P.; Colagiuri, S. The cost of diabetes in adults in Australia. Diabetes Res. Clin. Pract. 2013, 99, 385–390. [Google Scholar] [CrossRef]
- EuroQol Research Foundation. EQ-5D-5L User Guide. 2019. Available online: https://euroqol.org/publications/user-guides (accessed on 6 October 2022).
- Redekop, W.K.; Koopmanschap, M.A.; Stolk, R.P.; Rutten, G.E.; Wolffenbuttel, B.H.; Niessen, L.W. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care 2002, 25, 458–463. [Google Scholar] [CrossRef]
- Janssen, M.F.; Szende, A.; Cabases, J.; Ramos-Goñi, J.M.; Vilagut, G.; König, H.H. Population norms for the EQ-5D-3L: A cross-country analysis of population surveys for 20 countries. Eur. J. Health Econ. 2019, 20, 205–216. [Google Scholar] [CrossRef]
- Makrilakis, K.; Liatis, S.; Tsiakou, A.; Stathi, C.; Papachristoforou, E.; Perrea, D.; Katsilambros, N.; Kontodimopoulos, N.; Niakas, D. Comparison of health-related quality of Life (HRQOL) among patients with pre-diabetes, diabetes and normal glucose tolerance, using the 15D-HRQOL questionnaire in Greece: The DEPLAN study. BMC Endocr. Disord. 2018, 18, 32. [Google Scholar] [CrossRef]
- García-Molina, M.; Chicaíza-Becerra, L.A.; Quitian-Reyes, H.; Linares, A.; Ramírez, O. Cost-effectiveness of risk stratification tests for the treatment of acute myeloid leukemia in pediatric patients. Rev. Salud Pública 2017, 19, 657–663. [Google Scholar] [CrossRef]
- Australian Dentists Directory. Melbourne Dentists and Victorian Dentists. Available online: https://www.australiandentistsdirectory.com.au/melbourne-dentists-and-victorian-dentists#:~:text=There%20are%201701%20Victorian%20Dentists,Melbourne%20CBD%20and%20nearby%20suburbs (accessed on 6 October 2022).
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013, 346, f1049. [Google Scholar] [CrossRef]
- Wang, S.; Gum, D.; Merlin, T. Comparing the ICERs in Medicine Reimbursement Submissions to NICE and PBAC—Does the Presence of an Explicit Threshold Affect the ICER Proposed? Value Health 2018, 21, 938–943. [Google Scholar] [CrossRef]
- Neidell, M.; Lamster, I.B.; Shearer, B. Cost-effectiveness of diabetes screening initiated through a dental visit. Community Dent Oral Epidemiol. 2017, 45, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Barry, E.; Craig, D.; Airoldi, M.; Bevan, G.; Greenhalgh, T. Preventing type 2 diabetes: Systematic review of studies of cost-effectiveness of lifestyle programmes and metformin, with and without screening, for pre-diabetes. BMJ Open 2017, 7, e017184. [Google Scholar] [CrossRef] [PubMed]
- Barasch, A.; Gilbert, G.H.; Spurlock, N.; Funkhouser, E.; Persson, L.L.; Safford, M.M.; Group, D.C. Random plasma glucose values measured in community dental practices: Findings from the Dental Practice-Based Research Network. Clin. Oral Investig. 2013, 17, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Barasch, A.; Safford, M.M.; Qvist, V.; Palmore, R.; Gesko, D.; Gilbert, G.H.; Dental Practice-Based Research Network Collaborative Group. Random blood glucose testing in dental practice: A community-based feasibility study from The Dental Practice-Based Research Network. J. Am. Dent. Assoc. 2012, 143, 262–269. [Google Scholar] [CrossRef]
- Lalla, E.; Kunzel, C.; Burkett, S.; Cheng, B.; Lamster, I.B. Identification of unrecognized diabetes and pre-diabetes in a dental setting. J. Dent. Res. 2011, 90, 855–860. [Google Scholar] [CrossRef]
- Genco, R.J.; Schifferle, R.E.; Dunford, R.G.; Falkner, K.L.; Hsu, W.C.; Balukjian, J. Screening for diabetes mellitus in dental practices: A field trial. J. Am. Dent. Assoc. 2014, 145, 57–64. [Google Scholar] [CrossRef]
- Caro, J.J.; Getsios, D.; Caro, I.; Klittich, W.S.; O’Brien, J.A. Economic evaluation of therapeutic interventions to prevent Type 2 diabetes in Canada. Diabet Med. 2004, 21, 1229–1236. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Meigs, J.B.; Muller, D.C.; Nathan, D.M.; Blake, D.R.; Andres, R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003, 52, 1475–1484. [Google Scholar] [CrossRef]
- Balk, E.M.; Earley, A.; Raman, G.; Avendano, E.A.; Pittas, A.G.; Remington, P.L. Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann. Intern. Med. 2015, 163, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Craig, D.; Adler, A.; McPherson, K.; Greenhalgh, T. Economic evaluation of type 2 diabetes prevention programmes: Markov model of low- and high-intensity lifestyle programmes and metformin in participants with different categories of intermediate hyperglycaemia. BMC Med. 2018, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, P.W.; Milder, I.E.; Wielaard, F.; de Vries, J.H.M.; Baan, C.A.; van Oers, J.A.M.; Westert, G.P. A lifestyle intervention to reduce Type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet. Med. 2012, 29, e223–e231. [Google Scholar] [CrossRef] [PubMed]
- Ashra, N.; Spong, R.; Carter, P.; Davies, M.; Dunkley, A.; Gillies, C.; Greaves, C.; Khunti, K.; Sutton, S.; Yates, T. A Systematic Review and Metaanalysis Assessing the Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes Mellitus in Routine Practice; Public Health England: London, UK, 2015. [Google Scholar]


| Parameter | Value | Range for Sensitivity | Distribution |
|---|---|---|---|
| Probabilities | |||
| Proportion of patients with pre-diabetes in the high risk population, identified via intervention | 0.0625 | ||
| Proportion of patients with pre-diabetes in the high risk population, according to literature | 0.212 [12] | (0.152–0.232) | |
| Transition probability for normoglycaemia to pre-diabetes (no treatment) | 0.05065 [13] | ||
| Transition probability for pre-diabetes to normoglycaemia (no treatment) | 0.08969 [14,15] | ||
| Transition probability for pre-diabetes to diabetes (no treatment) | 0.11 [16] | (0.098–0.123) | Beta (alpha: 88.89, beta: 719.2) |
| Relative risk for the transition of pre-diabetes to normoglycaemia (due to lifestyle changes) | 1.4 [15,17] | ||
| Relative risk for the transition of pre-diabetes to diabetes (due to lifestyle changes) | 0.74 [18] | (0.58, 0.93) | Gamma (alpha: 100, lambda: 135.14) |
| Relative risk of mortality for pre-diabetes | 2.32 [20] | (1.24–3.40) | |
| Relative risk of mortality for type 2 diabetes | 3.45 [20] | (2.02–4.87) | Gamma (alpha: 100, lambda: 28.986) |
| Costs ($) | |||
| Implementing the intervention per high risk patient identified | $60 | Gamma (alpha: 100, lambda: 1.674) | |
| General practitioner visit | $38.75 [21] | ||
| Oral glucose tolerance test | $18.95 [22] | ||
| Pragmatic lifestyle intervention per high risk patient identified | $433.85 [24] | ||
| Annual direct medical cost per person with normoglycaemia | $2635 [23] | ||
| Annual direct medical cost per person with pre-diabetes | $2875 [23] | ||
| Annual direct medical cost per person with type 2 diabetes | $6091 [23] | Gamma (alpha: 100, lambda: 0.0164) | |
| Utilities | |||
| Normoglycaemia health state | 0.89 [25] | ||
| Pre-diabetes health state | 0.88 [26] | ||
| Type 2 diabetes health state | 0.78 [27] | SD:0.25 | Beta (alpha: 21.22, beta: 5.985) |
| Participants | % | |
|---|---|---|
| Wave | ||
| 1 | 305 | 38.1 |
| 2 | 496 | 61.9 |
| Participant Location | ||
| Metropolitan | 576 | 72.0 |
| Rural | 225 | 28.0 |
| Sex | ||
| Female | 491 | 61.4 |
| Male | 309 | 38.6 |
| Age Group | ||
| 34–44 years | 150 | 18.7 |
| 45–54 | 207 | 25.8 |
| 55–64 | 200 | 25.0 |
| 65–74 | 168 | 21.0 |
| 75 and more | 76 | 9.5 |
| Total | 801 | 100.0 |
| Total Cost | Medical Cost | Non-Medical | Total QALY | Number of T2D ^ | |
|---|---|---|---|---|---|
| Current practice | $38,469 * | $28,687 | $9783 | 10.561 | 3697 |
| 10% of intervention reach | |||||
| iDENTify | $38,462 | $28,686 | $9776 | 10.564 | 3689 |
| Difference | −$7.9 | −$1.1 | −$6.8 | 0.003 | 8 |
| ICER | Dominant | ||||
| 20% of intervention reach | |||||
| iDENTify | $38,454 | $28,684 | $9769 | 10.567 | 3680 |
| Difference | −$15.7 | −$2.2 | −$13.5 | 0.005 | 17 |
| ICER | Dominant | ||||
| 30% of intervention reach | |||||
| iDENTify | $38,446 | $28,683 | $9762 | 10.569 | 3672 |
| Difference | −$23.6 | −$3.3 | −$20.3 | 0.009 | 25 |
| ICER | Dominant | ||||
| 40% of intervention reach | |||||
| iDENTify | $38,438 | $28,682 | $9756 | 10.572 | 3663 |
| Difference | −$28.3 | −$1.2 | −$27.1 | 0.011 | 34 |
| ICER | Dominant | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Tan, E.; Mariño, R.; King, M.; Priede, A.; Adams, G.; Sicari, M.; Moodie, M. Cost-Effectiveness of Screening to Identify Pre-Diabetes and Diabetes in the Oral Healthcare Setting. Endocrines 2022, 3, 753-764. https://doi.org/10.3390/endocrines3040062
Gao L, Tan E, Mariño R, King M, Priede A, Adams G, Sicari M, Moodie M. Cost-Effectiveness of Screening to Identify Pre-Diabetes and Diabetes in the Oral Healthcare Setting. Endocrines. 2022; 3(4):753-764. https://doi.org/10.3390/endocrines3040062
Chicago/Turabian StyleGao, Lan, Elise Tan, Rodrigo Mariño, Michelle King, Andre Priede, Geoff Adams, Maria Sicari, and Marj Moodie. 2022. "Cost-Effectiveness of Screening to Identify Pre-Diabetes and Diabetes in the Oral Healthcare Setting" Endocrines 3, no. 4: 753-764. https://doi.org/10.3390/endocrines3040062
APA StyleGao, L., Tan, E., Mariño, R., King, M., Priede, A., Adams, G., Sicari, M., & Moodie, M. (2022). Cost-Effectiveness of Screening to Identify Pre-Diabetes and Diabetes in the Oral Healthcare Setting. Endocrines, 3(4), 753-764. https://doi.org/10.3390/endocrines3040062








