Abstract
Similar to previous pandemics, COVID-19 has been succeeded by well-documented post-infectious sequelae, including chronic fatigue, cough, shortness of breath, myalgia, and concentration difficulties, which may last 5 to 12 weeks or longer after the acute phase of illness. Both the psychological stress of SARS-CoV-2 infection and being diagnosed with COVID-19 can upregulate cortisol, a stress hormone that disrupts the efferocytosis effectors, macrophages, and natural killer cells, leading to the excessive accumulation of senescent cells and disruption of biological barriers. This has been well-established in cancer patients who often experience unrelenting fatigue as well as gut and blood–brain barrier dysfunction upon treatment with senescence-inducing radiation or chemotherapy. In our previous research from 2020 and 2021, we linked COVID-19 to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) via angiotensin II upregulation, premature endothelial senescence, intestinal barrier dysfunction, and microbial translocation from the gastrointestinal tract into the systemic circulation. In 2021 and 2022, these hypotheses were validated and SARS-CoV-2-induced cellular senescence as well as microbial translocation were documented in both acute SARS-CoV-2 infection, long COVID, and ME/CFS, connecting intestinal barrier dysfunction to disabling fatigue and specific infectious events. The purpose of this narrative review is to summarize what is currently known about host immune responses to translocated gut microbes and how these responses relate to fatiguing illnesses, including long COVID. To accomplish this goal, we examine the role of intestinal and blood–brain barriers in long COVID and other illnesses typified by chronic fatigue, with a special emphasis on commensal microbes functioning as viral reservoirs. Furthermore, we discuss the role of SARS-CoV-2/Mycoplasma coinfection in dysfunctional efferocytosis, emphasizing some potential novel treatment strategies, including the use of senotherapeutic drugs, HMGB1 inhibitors, Toll-like receptor 4 (TLR4) blockers, and membrane lipid replacement.
1. Introduction
In the post-pandemic era, residual or long COVID-19 sequelae have been gradually emerging as many patients experience prolonged fatigue, cough, shortness of breath, myalgia, and problems with concentration long after the acute illness phase [1]. From a biological pathway perspective, as both SARS-CoV-2 infection and the associated psychological stress upregulate cortisol, the function of macrophages and natural killer (NK) cells may be impaired, disrupting the clearance of senescent, damaged, or virus-infected cells. This may lead to biological barrier dysfunction and chronic fatigue, phenomena well-documented in cancer survivors treated with cellular senescence-inducing chemotherapy or radiation [2,3,4,5].
At the molecular level, upregulated cortisol lowers the expression of claudin-1 (CLDN1), an intestinal tight junction protein, facilitating microbial translocation outside of the gastrointestinal (GI) tract [6]. In the central nervous system (CNS), P-glycoprotein (P-gp), a cortisol substrate, functions as a gatekeeper of the blood–brain barrier (BBB), likely accounting for hypercortisolemia-increased barrier permeability [7,8,9]. Interestingly, in the GI tract, gut microbiota and lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria, regulate P-gp, connecting this protein to microbial translocation [10,11].
Recent studies have found a direct relationship between circulating levels of cortisol and premature cellular senescence, a phenotype characterized by permanent cell cycle arrest, active metabolism, and a detrimental secretome [12]. The accumulation of senescent cells was found to be associated not only with organismal aging but also with chronic fatigue, pain, and depression, documented in cancer survivors [2]. On the other hand, enhanced elimination of senescent cells via senotherapeutics can correct barrier dysfunction, lowering fatigue [13,14,15].
NK cells and macrophages can execute the phagocytic engulfment (efferocytosis) of damaged or dead cells, including malignant, virus-infected, and senescent cells. Dysfunctional efferocytosis has been associated with biological barrier disruption, inflammatory bowel disease (IBD), and a constellation of symptoms reminiscent of long COVID and other fatiguing illnesses [16,17,18,19,20,21]. Indeed, NK cell dysfunction is one of the most consistent findings in long COVID-19, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Gulf War illnesses (GWI), and fibromyalgia (FM), linking these pathologies to dysfunctional efferocytosis [22,23,24,25,26,27]. Moreover, NK cells had been previously linked to fatigue-associated thyroid, adrenal, hypothalamic, and pituitary disorders, thus connecting these neuroendocrine -related pathologies with dysfunctional immunity [28,29,30,31,32]. Furthermore, NK cells express estrogen, prolactin, and cortisol receptors as well as a functional renin-angiotensin-system (RAS), rendering them sensitive to hormonal fluctuations [33,34,35]. NK cells are capable of paracrine signaling and they secrete biomolecules, including perforin, granzyme B, and the high mobility group box 1 protein (HMGB1) that can facilitate the elimination of damaged, senescent, and/or malignant cells [36,37,38].
Microbial translocation markers, such as LPS, lipopolysaccharide binding protein (LPB), soluble CD14 (sCD14), and HMGB1, were found to be elevated in long COVID, highlighting the role of dysfunctional gut barrier in this condition [39,40,41]. Indeed, accumulation of senescent cells has been associated with HMGB1 spillover into the extracellular space where it can act as an inflammagen and barrier disruptor [42,43,44,45,46]. Therefore, upregulated HMGB1, documented in ME/CFS, FM, and GWI, and COVID-19, directly links dysfunctional efferocytosis to chronic fatigue [47,48,49,50]. For example, gut HMGB1 disrupts the barrier tight junctions and is considered a biomarker of inflammatory bowel disease (IBD), a condition associated with both fatigue and increased GI tract permeability. This may explain certain SARS-CoV-2 bacteriophage-like properties as this virus can likely penetrate the microbial cell walls [51,52,53,54,55,56,57].
In this article, we will take a closer look at the role of dysfunctional NK cells and efferocytosis in long COVID and other fatiguing illnesses. We also discuss the comorbidity of Mycoplasma species and SARS-CoV-2 as well as their coinfection, barrier rehabilitation via senotherapeutic drugs, HMGB1 inhibitors, Toll-like receptor 4 (TLR-4) blockers and membrane lipid replacement (MLR).
2. Efferocytosis and Biological Barriers
Each day billions of cells throughout the body undergo apoptosis and are removed by professional phagocytes, macrophages, monocytes, and neutrophils as well as non-professional phagocytes, including the intestinal epithelial cells (IECs) and the M2 microglia in the BBB [58,59,60]. Professional and non-professional phagocytes are assisted by NK cells that can eliminate defective and pathogen-infected cells without prior sensitization [61,62,63]. NK cells maintain the integrity of BBB and the intestinal barrier as they can promptly clear damaged cells, preventing inflammation and barrier disruption [64,65]. To accomplish this, NK cells perforate the membrane of targeted cells by releasing HMGB1, perforin, and granzyme, triggering apoptosis by Ca2+ influx [66,67]. Upregulated cytosolic Ca2+ is known to activate TMEM16F, an enzyme that flips phosphatidylserine (PS) to the outer leaflet of the cell membrane, providing a distress signal, that attracts immune cells, promoting phagocytosis [68]. Exposed PS (ePS) comprises an “eat me” or “fuse with me” signal that can lead to either cell death or syncytia formation, depending on the degree of cell membrane damage [69]. For example, less damaged cells can fuse with each other for protection, a phenomenon documented in in many tissues, including the CNS [70] (Figure 1). Several studies have demonstrated that senescent and cancer cells can avoid elimination by expressing CD47, a “don’t eat me” signal, that inhibits phagocytosis [71,72,73].
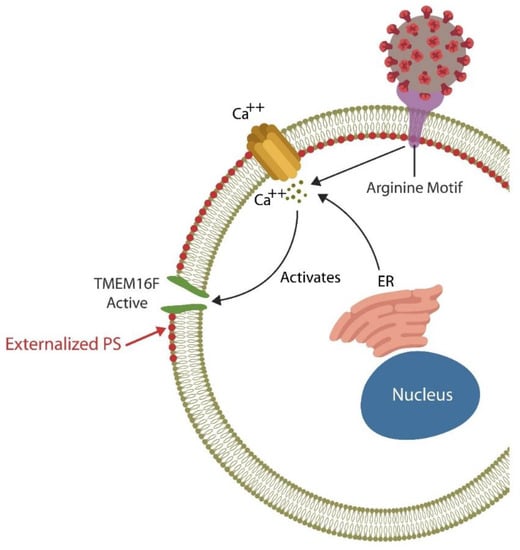
Figure 1.
The SARS-CoV-2 receptor binding site (RBS) contains a double arginine insert (PRRA) or arginine motif, that perforates the cell membrane, triggering Ca2+ influx from both the endoplasmic reticulum (ER) and the extracellular compartment. Upregulated cytosolic Ca2+ activates TMEN16F, externalizing phosphatidylserine (ePS), an “eat me” or “fuse me” signal that leads to cell death (if the damage is irreparable) or cell–cell fusion (if the cell can be repaired). Cell–cell fusion or syncytia formation induces premature cellular senescence, disrupting biological barriers. The virus benefits from ePS as this comprises a global immunosuppressive signal, allowing its undetected entry into host cells.
2.1. Blood–Brain Barrier
The BBB, a highly regulated interface between the circulatory system and the CNS, consists of cerebral endothelial cells (ECs) that regulate the inward and outward movement of molecules and ions into the CNS [74]. BBB disruption enables viral entry into the brain along with inflammatory cells, and/or deleterious molecules that can trigger infection. Indeed, members of at least 11 viral families, including the human immunodeficiency virus-1 (HIV-1), T-cell leukemia virus, lymphocytic choriomeningitis virus, West Nile virus, and others, can enter the brain, causing encephalitis [75,76,77].
COVID-19-mediated accumulation of senescent ECs compromises the BBB, allowing viral and microbial access to the CNS [78]. In contrast, enhanced elimination of senescent cells via senolytic drugs decreases COVID-19 mortality in rodents, highlighting the role of senescent cells in BBB dysfunction [79,80]. In addition, the S protein of the SARS-CoV-2 virus was demonstrated to directly bind bacterial LPS, outlining a virus-mediated mechanism of endotoxin entry into the CNS [80,81]. LPS-induced neuroinflammation has been associated with microglial fusion and multinucleation, generating highly phagocytic phenotypes that can cause collateral damage by eliminating viable neurons [82,83]. Indeed, long COVID has been associated with LPS-activated microglia (M1 phenotype), neuroinflammation and neuronal death [82,83,84,85]. On the other hand, the M2 microglial phenotype has been shown to repair the damage, protecting the neurons [58].
2.2. Intestinal Barrier
Many viruses, including SARS-CoV-2, enhance infectivity by usurping both the physiological cell–cell fusion and efferocytosis, disrupting biological barriers [75,86]. For example, the SARS-CoV-2 virus thrives in infected cells and likely inhibits their clearance, causing inflammation and barrier dysfunction [87]. In addition, SARS-CoV-2 promotes pathological cell–cell fusion and syncytia formation by generating cell membrane pores via the PRRA (proline-arginine-arginine-alanine) motif situated at the furin-cleavage site (FCS). This system is reminiscent of microbial twin arginine translocation pathway, a pore-forming mechanism implicated in bacterial virulence [88,89,90]. Moreover, SARS-CoV-2 fusion with Mycoplasma, an arginine dependent microorganism, may explain the high comorbidity of these very different infections [91]. Cell membrane pores lead to ePS, a global immunosuppressive signal, that helps the virus exploit host defenses [92]. The subsequent, syncytia formation can then induce premature cellular senescence and the release of senescence-associated secretory phenotype (SASP), a pathological secretome that disrupts endothelial barriers by promoting premature ECs senescence, a phenotype documented in both ME/CFS and COVID-19 [42,93,94,95].
Senescence-induced pathological syncytia can trigger lymphopenia by cell-in-cell phenomena, elimination of viable lymphocytes, including NK cells, a frequent finding in ME/CFS [96,97,98]. In addition, as cellular senescence upregulates HMGB1, it may further predispose to fatiguing disorders [44,47,48,49,99,100,101,102].
In the GI tract, IECs comprise a single layer of tightly linked columnar cells that are short-lived and need to be replaced every 4 to 5 days to maintain an adequate barrier function [103]. Moreover, IECs acting as non-professional phagocytes, can engulf the translocating microbes and/or antigens, preventing microbial translocation outside the GI tract [104]. However, accumulation of uncleared, defective IECs can trigger inflammation, predisposing to IBD and other illnesses marked by dysfunctional barrier [105]. Macrophages, intestinal NK cells, and Paneth cells contribute to barrier integrity by promptly removing damaged IECs, thus averting necrosis and inflammation-mediated pathology [21,106,107,108]. Interestingly, IECs were demonstrated to produce cortisol, a steroid hormone that lowers the expression of CLDN1 that in return increases intestinal permeability [109,110,111].
The role of ANG II: COVID-19-upregulated angiotensin II (ANG II) can disrupt efferocytosis inducing ECs senescence and vascular barrier dysfunction via angiotensin II type 1 receptors (AT-1Rs) which can enhance both cortisol and HMGB1 production, [112,113] (Figure 2).
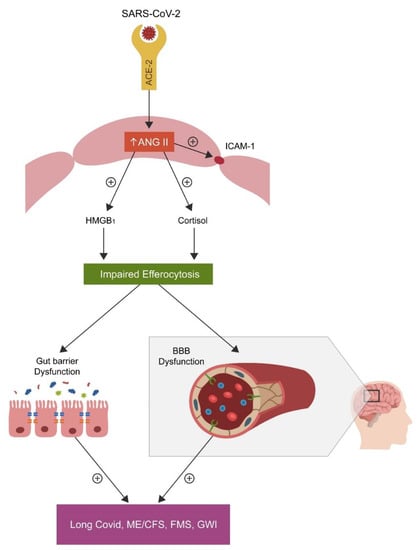
Figure 2.
SARS-CoV-2 attachment to ACE-2, blocks this enzyme, causing angiotensin II (ANG II) accumulation by inhibiting its hydrolysis. Upregulated ANG II, increases both cortisol and HMGB1, disrupting the efferocytosis of senescent cells. Accumulation of senescent cells triggers inflammation and biological barrier disruption, a common pathology found not only in the disorders marked by chronic fatigue but also in neuropsychiatric and autoimmune diseases.
Increased cytosolic ANG II disrupts the function of NK cells and, as these cells express a viable RAS, including ACE-2, the virus may directly infect these immune cells [114,115]. In the CNS, ANG II disrupts the BBB via AT1 receptors; in contrast, Losartan, an AT1 antagonist, can repair this and the intestinal biological barrier [116,117].
3. Biological Barriers and Chronic Fatigue
In previous research (2020 and 2021), we linked COVID-19 to ME/CFS via ANG II, endothelial senescence, intestinal barrier dysfunction, and microbial translocation from the GI tract into host systemic circulation [118,119]. Subsequently, these notions were validated by various groups and virus-induced cellular senescence as well as microbial translocation were documented in both acute COVID-19 illness and long COVID, linking intestinal barrier dysfunction to chronic fatigue [120,121,122,123,124,125,126,127]. Indeed, abnormal intestinal permeability has been documented in GWI, COVID-19 and FM, connecting these conditions to microbial translocation [127,128,129]. Aside from fatiguing illnesses, increased microbial migration outside the GI tract has also been demonstrated in autoimmune disorders, cancers, and neuropsychiatric conditions, including schizophrenia and Alzheimer’s disease (AD) [130,131,132,133,134].
Numerous gut microbes express proteins that are identical or similar to those of the human host, eliciting antibodies upon translocation that could be misconstrued as autoantibodies. For example, several intestinal microbial species express QseC, an adrenergic receptor, indicating that antibodies against translocated QseC could trigger human pathology [135]. Indeed, autoantibodies against β1 adrenergic receptors were found in patients with ME/CFS as well as in certain cardiovascular diseases, linking these conditions to microbial translocation [136,137]. In addition, bacteria and archaea synthesize acetylcholine (ACh) and express nicotinic or muscarinic receptors that can elicit antibodies upon translocation, blurring the distinction between autoimmunity and immune reactions to gut microbes at extra-intestinal locations [138]. For example, antimuscarinic autoantibodies, documented in ME/CFS, may represent antibodies against translocating microbes expressing ACh receptors [139]. Indeed, ACh receptor autoantibodies, markers of myasthenia gravis and Lambert–Eaton syndrome, have also been documented in Mycoplasma pneumoniae and influenza, suggesting that, rather than autoantibodies, these could be conventional antibodies against translocated pathogens [140,141,142,143,144]. Mycoplasma infection has also been associated with autoantibodies against N-methyl-D-aspartate (NMDA) receptors, markers of neuropsychiatric disorders, including schizophrenia, connecting this illness to gut microbes [145,146]. Furthermore, microbiota-expressing metabotropic glutamate receptors subtype 2 (mGluR2), linked to both SARS-CoV-2 and Rabies (RABV) viruses, may elicit antibodies rather than autoantibodies, further blurring the border between immunity and autoimmunity [147,148]. For example, mGluR2 autoantibodies, documented in paraneoplastic syndrome as well as in Mycoplasma and Herpes virus infection, may be conventional immunoglobulins against translocated microbes [149,150,151]. Together this data raises an important question: are antibodies against translocated microbial proteins being misidentified as autoantibodies [152,153]?
The human gut microbiome is composed of bacteria, yeasts, fungi, and viruses, an ecosystem in which microbes can inhibit some viral pathogens, while promoting others [154,155]. Most gut viruses are bacteriophages (bacteria-infecting viruses); however, others including SARS-CoV-2, may display bacteriophage-like properties and enter selective microbes, including the cell wall-deficient Mycoplasmas [156,157,158]. In addition, angiotensin converting enzyme-2 (ACE-2), the SARS-CoV-2 entry portal, is expressed by some gut microbes, indicating that the SARS-CoV-2 virus may enter some microbiota, potentially utilizing them as reservoirs [22]. Indeed, long COVID was found to reactivate Epstein–Barr virus (EBV), suggesting that translocating microbes, containing SARS-CoV-2, could play a key role in maintaining a state of latent infection [159].
When thinking about this problem, studying the microbiome may allow us to reconceptualize some autoimmune disorders as conventional immunity against translocating microbes or their antigens, a model that has already been proposed in the etiopathogenesis of systemic lupus erythematosus (SLE) [160,161]. This is significant as methotrexate, a drug often prescribed to patients with autoimmune disorders, can increase intestinal permeability, further facilitating microbial translocation, while at the same time, lowering host immune defenses that oppose these agents [162,163,164].
4. Rethinking Mycoplasma
Mycoplasmas, members of the Mollicutes class of bacteria, are among the simplest and smallest known self-replicating microorganisms [165]. Their genomes, containing about 400–600 genes, are comprised of either a single stranded RNA or a double stranded DNA nucleic acid. Mycoplasmas can be commensal or pathogenic, the former dwelling superficially, for example in the oral cavity, while the latter inside host cells [166,167]. Like SARS-CoV-2, some Mycoplasma species release immune modulators and proinflammatory cytokines that can disrupt host immunity or cause hyperinflammatory reactions (“cytokine storms”) [168,169,170,171]. In addition, some Mycoplasma species synthesize arginine deaminase (ADI), an enzyme that can further disrupt host immunity [172,173].
The infection with SARS-CoV-2 virus results in variable patient outcomes, ranging from few or no symptoms, in some individuals, to critical illness and death in a small number of patients. As viral infections are often accompanied by secondary bacterial contagion, coinfection may contribute to the majority of unfavorable outcomes. Indeed, Mycoplasma comorbidity has been associated with poor COVID-19 prognosis, while epidemiological studies show up to 47% comorbidity between SARS-CoV-2 and Mycoplasma [174,175,176,177,178,179,180]. In addition, like SARS-CoV-2, Mycoplasma infections have been associated with BBB dysfunction and IBD, connecting this cell wall-deficient bacterium to biological barrier dysfunction [181,182,183].
Coinfection with Mycoplasma species has been demonstrated in other viral illnesses, including HIV-1, HHV-6, and various influenza strains as well as in some fatiguing illnesses and cancers. This suggests that Mycoplasma may thrive in defective cells and probably induce further cellular damage by disrupting efferocytosis [184,185,186,187]. There are also significant overlaps in the clinical picture, laboratory, and imaging studies between SARS-CoV-2 and Mycoplasma infections, further complicating the differential diagnosis [188,189,190]. Moreover, diagnostic tests, including Mycoplasma species serology, culture, and even nucleic acid amplification, such as PCR, are marked by numerous limitations [191,192,193]. In this regard, false-positive and -negative COVID-19 serological test results have been reported in many patients with Mycoplasma pneumoniae infection, highlighting the limitation of these assays [194,195]. The next generation sequencing by shot gun methodology appears promising for differentiating Mycoplasma from SARS-CoV-2 and may have a place in the diagnosis of long COVID [196]. However, leukopenia, lymphocytopenia, thrombocytopenia, and thromboembolism were documented in both SARS-CoV-2 and Mycoplasma infections, further highlighting their intertwined etiopathogenesis [197,198,199,200]. Furthermore, certain anti-microbial treatments, such as azithromycin or tetracyclines, were found beneficial for both Mycoplasma and COVID-19, further suggesting a likely silent partnership between these quite different infections [201,202].
Several Mycoplasma species express the integrin motif, Arg-Gly-Asp, or RGD, a cell attachment sequence that connects these pathogens to the host extracellular matrix (ECM) proteins, including integrins, laminins, and fibronectin (FBN) [169,203,204,205,206]. A recent milestone in COVID-19 pathogenesis was the revelation that SARS-CoV-2 receptor binding site (RBS) contains an RGD motif that could facilitate viral entry in host cells [207,208,209]. Since both SARS-CoV-2 and Mycoplasma species bind FBN and express the RGD motif, they may fuse with each other, engendering a combined pathology [206,209,210]. In addition, it has been established that Mycoplasma fermentans incognitus strain stimulates tissue plasminogen activator (tPA) which converts plasminogen to plasmin, a protein that, like furin, can cleave the SARS-CoV-2 S antigen at the S1/S2 site, triggering pathological cell–cell fusion [211,212,213]. Elevated plasmin and plasminogen levels are common findings in severe COVID-19 illness as well as in patients with various chronic diseases, including hypertension, diabetes, and cardiovascular diseases. This may account for an unfavorable COVID-19 prognosis in patients with these disorders [214,215]. Moreover, SARS-CoV-2 may benefit from its association with Mycoplasma as this bacterium can directly block host immunoglobulins, protecting the virus [216,217]. This finding may be significant, as COVID-19 vaccines may be less effective in patients infected with Mycoplasma.
Do Mycoplasma and COVID-19 Comprise a Binary Biological Weapon?
The pathogenic Mycoplasma fermentans incognitus strain, or Lo’s Mycoplasma, was patented by Shyh-Ching Lo in 1993 (Patent Number 5,242,820) and several scientists and clinicians have linked this pathogen to over 45% of GWI cases [218]. This connection was never ruled out, even though Shyh-Ching Lo published in 2000 that there was no serological connection between Mycoplasma fermentans and GWI. However, serological detection of Mycoplasma fermentans is fraught with difficulties that even Lo admits, and his study may have been marred by potential conflict of interest [219]. Indeed, significant fractions of ME/CFS and FM cases have been associated with Mycoplasma fermentans infections as well as those of other Mycoplasma species, indicating that this pathogen may be involved in various chronic illnesses [220,221,222]. Thus, the association of long COVID with Mycoplasma infections may establish this bacterial pathogen as a common coinfection in most fatiguing illnesses [175,223]. Moreover, Mycoplasma infections are associated with increased susceptibility to SLE, a condition also associated with excessive fatigue, linking this microorganism to other illnesses marked by exhaustion [224,225,226]. This could be important, because SLE has been associated with microbial translocation from various niches, possibly linking this autoimmune disease to Mycoplasma colonization [160,175]. Furthermore, lipid-associated membrane proteins (LAMPs) of Mycoplasma fermentans and Mycoplasma hominis have been shown to increase cortisol secretion, further connecting this bacterium to biological barrier dysfunction and chronic fatigue [227]. In 1995, the Institute for Genome Research in Rockville, Maryland completed the nucleotide sequencing of the Mycoplasma genitalium genome, opening the way for the manipulation of this pathogen [228]. Indeed, in 2010, a completely synthetic Mycoplasma mycoides JCVI-syn1.0. was created in the laboratory, contributing further to the potential weaponization of this pathogen [229]. In this regard, Mycoplasma/virus combinations appear suitable for the development of binary biological weapons comprised of independent microorganisms that are considered safe to handle separately, but lethal when mixed, as documented by several studies, including the US Airforce Counterproliferation Center Future Warfare Series No. 53 from 2010 Institute for Molecular Medicine (https://apps.dtic.mil/sti/pdfs/ADA556597.pdf, accessed on 29 September 2022) [230,231]. HIV-1 and Mycoplasma fermentans could be an example of this combination. Since SARS-CoV-2/Mycoplasma comorbidity predicts poor COVID-19 prognosis, it is possible that these infections could be further developed as binary biological weapons [232]. Indeed, the SARS-CoV-2 virus is highly contagious and should only be studied in biosafety level 3 (BSL3) laboratories; therefore, the larger scientific community cannot easily study this pathogen or its combinations [233]. Indeed, a microbial cofactor in COVID-19 disease cannot be ruled out, especially since most Mycoplasma species lack reliable antibody tests and are often associated with false-positive COVID-19 serology [193,194,195]. Moreover, given the possibility of Mycoplasma symbiosis and partnership with other pathogens, including Trichomonas vaginalis, influenza, and HIV, it might be tempting to create a SARS-CoV-2/Mycoplasma coinfection partnership similar to that found in HIV-1 [234,235,236].
Like SARS viruses, Mycoplasma species can also disrupt biological barriers, enabling microbial translocation into host tissues, including the brain. This pathology overlaps with “disorders of unknown etiology” that affect multiple organs and exacerbate many preexistent chronic conditions. Since both Mycoplasmas and SARS-CoV-2 induce symptoms that are difficult to connect to a specific etiology, they may be ideal candidates for the development of possible binary biological weapons.
5. Interventions
In our previous article on ME/CFS, we introduced some novel treatment strategies for barrier dysfunction, including senotherapeutics, short chain fatty acids (SCFAs), milk fat globule membranes (MFGM), β-glucan, and fecal microbial transplantation (FMT) [118]. Here, after a short discussion of some senotherapeutic strategies, we introduce HMGB1 inhibitors, TLR4 antagonists, and MLR.
5.1. Senotherapeutic Strategies
Senescent cells play a key role in organismal aging, while efferocytosis maintains the homeostasis of biological barriers by clearing senescent or damaged cells. Unchecked accumulation of senescent cells can spread the premature aging phenotype to the neighboring healthy cells via SASP paracrine signaling.
Senotherapeutic agents can be divided into senolytics and senomorphics, the former selectively eliminate senescent cells, while the later delete the senescent markers p16INK4a and p21CIP1, restoring the cells to pre-senescent status [237,238]. Here, we introduce a third senotherapeutic category, efferocytosis enhancers comprised of: syncytia inhibitors and blockers of negative efferocytosis regulators. The former subcategory includes TMEM16F inhibitors, while the later contains inhibitors of anti-efferocytotic receptors.
5.2. TMEM16F Inhibitors
Include drugs like Niclosamide, a widely used anthelmintic agent that inhibits PS externalization, averting both viral fusion with host cells and pathological cell–cell fusion [239,240].
5.3. Negative Efferocytosis Regulator Blockers
Senescent and cancer cells can avert elimination by expressing CD47, a “don’t eat me” marker that inhibits the key efferocytosis driver, MER tyrosine kinase (MERTK), thus blocking the clearance of damaged cells [240,241,242,243]. The recently designed CD47 inhibitors, including Hu5F9 and TTI-621, facilitate efferocytosis by blocking the expression of “do not eat me” signals, [244]. These compounds are currently in phase I and II clinical trials, respectively, and are anticipated to receive approval for anticancer indications (NCT04996004 and NCT02216409).
5.4. HMGB1 Antagonists
In the intracellular compartment, HMGB1 acts as a transcription factor that can facilitate the expression of many genes, including those involved in inflammation and immune responses [245]. Hyperacetylation of HMGB1 causes translocation of this protein from the nucleus into the cytosol where it can act as a danger associated molecular pattern (DAMP), or alarmin [246]. From the cytosol, HMGB1 can be released into the extracellular compartment by disintegrating cells or by secretion from lymphocytes, including NK cells. Extracellular HMGB1 has been associated with illnesses that have fatigue as a major symptom, such as rheumatoid arthritis, atherosclerosis, and certain cancers [245]. HMGB1 attaches to several receptors, including TLR4 and the receptor for advanced glycation end-products (RAGE). Binding to these receptors induces premature cellular senescence in many cell types, including ECs and the result is disruption of endothelial barrier [247,248,249,250,251]. In the GI tract, dysfunctional HMGB1 signaling with RAGE and TLR4, promotes IBD, chronic pain, and the illnesses FM, ME/CFS, long COVID, and GWI (in animal models of this disease) [252,253,254,255,256,257,258].
HMGB1 antagonists are likely beneficial for fatigue-related disorders via anti-inflammatory and pro-efferocytotic properties. These agents include:
Glycyrrhizin (glycyrrhizic acid), a HMGB1 inhibitor and a traditional medicine, extract from the Glycyrrhiza glabra plant, possesses anti-inflammatory, antioxidant, and antimicrobial properties, suggesting it could be beneficial for patients with chronic fatigue [259].
Gabexate mesylate is a synthetic protease inhibitor that blocks HMGB1. This inhibitor showed promising results in preclinical studies, especially for the treatment of neuropathic pain and gut barrier dysfunction [260,261].
Anti-HMGB1 monoclonal antibodies, highly specific antibodies that have been studied for the treatment of several CNS diseases, including stroke, traumatic brain injury (TBI), Parkinson’s disease, epilepsy, and AD, suggesting potentially beneficial results for ME/CFS and similar illnesses [262].
DNA and DNA-like oligonucleotide duplexes, nucleic acids that have been studied in rodents for their anti-inflammatory properties, suggesting a potential role in illnesses marked by inflammation and chronic fatigue [245].
Peptide (HBP08) is a novel pharmacological agent that targets chronic inflammation and fatigue, suggesting that it could be developed as a potential therapy for ME/CFS [263].
N-butanol extracts of Morinda citrifolia, that were found to lower intestinal inflammation, and pain in animal models, suggesting that such extracts could be developed for the treatment of chronic fatigue [264,265,266].
5.5. TLR4 Antagonists
TLR4 is a sensor for HMGB1 and LPS, molecules implicated in chronic fatigue, pain, and depression [267]. In addition, TLR4 alters efferocytosis and exacerbates Mycoplasma infections, suggesting that biological barriers could be enhanced by inhibiting this protein [268,269] (Table 1). Several TLR4 antagonists are in development as potential therapeutics for IBD, including:
Rhodobacter sphaeroides LPS, a non-toxic molecule that competes with the toxic LPS of Gram-negative bacteria, suggesting a potential benefit as an inhibitor of intestinal barrier disruption [270].
Eritoran (E5564), a synthetic anti-LPS molecule that is considered a second generation TLR4 inhibitor; it has a long duration of action and superior inhibitory properties [271].
TAK-242 is a TLR 4 signaling inhibitor that prevents LPS-induced muscle wasting in mice and probably influences fatigue in humans [272].

Table 1.
Biological barrier enhancers.
Table 1.
Biological barrier enhancers.
| Interventions | Mechanism | References |
|---|---|---|
| Senolytic agents | Selective elimination of senescent cells | [237] |
| Senpmorphic agents | Delete senescent markers | [238] |
| TMEM16F inhibitors | Block PS externalization | [239,240] |
| CD47 inhibitors | Promote efferocytosis | [244] |
| HMGB1 antagonists | Inhibit RAGE and TLR4 signaling | [245,246] |
| TLR4 antagonists | Promote efferocytosis | [270] |
| MLR | Replace oxidated membrane lipids | [273,274] |
5.6. Membrane Lipid Replacement (MLR)
SARS-CoV-2-induced cellular senescence causes a phenotype typified by upregulated cytosolic iron which predisposes cells to phospholipid peroxidation of their unsaturated cell membrane glycerolphospholipids and causes ferroptosis [273,274]. Ferroptosis is an iron-induced form of programmed cell death caused by the unchecked accumulation of oxidized lipids in the absence of glutathione peroxidase 4 (GPX4) [275]. Oxidized lipids act as foreign molecules that activate host PRR, triggering chronic inflammation, neuropathic pain, depression, and neurodegeneration [276,277,278]. Rescue from ferroptotic cell death can be achieved by lowering intracellular iron, increasing GPX4, or replacing cell membrane oxidized lipids. As the SARS-CoV-2 virus upregulates intracellular iron by hijacking host lysosomes and ferritinophagy (ferritin autophagy), restoring cellular iron homeostasis would require lysosomal rehabilitation, a currently unavailable modality. This is illustrated by the paucity of effective treatments for lysosomal disorders [279]. In addition, ferroptotic pores enhance lipid peroxidation by Ca2+ influx, further lowering GPX4 concentrations [280,281]. Therefore, once activated, ferroptotic cell death takes on a life of its own by initiating a vicious circle of body fat “rusting” and cell death [282]. In addition, enhanced lipid peroxidation and ferroptosis have been associated with ME/CFS, FM, GWI, chronic pain, and neuropsychiatric disorders [283,284,285,286,287]. Interestingly, excess glucocorticoids and ANG II, predispose patients to ferroptosis, while ferroptosis-disintegrating cells release HMGB1, linking this type of programed cell death to biological barrier dysfunction [288,289,290].
Natural membrane phospholipid supplementation with fructooligosaccharide-protected glycerolphospholipids, containing unsaturated fatty acids, was demonstrated to safely restore the homeostasis of biological barriers, limiting microbial translocation [291]. The aim of MLR is substitution of ferroptosis-prone polyunsaturated ether phospholipids (PUFA-ePLs) and oxidized lipids with healthy unsaturated glycerolphospholipids [292,293] (Table 1).
6. Discussion
The concept of microbial translocation as a key mechanism of chronic systemic immune activation, and disease was studied extensively in the HIV infection, a condition associated with chronic fatigue and increased prevalence of ME/CFS [294,295]. COVID-19, like HIV, causes intestinal barrier disruption, impaired efferocytosis, and accumulation of senescent, apoptotic, and necrotic cells that were previously associated with dysfunctional immune responses [296,297]. Indeed, the newly discovered innate lymphoid cells 3 (ILC3) that release interleukin 22 (IL22), a protector of intestinal barrier, have been implicated in both COVID-19 and HIV, linking dysfunctional mucosal immunity to these viral infections [298,299]. As loss of IL22 was associated with premature cellular senescence, this mechanism may account for the dysfunctional efferocytosis and gut barrier dysfunction in long COVID [300]. Moreover, both IL22 and IL10 protect gut mucosal immunity and act on the same receptors, loss of these cytokines may trigger the pathogenesis of long COVID and ME/CFS [301,302]. These findings are in line not only with our earlier hypothesis but also with the results novel studies that have connected dysfunctional efferocytosis with fatiguing illnesses, including FM, ME/CFS, and GWIs [303,304,305].
7. Conclusions
At the cellular level, life is made possible by cell membranes that separate the intracellular from extracellular compartments and intracellular membranes that separate various organelles from the cell cytoplasm [292]. At the tissue and organismal levels, the gut barrier, comprised of a single layer of epithelial cells, separates luminal prokaryotes from host eukaryotic cells. Although during the development and early life, a limited amount of microbial translocation is thought to help “educate” the immune system to distinguish “self” from “non-self” antigens, later in life gut microbes are immunologically tolerated only in the GI tract.
Weakening of biological barriers and microbial translocation into the systemic circulation, can result in the development of various pathologies, including premature cellular senescence, redox dysfunction, autoimmunity, and elevated inflammatory markers that can be manifested clinically in a variety of forms, such as long COVID, ME/CFS, FMS, GWI, IBD, and even some neuropsychiatric disorders [293]. Ferroptotic signatures, found in these illnesses “of unknown etiology”, point to lipid pathologies, a modifiable risk factor, that may be reversed via novel, strategies, including enhanced clearance of senescent cells, MLR, HMGB1 inhibitors, and TLR4 receptor blockers.
This research connects long COVID to other fatiguing illnesses, including FM, ME/CFS, and GWIs, emphasizing the role of microbial translocation outside the GI tract as the driver of these pathologies. In contrast, correcting the barrier function could ameliorate clinical symptoms as demonstrated in GWIs [293].
Author Contributions
Conceptualization, A.S., G.L.N., Z.K. and S.H.; methodology, C.V.A. and L.R.; resources, J.J.A.; writing, A.S. and C.O.; writing—review and editing, C.M.Z.-M.d.C. and J.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raveendran, A.V. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab. Syndr. 2021, 15, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, S.; Arana Chicas, E.; Shafqat, A.; Hashmi, S.K. The Achilles’ heel of cancer survivors: Fundamentals of accelerated cellular senescence. J. Clin. Investig. 2022, 132, e158452. [Google Scholar] [CrossRef] [PubMed]
- Ekedahl, H.; Isaksson, S.; Ståhl, O.; Bogefors, K.; Romerius, P.; Eberhard, J.; Giwercman, A. Low-grade inflammation in survivors of childhood cancer and testicular cancer and its association with hypogonadism and metabolic risk factors. BMC Cancer 2022, 22, 157. [Google Scholar] [CrossRef] [PubMed]
- Reinertsen, K.V.; Loge, J.H.; Brekke, M.; Kiserud, C.E. Chronic fatigue in adult cancer survivors. Tidsskr. Nor. Laegeforen. 2017, 137. [Google Scholar] [CrossRef]
- Bøhn, S.H.; Thorsen, L.; Kiserud, C.E.; Fosså, S.D.; Lie, H.C.; Loge, J.H.; Wisløff, T.; Haugnes, H.S.; Reinertsen, K.V. Chronic fatigue and associated factors among long-term survivors of cancers in young adulthood. Acta Oncol. 2019, 58, 753–762. [Google Scholar] [CrossRef]
- Zheng, G.; Victor Fon, G.; Meixner, W.; Creekmore, A.; Zong, Y.; Dame, M.K.; Colacino, J.; Dedhia, P.H.; Hong, S.; Wiley, J.W. Chronic stress and intestinal barrier dysfunction: Glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci. Rep. 2017, 7, 4502. [Google Scholar] [CrossRef]
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef]
- Mason, B.L.; Pariante, C.M.; Thomas, S.A. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology 2008, 149, 5244–5253. [Google Scholar] [CrossRef][Green Version]
- Ueda, K.; Okamura, N.; Hirai, M.; Tanigawara, Y.; Saeki, T.; Kioka, N.; Komano, T.; Hori, R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J. Biol. Chem. 1992, 267, 24248–24252. [Google Scholar] [CrossRef]
- Foley, S.E.; Tuohy, C.; Dunford, M.; Grey, M.J.; De Luca, H.; Cawley, C.; Szabady, R.L.; Maldonado-Contreras, A.; Houghton, J.M.; Ward, D.V.; et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome 2021, 9, 183. [Google Scholar] [CrossRef]
- Moriguchi, J.; Kato, R.; Nakagawa, M.; Hirotani, Y.; Ijiri, Y.; Tanaka, K. Effects of lipopolysaccharide on intestinal P-glycoprotein expression and activity. Eur. J. Pharmacol. 2007, 565, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Yiallouris, A.; Tsioutis, C.; Agapidaki, E.; Zafeiri, M.; Agouridis, A.P.; Ntourakis, D.; Johnson, E.O. Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Front Endocrinol (Lausanne). Front. Endocrinol. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Lewis-McDougall, F.C.; Ruchaya, P.J.; Domenjo-Vila, E.; Teoh, T.S.; Prata, L.; Cottle, B.J.; Clark, J.E.; Punjabi, P.P.; Awad, W.; Torella, D.; et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 2019, 18, e12931. [Google Scholar] [CrossRef] [PubMed]
- Short, S.; Fielder, E.; Miwa, S.; von Zglinicki, T. Senolytics and senostatics as adjuvant tumour therapy. eBioMedicine 2019, 41, 683–692. [Google Scholar] [CrossRef]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1895–1905. [Google Scholar] [CrossRef]
- Jeyapalan, J.C.; Sedivy, J.M. Cellular senescence and organismal aging. Mech. Ageing Dev. 2008, 129, 467–474. [Google Scholar] [CrossRef]
- LeBrasseur, N.K.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Frailty Pathophysiol. Phenotype Patient Care 2015, 83, 11–18. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, Y.-K.; Lim, J.J.; Byun, H.-O.; Park, I.; Kim, G.-H.; Xu, W.G.; Wang, H.-J.; Yoon, G. Mitochondrial Respiratory Dysfunction Induces Claudin-1 Expression via Reactive Oxygen Species-mediated Heat Shock Factor 1 Activation, Leading to Hepatoma Cell Invasiveness. J. Biol. Chem. 2015, 290, 21421–21431. [Google Scholar] [CrossRef]
- Martínez-Cué, C.; Rueda, N. Cellular senescence in neurodegenerative diseases. Front. Cell Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef]
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112. [Google Scholar] [CrossRef]
- Martin-Rodriguez, O.; Gauthier, T.; Bonnefoy, F.; Couturier, M.; Daoui, A.; Chagué, C.; Valmary-Degano, S.; Gay, C.; Saas, P.; Perruche, S. Pro-Resolving Factors Released by Macrophages After Efferocytosis Promote Mucosal Wound Healing in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 754475. [Google Scholar] [CrossRef]
- Verma, V.; Drury, G.L.; Parisien, M.; Özdağ Acarli, A.N.; Al-Aubodah, T.A.; Nijnik, A.; Wen, X.; Tugarinov, N.; Verner, M.; Klares, R., 3rd; et al. Unbiased immune profiling reveals a natural killer cell-peripheral nerve axis in fibromyalgia. Pain 2022, 163, e821–e836. [Google Scholar] [CrossRef] [PubMed]
- Bi, J. NK cell dysfunction in patients with COVID-19. Cell Mol. Immunol. 2022, 19, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Galán, M.; Vigón, L.; Fuertes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent Overactive Cytotoxic Immune Response in a Spanish Cohort of Individuals With Long-COVID: Identification of Diagnostic Biomarkers. Front. Immunol. 2022, 13, 848886. [Google Scholar] [CrossRef]
- Whistler, T.; Fletcher, M.A.; Lonergan, W.; Zeng, X.R.; Lin, J.M.; Laperriere, A.; Vernon, S.D.; Klimas, N.G. Impaired immune function in Gulf War Illness. BMC Med. Genom. 2009, 2, 12. [Google Scholar] [CrossRef]
- Rivas, J.L.; Palencia, T.; Fernández, G.; García, M. Association of T and NK Cell Phenotype With the Diagnosis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Immunol. 2018, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Sung, A.P.; Tang, J.J.; Guglielmo, M.J.; Smith-Gagen, J.; Bateman, L.; Navarrete-Galvan, L.; Redelman, D.D.; Hudig, D. Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) in Familial Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Fatigue 2020, 8, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Sunwoo, J.B. Natural Killer Cells and Thyroid Diseases. Endocrinol. Metab. Seoul. 2019, 34, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Bancos, I.; Hazeldine, J.; Chortis, V.; Hampson, P.; Taylor, A.E.; Lord, J.M.; Arlt, W. Primary adrenal insufficiency is associated with impaired natural killer cell function: A potential link to increased mortality. Eur. J. Endocrinol. 2017, 176, 471–480. [Google Scholar] [CrossRef]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, I.T.; Ravelli, R.B.G.; van Schayck, J.P.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Sanno, N.; Itoh, J.; Teramoto, A.; Itoh, Y.; Hori, S.; Osamura, R.Y. Immunohistochemical detection of human natural killer cell like immunoreactivity in human pituitary adenomas, using monoclonal antibody NK-1. J. Neurooncol. 1997, 35, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Belluardo, N.; Mudó, G.; Cella, S.; Santoni, A.; Forni, G.; Bindoni, M. Hypothalamic control of certain aspects of natural immunity in the mouse. Immunology 1987, 62, 321–327. [Google Scholar] [PubMed]
- Godoy-Pacheco, A.; García-Chagollán, M.; Ramírez-De-Arellano, A.; Hernández-Silva, C.D.; Villegas-Pineda, J.C.; Ramírez-López, I.G.; Zepeda-Nuño, J.S.; Aguilar-Lemarroy, A.; Pereira-Suárez, A.L. Differential modulation of natural killer cell cytotoxicity by 17β-estradiol and prolactin through the NKG2D/NKG2DL axis in cervical cancer cells. Oncol. Lett. 2022, 24, 288. [Google Scholar] [CrossRef] [PubMed]
- Mavoungou, E.; Bouyou-Akotet, M.K.; Kremsner, P.G. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30). Clin. Exp. Immunol. 2005, 139, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, M.; McDermott, D.H.; Sechler, J.M.; Tinckam, K.; Takakura, A.; Carpenter, C.B.; Milford, E.; Abdi, R. Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II-induced inflammation. J. Am. Soc. Nephrol. 2007, 18, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, A.R.; Hazime, K.S.; Worboys, J.D.; Niembro-Vivanco, O.; Davis, D.M. Synaptic secretion from human natural killer cells is diverse and includes supramolecular attack particles. Proc. Natl. Acad. Sci. USA 2020, 117, 23717–23720. [Google Scholar] [CrossRef] [PubMed]
- Gdynia, G.; Sauer, S.; Kopitz, J.; Fuchs, D.; Duglova, K.; Ruppert, T.; Miller, M.; Pahl, J.; Cerwenka, A.; Enders, M.; et al. The HMGB1 protein induces a metabolic type of tumour cell death by blocking aerobic respiration. Nat. Commun. 2016, 7, 10764. [Google Scholar] [CrossRef]
- Cerwenka, A.; Kopitz, J.; Schirmacher, P.; Roth, W.; Gdynia, G. HMGB1: The metabolic weapon in the arsenal of NK cells. Mol. Cell Oncol. 2016, 3, e1175538. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deitchman, A.N.; Torres, L.; Iyer, N.S.; Munter, S.E.; Nixon, C.C.; Donatelli, J.; Thanh, C.; Takahashi, S.; Hakim, J.; et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021, 36, 109518. [Google Scholar] [CrossRef]
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Strömdahl, A.C.; Cerps, S.; Uller, L.; Kjellström, S.; Bond, P.J.; Schmidtchen, A.A.; et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020, 12, 916–932. [Google Scholar] [CrossRef]
- Štros, M.; Polanská, E.V.; Hlaváčová, T.; Skládal, P. Progress in Assays of HMGB1 Levels in Human Plasma-The Potential Prognostic Value in COVID-19. Biomolecules 2022, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, U.; Nchioua, R.; Prata, L.G.P.L.; Zhu, Y.; Gerdes, E.O.W.; Giorgadze, N.; Pirtskhalava, T.; Parker, E.; Xue, A.; Espindola-Netto, J.M.; et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging 2021, 13, 21838–21854. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Kawahara, M.; Malhotra, G.K.; Schaum, N.; Huang, J.; Ved, U.; Beausejour, C.M.; Coppe, J.P.; Rodier, F.; Campisi, J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013, 201, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Sofiadis, K.; Josipovic, N.; Nikolic, M.; Kargapolova, Y.; Übelmesser, N.; Varamogianni-Mamatsi, V.; Zirkel, A.; Papadionysiou, I.; Loughran, G.; Keane, J.; et al. HMGB1 coordinates SASP-related chromatin folding and RNA homeostasis on the path to senescence. Mol. Syst. Biol. 2021, 17, e9760. [Google Scholar] [CrossRef]
- Banerjee, S.; Friggeri, A.; Liu, G.; Abraham, E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J. Leukoc. Biol. 2010, 88, 973–979. [Google Scholar] [CrossRef]
- Friggeri, A.; Yang, Y.; Banerjee, S.; Park, Y.J.; Liu, G.; Abraham, E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am. J. Physiol. Cell Physiol. 2010, 299, C1267–C1276. [Google Scholar] [CrossRef]
- Nguyen, T.; Johnston, S.; Chacko, A.; Gibson, D.; Cepon, J.; Smith, P.; Staines, D.; Marshall-Gradisnik, S. Novel characterisation of mast cell phenotypes from peripheral blood mononuclear cells in chronic fatigue syndrome/myalgic encephalomyelitis patients. Asian Pac. J. Allergy Immunol. 2017, 35, 75–81. [Google Scholar] [CrossRef][Green Version]
- Oktayoglu, P.; Tahtasiz, M.; Bozkurt, M.; Em, S.; Ucar, D.; Yazmalar, L.; Mete, N.; Nas, K.; Gezer, O. Serum levels of high mobility group box 1 protein and its association with quality of life and psychological and functional status in patients with fibromyalgia. Int. J. Rheum. Dis. 2013, 16, 403–407. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Thang, M.; Greve, H.J.; Mumaw, C.L.; Messenger, E.J.; Ahmed, C.; Quinn, E.; Sullivan, K.; Block, M.L. Circulating HMGB1 is elevated in veterans with Gulf War Illness and triggers the persistent pro-inflammatory microglia phenotype in male C57Bl/6J mice. Transl. Psychiatry 2021, 11, 390. [Google Scholar] [CrossRef]
- Hsiao, I.H.; Lin, Y.W. Electroacupuncture Reduces Fibromyalgia Pain by Attenuating the HMGB1, S100B, and TRPV1 Signalling Pathways in the Mouse Brain. Evid. Based Complement. Altern. Med. 2022, 2022, 2242074. [Google Scholar] [CrossRef]
- Palone, F.; Vitali, R.; Cucchiara, S.; Pierdomenico, M.; Negroni, A.; Aloi, M.; Nuti, F.; Felice, C.; Armuzzi, A.; Stronati, L. Role of HMGB1 as a suitable biomarker of subclinical intestinal inflammation and mucosal healing in patients with inflammatory bowel disease. Inflamm. Bowel. Dis. 2014, 20, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, D.; Han, W.; Guo, C. High-mobility group box-1 inhibition stabilizes intestinal permeability through tight junctions in experimental acute necrotizing pancreatitis. Inflamm. Res. 2019, 68, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Zaiatz Bittencourt, V.; Jones, F.; Tosetto, M.; Doherty, G.A.; Ryan, E.J. Dysregulation of Metabolic Pathways in Circulating Natural Killer Cells Isolated from Inflammatory Bowel Disease Patients. J. Crohns. Colitis. 2021, 15, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Van den Eede, G. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550. [Google Scholar] [CrossRef]
- Groff, A.; Kavanaugh, M.; Ramgobin, D.; McClafferty, B.; Aggarwal, C.S.; Golamari, R.; Jain, R. Gastrointestinal Manifestations of COVID-19: A Review of What We Know. Ochsner J. 2021, 21, 177–180. [Google Scholar] [CrossRef]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708. [Google Scholar] [CrossRef]
- Aktaş, E.; Özdemir Özgentürk, N. Revealing In Silico that Bacteria’s Outer Membrane Proteins may Help our Bodies Replicate and Carry Severe Acute Respiratory Syndrome Coronavirus 2. Bioinform. Biol. Insights 2022, 16, 11779322221116320. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef]
- Lim, J.J.; Grinstein, S.; Roth, Z. Diversity and Versatility of Phagocytosis: Roles in Innate Immunity, Tissue Remodeling, and Homeostasis. Front. Cell Infect. Microbiol. 2017, 7, 191. [Google Scholar] [CrossRef]
- Belizário, J.E.; Neyra, J.M.; Setúbal Destro Rodrigues, M.F. When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection. Innate Immun. 2018, 24, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Vann, J.M.; Proctor, R.A. Phagocytosis of bacteria by endothelial cells. In Pathogenesis of Wound and Biomaterial-Associated Infections; Wadström, T., Eliasson, I., Holder, I., Ljungh, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar] [CrossRef]
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Büttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlötzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.J.; Ghazanfari, N.; Constantinescu, P.; Mantamadiotis, T.; Barrow, A.D. The Role of NK Cells and Innate Lymphoid Cells in Brain Cancer. Front. Immunol. 2020, 11, 1549. [Google Scholar] [CrossRef]
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Bricogne, C.; Fine, M.; Pereira, P.M.; Sung, J.; Tijani, M.; Wang, Y.; Henriques, R.; Collins, M.K.; Hilgemann, D.W. TMEM16F activation by Ca2+ triggers plasma membrane expansion and directs PD-1 trafficking. Sci. Rep. 2019, 9, 619. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Chernomordik, L.V. Flagging fusion: Phosphatidylserine signaling in cell-cell fusion. J. Biol. Chem. 2021, 296, 100411. [Google Scholar] [CrossRef]
- Kemp, K.; Wilkins, A.; Scolding, N. Cell fusion in the brain: Two cells forward, one cell back. Acta Neuropathol. 2014, 128, 629–638. [Google Scholar] [CrossRef]
- Schürch, C.M.; Forster, S.; Brühl, F.; Yang, S.H.; Felley-Bosco, E.; Hewer, E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology 2017, 7, e1373235. [Google Scholar] [CrossRef]
- Song, P.; An, J.; Zou, M.H. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Barrera, L.; Montes-Servín, E.; Hernandez-Martinez, J.M.; García-Vicente, M.L.Á.; Montes-Servín, E.; Herrera-Martínez, M.; Crispín, J.C.; Borbolla-Escoboza, J.R.; Arrieta, O. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br. J. Cancer 2017, 117, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.R.; Hsu, T.H. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012, 20, 282–290. [Google Scholar] [CrossRef]
- Strazza, M.; Pirrone, V.; Wigdahl, B.; Nonnemacher, M.R. Breaking down the barrier: The effects of HIV-1 on the blood-brain barrier. Brain Res. 2011, 1399, 96–115. [Google Scholar] [CrossRef]
- Diamond, M.S.; Klein, R.S. West Nile virus: Crossing the blood-brain barrier. Nat Med. 2004, 10, 1294–1295. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, J.H.; Jo, C.; Kim, K.C.; Koh, Y.H. SARS-CoV-2 spike S1 subunit protein-mediated increase of beta-secretase 1 (BACE1) impairs human brain vessel cells. Biochem. Biophys. Res. Commun. 2022, 626, 66–71. [Google Scholar] [CrossRef]
- Camell, C.D.; Yousefzadeh, M.J.; Zhu, Y.; Prata, L.G.P.L.; Huggins, M.A.; Pierson, M.; Zhang, L.; O’Kelly, R.D.; Pirtskhalava, T.; Xun, P.; et al. Senolytics reduce coronavirus-related mortality in old mice. Science 2021, 373, eabe4832. [Google Scholar] [CrossRef]
- Adesse, D.; Gladulich, L.; Alvarez-Rosa, L.; Siqueira, M.; Marcos, A.C.; Heider, M.; Motta, C.S.; Torices, S.; Toborek, M.; Stipursky, J. Role of aging in Blood–Brain Barrier dysfunction and susceptibility to SARS-CoV-2 infection: Impacts on neurological symptoms of COVID-19. Fluids Barriers CNS 2022, 19, 63. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Dorneles, G.P.; Filho, P.C.S.; da Silva, I.M.; Schipper, L.L.; Postiga, I.A.; Neves, C.A.M.; Junior, L.C.R.; Peres, A.; de Souto, J.T.; et al. Increased LPS levels coexist with systemic inflammation and result in monocyte activation in severe COVID-19 patients. Int. Immunopharmacol. 2021, 100, 108125. [Google Scholar] [CrossRef]
- Gomes-Leal, W. Why microglia kill neurons after neural disorders? The friendly fire hypothesis. Neural. Regen. Res. 2019, 14, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Hornik, T.C.; Neniskyte, U.; Brown, G.C. Inflammation induces multinucleation of Microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J. Neurochem. 2014, 128, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, S.; Lopes-Virella, M.F.; Wang, Z. LPS and palmitic acid Co-upregulate microglia activation and neuroinflammatory response. Compr. Psychoneuroendocrinol. 2021, 6, 100048. [Google Scholar] [CrossRef] [PubMed]
- Tate, W.; Walker, M.; Sweetman, E.; Helliwell, A.; Peppercorn, K.; Edgar, C.; Blair, A.; Chatterjee, A. Molecular Mechanisms of Neuroinflammation in ME/CFS and Long COVID to Sustain Disease and Promote Relapses. Front. Neurol. 2022, 13, 877772. [Google Scholar] [CrossRef]
- Sencio, V.; Gallerand, A.; Gomes Machado, M.; Deruyter, L.; Heumel, S.; Soulard, D.; Barthelemy, J.; Cuinat, C.; Vieira, A.T.; Barthelemy, A.; et al. Influenza Virus Infection Impairs the Gut’s Barrier Properties and Favors Secondary Enteric Bacterial Infection through Reduced Production of Short-Chain Fatty Acids. Infect. Immun. 2021, 89, e0073420. [Google Scholar] [CrossRef]
- Salina, A.C.G.; Dos-Santos, D.; Rodrigues, T.S.; Fortes-Rocha, M.; Freitas-Filho, E.G.; Alzamora-Terrel, D.L.; Castro, I.M.S.; Fraga da Silva, T.F.C.; de Lima, M.H.F.; Nascimento, D.C.; et al. Efferocytosis of SARS-CoV-2-infected dying cells impairs macrophage anti-inflammatory functions and clearance of apoptotic cells. Elife 2022, 11, e74443. [Google Scholar] [CrossRef]
- Liu, S.; Selvaraj, P.; Lien, C.Z.; Nunez, I.A.; Wu, W.W.; Chou, C.K.; Wang, T.T. The PRRA insert at the S1/S2 site modulates cellular tropism of SARS-CoV-2 and ACE2 usage by the closely related Bat RaTG13. J. Virol. 2021, 95, e01751-20. [Google Scholar] [CrossRef]
- Lee, P.A.; Tullman-Ercek, D.; Georgiou, G. The bacterial twin-arginine translocation pathway. Annu. Rev. Microbiol. 2006, 60, 373–395. [Google Scholar] [CrossRef]
- Yan, X.; Hu, S.; Yang, Y.; Xu, D.; Li, H.; Liu, W.; He, X.; Li, G.; Cai, W.; Bu, Z. The Twin-Arginine Translocation System Is Important for Stress Resistance and Virulence of Brucella melitensis. Infect. Immun. 2020, 88, e00389-20. [Google Scholar] [CrossRef]
- Pereyre, S.; Sirand-Pugnet, P.; Beven, L.; Charron, A.; Renaudin, H.; Barré, A.; Avenaud, P.; Jacob, D.; Couloux, A.; Barbe, V.; et al. Life on arginine for Mycoplasma hominis: Clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet. 2009, 5, e1000677. [Google Scholar] [CrossRef]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Gal, H.; Krizhanovsky, V. Cell fusion induced senescence. Aging 2014, 6, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Urata, R.; Ikeda, K.; Yamazaki, E.; Ueno, D.; Katayama, A.; Shin-Ya, M.; Ohgitani, E.; Mazda, O.; Matoba, S. Senescent endothelial cells are predisposed to SARS-CoV-2 infection and subsequent endothelial dysfunction. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajeevan, M.S.; Murray, J.; Oakley, L.; Lin, J.S.; Unger, E.R. Association of chronic fatigue syndrome with premature telomere attrition. J. Transl. Med. 2018, 16, 44. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Niu, Z.; Zhang, B.; Wang, C.; Yao, X.; Peng, H.; Franca, D.N.; Wang, Y.; Zhu, Y.; et al. SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination. Cell Death Differ. 2021, 28, 2765–2777. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Wei, C. Cell-in-cell: An Emerging Player in COVID-19 and Immune Disorders. Natl. Sci. Open 2022, 1, 20220001. [Google Scholar] [CrossRef]
- Fluge, Ø.; Rekeland, I.G.; Lien, K.; Thürmer, H.; Borchgrevink, P.C.; Schäfer, C.; Sørland, K.; Aßmus, J.; Ktoridou-Valen, I.; Herder, I.; et al. B-Lymphocyte Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Tern. Med. 2019, 170, 585–593. [Google Scholar] [CrossRef]
- Sweetman, E.; Kleffmann, T.; Edgar, C.; de Lange, M.; Vallings, R.; Tate, W. A SWATH-MS analysis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J. Transl. Med. 2020, 18, 365. [Google Scholar] [CrossRef]
- Hassett, A.L.; Clauw, D.J.; Williams, D.A. Premature Aging in Fibromyalgia. Curr. Aging Sci. 2015, 8, 178–185. [Google Scholar] [CrossRef]
- Zundel, C.G.; Krengel, M.H.; Heeren, T.; Yee, M.K.; Grasso, C.M.; Janulewicz Lloyd, P.A.; Coughlin, S.S.; Sullivan, K. Rates of Chronic Medical Conditions in 1991, Gulf War Veterans Compared to the General Population. Int. J. Environ. Res. Public Health 2019, 16, 949. [Google Scholar] [CrossRef]
- Clark, I.A. Chronic cerebral aspects of long COVID, post-stroke syndromes and similar states share their pathogenesis and perispinal etanercept treatment logic. Pharmacol. Res. Perspect. 2022, 10, e00926, Erratum in: Pharmacol Res Perspect. 2022, 10, e00942. [Google Scholar] [CrossRef]
- Subramanian, S.; Geng, H.; Tan, X.D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao 2020, 72, 308–324. [Google Scholar] [PubMed]
- Geng, H.; Bu, H.F.; Liu, F.; Wu, L.; Pfeifer, K.; Chou, P.M.; Wang, X.; Sun, J.; Lu, L.; Pandey, A.; et al. In Inflamed Intestinal Tissues and Epithelial Cells, Interleukin 22 Signaling Increases Expression of H19 Long Noncoding RNA, Which Promotes Mucosal Regeneration. Gastroenterology 2018, 155, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, S.; Ivanov, A.I. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Shankman, L.S.; Fleury, S.T.; Evans, W.B.; Penberthy, K.K.; Arandjelovic, S.; Blumberg, R.S.; Agaisse, H.; Ravichandran, K.S. Efferocytosis by Paneth cells within the intestine. Curr. Biol. 2021, 31, 2469–2476.e5. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Vosshenrich, C.A.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–970. [Google Scholar] [CrossRef]
- Wen, S.; Ling, Y.; Yang, W.; Shen, J.; Li, C.; Deng, W.; Liu, W.; Liu, K. Necroptosis is a key mediator of enterocytes loss in intestinal ischaemia/reperfusion injury. J. Cell Mol. Med. 2017, 21, 432–443. [Google Scholar] [CrossRef]
- Mueller, M.; Cima, I.; Noti, M.; Fuhrer, A.; Jakob, S.; Dubuquoy, L.; Schoonjans, K.; Brunner, T. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J. Exp. Med. 2006, 203, 2057–2062. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, S.P.; Hu, Y.; Smith, D.E.; Wiley, J.W.; Hong, S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol. Motil. 2013, 25, e127–e139. [Google Scholar] [CrossRef]
- Cima, I.; Corazza, N.; Dick, B.; Fuhrer, A.; Herren, S.; Jakob, S.; Ayuni, E.; Mueller, C.; Brunner, T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 2004, 200, 1635–1646. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhou, D.; Zhang, L.S.; Deng, F.X.; Shu, S.; Wang, L.J.; Wu, Y.; Guo, N.; Zhou, J.; et al. Angiotensin II deteriorates advanced atherosclerosis by promoting MerTK cleavage impairing efferocytosis through the AT1R/ROS/p38 MAPK/ADAM17 pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C776–C787. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef] [PubMed]
- Miesbach, W. Pathological Role of Angiotensin II in Severe COVID-19. TH Open 2020, 4, e138–e144. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, S.; Schwenk, M.; Hausding, M.; Karbach, S.H.; Schmidgen, M.I.; Brandt, M.; Knorr, M.; Hu, H.; Kröller-Schön, S.; Schönfelder, T.; et al. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, V.C.; Son, S.J.; Ahmadi, S.; Filosa, J.A.; Stern, J.E. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 2014, 63, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.J.; Shi, Y.Y.; Wang, E.B.; Zhu, T.; Zhao, Q. AT1R blocker losartan attenuates intestinal epithelial cell apoptosis in a mouse model of Crohn’s disease. Mol. Med. Rep. 2016, 13, 1156–1162. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Zapata Martín Del Campo, C.M.; Pereida, S.; Maurer, S.; Maldonado, J.C.; Kozlakidis, Z. Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis. Front. Cell Neurosci. 2021, 15, 673217. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Jafri, N.; Diaz, E.L.; Campo Maldonado, J.E. Intoxication With Endogenous Angiotensin II: A COVID-19 Hypothesis. Front. Immunol. 2020, 11, 1472. [Google Scholar] [CrossRef]
- D’Agnillo, F.; Walters, K.A.; Xiao, Y.; Sheng, Z.M.; Scherler, K.; Park, J.; Gygli, S.; Rosas, L.A.; Sadtler, K.; Kalish, H.; et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021, 13, eabj7790. [Google Scholar] [CrossRef]
- Lee, S.; Yu, Y.; Trimpert, J.; Benthani, F.; Mairhofer, M.; Richter-Pechanska, P.; Wyler, E.; Belenki, D.; Kaltenbrunner, S.; Pammer, M.; et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 2021, 599, 283–289. [Google Scholar] [CrossRef]
- Evangelou, K.; Veroutis, D.; Paschalaki, K.; Foukas, P.G.; Lagopati, N.; Dimitriou, M.; Papaspyropoulos, A.; Konda, B.; Hazapis, O.; Polyzou, A.; et al. Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: Possible implications for viral mutagenesis. Eur. Respir. J. 2022, 60, 2102951. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Schmidt, M.O.; Kallakury, B.; Jain, S.; Mehdikhani, S.; Levi, M.; Mendonca, M.; Welch, W.; Riegel, A.T.; Wilcox, C.S.; et al. Low Dose Chronic Angiotensin II Induces Selective Senescence of Kidney Endothelial Cells. Front. Cell Dev. Biol. 2021, 9, 782841. [Google Scholar] [CrossRef] [PubMed]
- Lekva, T.; Ueland, T.; Halvorsen, B.; Murphy, S.L.; Dyrhol-Riise, A.M.; Tveita, A.; Finbråten, A.K.; Mathiessen, A.; Müller, K.E.; Aaløkken, T.M.; et al. Markers of cellular senescence is associated with persistent pulmonary pathology after COVID-19 infection. Infect. Dis. 2022, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Venzon, M.; Bernard-Raichon, L.; Klein, J.; Axelrad, J.E.; Zhang, C.; Hussey, G.A.; Sullivan, A.P.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation. Biorxiv 2022. [Google Scholar] [CrossRef]
- Oliva, A.; Miele, M.C.; Di Timoteo, F.; De Angelis, M.; Mauro, V.; Aronica, R.; Al Ismail, D.; Ceccarelli, G.; Pinacchio, C.; d’Ettorre, G.; et al. Persistent Systemic Microbial Translocation and Intestinal Damage During Coronavirus Disease-19. Front. Immunol. 2021, 12, 708149. [Google Scholar] [CrossRef] [PubMed]
- Giron, L.B.; Peluso, M.J.; Ding, J.; Kenny, G.; Zilberstein, N.F.; Koshy, J.; Hong, K.Y.; Rasmussen, H.; Miller, G.E.; Bishehsari, F.; et al. Markers of fungal translocation are elevated during post-acute sequelae of SARS-CoV-2 and induce NF-κB signaling. JCI Insight. 2022, 7, e160989. [Google Scholar] [CrossRef] [PubMed]
- Kates, A.; Keating, J.; Baubie, K.; Putman-Buehler, N.; Watson, L.; Godfrey, J.; Deblois, C.L.; Suen, G.; Cook, D.B.; Rabago, D.; et al. Examining the association between the gastrointestinal microbiota and Gulf War illness: A prospective cohort study. PLoS ONE 2022, 17, e0268479. [Google Scholar] [CrossRef]
- Minerbi, A.; Fitzcharles, M.A. Gut microbiome: Pertinence in fibromyalgia. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S123), 99–104. [Google Scholar]
- Zhang, B.; Verne, M.L.; Fields, J.Z.; Verne, G.N.; Zhou, Q. Intestinal Hyperpermeability in Gulf War Veterans With Chronic Gastrointestinal Symptoms. J. Clin. Gastroenterol. 2019, 53, e298–e302. [Google Scholar] [CrossRef]
- Fine, R.L.; Manfredo Vieira, S.; Gilmore, M.S.; Kriegel, M.A. Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes 2020, 11, 217–230. [Google Scholar] [CrossRef]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Bacterial Translocation in Gastrointestinal Cancers and Cancer Treatment. Biomedicines 2022, 10, 380. [Google Scholar] [CrossRef]
- Zhu, F.; Ju, Y.; Wang, W.; Wang, Q.; Guo, R.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef]
- Clarke, M.B.; Hughes, D.T.; Zhu, C.; Boedeker, E.C.; Sperandio, V. The QseC sensor kinase: A bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 10420–10425. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Hernandez, A.F. Targeting anti-beta-1-adrenergic receptor antibodies for dilated cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.D.; Fluge, Ø.; et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef]
- Wessler, I.K.; Kirkpatrick, C.J. Non-neuronal acetylcholine involved in reproduction in mammals and honeybees. J. Neurochem. 2017, 142 (Suppl. S2), 144–150. [Google Scholar] [CrossRef]
- Bynke, A.; Julin, P.; Gottfries, C.G.; Heidecke, H.; Scheibenbogen, C.; Bergquist, J. Autoantibodies to beta-adrenergic and muscarinic cholinergic receptors in Myalgic Encephalomyelitis (ME) patients—A validation study in plasma and cerebrospinal fluid from two Swedish cohorts. Brain Behav. Immun. Health 2020, 7, 100107. [Google Scholar] [CrossRef]
- Iwasa, K.; Yoshikawa, H.; Hamaguchi, T.; Sakai, K.; Shinohara-Noguchi, M.; Samuraki, M.; Takahashi, K.; Yanase, D.; Ono, K.; Ishida, C.; et al. Time-series analysis: Variation of anti-acetylcholine receptor antibody titer in myasthenia gravis is related to incidence of Mycoplasma pneumoniae and influenza virus infections. Neurol. Res. 2018, 40, 102–109. [Google Scholar] [CrossRef]
- Elsais, A.; Wyller, V.B.; Loge, J.H.; Kerty, E. Fatigue in myasthenia gravis: Is it more than muscular weakness? BMC Neurol. 2013, 13, 132. [Google Scholar] [CrossRef]
- Alekseeva, T.M.; Gavrilov, Y.V.; Kreis, O.A.; Valko, P.O.; Weber, K.P.; Valko, Y. Fatigue in patients with myasthenia gravis. J. Neurol. 2018, 265, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- BJordan, T.; Mehl, T.L.K.; Schweden, U.; Menge, S. Assessment of physical fatigability and fatigue perception in myasthenia gravis. Muscle Nerve 2017, 55, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, A.M.; Verschuuren, J.J.G.M.; Tannemaat, M.R. Fatigue in patients with myasthenia gravis. A systematic review of the literature. Neuromuscul. Disord. 2020, 30, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Gable, M.S.; Gavali, S.; Radner, A.; Tilley, D.H.; Lee, B.; Dyner, L.; Collins, A.; Dengel, A.; Dalmau, J.; Glaser, C.A. Anti-NMDA receptor encephalitis: Report of ten cases and comparison with viral encephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Huang, J.; Luo, X.; Chen, S.; Cui, Y.; An, H.; Xiu, M.; Tan, S.; Wang, Z.; Yuan, Y.; et al. Elevated serum anti-NMDA receptor antibody levels in first-episode patients with schizophrenia. Brain Behav. Immun. 2019, 81, 213–219. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Wang, X.; Wen, Z.; Shuai, L.; Luo, J.; Wang, C.; Sun, Z.; Liu, R.; Ge, J.; et al. SARS-CoV-2 uses metabotropic glutamate receptor subtype 2 as an internalization factor to infect cells. Cell Discov. 2021, 7, 119. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liu, R.; Shuai, L.; Wang, X.; Luo, J.; Wang, C.; Chen, W.; Wang, X.; Ge, J.; et al. Metabotropic glutamate receptor subtype 2 is a cellular receptor for rabies virus. PLoS Pathog. 2018, 14, e1007189. [Google Scholar] [CrossRef]
- Ruiz-García, R.; Martínez-Hernández, E.; Joubert, B.; Petit-Pedrol, M.; Pajarón-Boix, E.; Fernández, V.; Salais, L.; Del Pozo, M.; Armangué, T.; Sabater, L.; et al. Paraneoplastic cerebellar ataxia and antibodies to metabotropic glutamate receptor 2. Neurol. Neuroimmunol. Neuroinflamm. 2019, 7, e658. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Palazzo, E.; Maione, S.; Neugebauer, V. Group II Metabotropic Glutamate Receptors: Role in Pain Mechanisms and Pain Modulation. Front. Mol. Neurosci. 2018, 11, 383. [Google Scholar] [CrossRef]
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol. Rev. 2017, 97, 839–887. [Google Scholar] [CrossRef]
- Thye, A.Y.; Law, J.W.; Tan, L.T.; Thurairajasingam, S.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients 2022, 14, 1647. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Xia, Z.; Jiao, X.; Deng, J.; Zhang, L.; Li, J. Altered Gut Microbiota in Myasthenia Gravis. Front. Microbiol. 2018, 9, 2627. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Pfeiffer, J.K. Viruses and the Microbiota. Annu. Rev. Virol. 2014, 1, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Guerin, E.; Hill, C. Shining Light on Human Gut Bacteriophages. Front. Cell Infect. Microbiol. 2020, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Van den Eede, G. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Research 2021, 10, 370. [Google Scholar] [CrossRef]
- Vishnyakov, I.E. Cell-in-Cell Phenomena in Wall-Less Bacteria: Is It Possible? Int. J. Mol. Sci. 2022, 23, 4345. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef]
- Ma, L.; Morel, L. Loss of Gut Barrier Integrity In Lupus. Front. Immunol. 2022, 13, 919792. [Google Scholar] [CrossRef]
- Ogunrinde, E.; Zhou, Z.; Luo, Z.; Alekseyenko, A.; Li, Q.Z.; Macedo, D.; Kamen, D.L.; Oates, J.C.; Gilkeson, G.S.; Jiang, W. A Link Between Plasma Microbial Translocation, Microbiome, and Autoantibody Development in First-Degree Relatives of Systemic Lupus Erythematosus Patients. Arthritis Rheumatol. 2019, 71, 1858–1868. [Google Scholar] [CrossRef]
- Carneiro-Filho, B.A.; Lima, I.P.; Araujo, D.H.; Cavalcante, M.C.; Carvalho, G.H.; Brito, G.A.; Lima, V.; Monteiro, S.M.N.; Santos, F.N.; Ribeiro, R.A.; et al. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig. Dis. Sci. 2004, 49, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Shi, B.; Xue, H.; Li, Y.; Yang, X.; Yu, B.; Xu, Z.; Liu, F.; Li, J. Confirmation and prevention of intestinal barrier dysfunction and bacterial translocation caused by methotrexate. Dig. Dis. Sci. 2006, 51, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- De Arumahandi Silva, A.N.; Frommert, L.M.; Albach, F.N.; Klotsche, J.; Scholz, V.; Jeworowski, L.M.; Schwarz, T.; Ten Hagen, A.; Zernicke, J.; Corman, V.M.; et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann. Rheum. Dis. 2022, 81, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef]
- Fadiel, A.; Eichenbaum, K.D.; Semary, N.E.; Epperson, B. Mycoplasma genomics: Tailoring the genome for minimal life requirements through reductive evolution. Front. Biosci. 2007, 12, 2020–2028. [Google Scholar] [CrossRef]
- Glass, J.I.; Assad-Garcia, N.; Alperovich, N.; Yooseph, S.; Lewis, M.R.; Maruf, M.; Hutchison, C.A.; Smith, H.O.; Venter, J.C. Essential genes of a minimal bacterium. Proc. Natl. Acad. Sci. USA 2006, 103, 425–430. [Google Scholar] [CrossRef]
- Rawadi, G.; Roman-Roman, S. Mycoplasma membrane lipoproteins induced proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect. Immun. 1996, 64, 637–643. [Google Scholar] [CrossRef]
- Kotecha, A.; Wang, Q.; Dong, X.; Ilca, S.L.; Ondiviela, M.; Zihe, R.; Seago, J.; Charleston, B.; Fry, E.E.; Abrescia, N.G.; et al. Rules of engagement between αvβ6 integrin and foot-and-mouth disease virus. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Fan, W.; Qian, P.; Wang, D.; Zhi, X.; Wei, Y.; Chen, H.; Li, X. Integrin αvβ3 promotes infection by Japanese encephalitis virus. Res. Vet. Sci. 2017, 111, 67–74. [Google Scholar] [CrossRef]
- He, Q.Q.; Ren, S.; Xia, Z.C.; Cheng, Z.K.; Peng, N.F.; Zhu, Y. Fibronectin facilitates enterovirus 71 infection by mediating viral entry. J. Virol. 2018, 92, e02251-17. [Google Scholar] [CrossRef]
- Rechnitzer, H.; Rottem, S.; Herrmann, R. Reconstitution of an active arginine deiminase pathway in Mycoplasma pneumoniae M129. Infect. Immun. 2013, 81, 3742–3749. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hu, H. Arginine Deiminase Induces Immunogenic Cell Death and Is Enhanced by N-acetylcysteine in Murine MC38 Colorectal Cancer Cells and MDA-MB-231 Human Breast Cancer Cells In Vitro. Molecules 2021, 26, 511. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; de Mattos, G.F. COVID-19 Coronavirus: Is Infection along with Mycoplasma or Other Bacteria Linked to Progression to a Lethal Outcome? Int. J. Clin. Med. 2020, 11, 5. [Google Scholar] [CrossRef]
- Gayam, V.; Konala, V.M.; Naramala, S.; Garlapati, P.R.; Merghani, M.A.; Regmi, N.; Balla, M.; Adapa, S. Presenting characteristics, comorbidities, and outcomes of patients coinfected with COVID-19 and Mycoplasma pneumoniae in the USA. J. Med. Virol. 2020, 92, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Choubey, A.; Sagar, D.; Cawley, P.; Miller, K. Retrospective review analysis of COVID-19 patients co-infected with Mycoplasma pneumoniae. Lung India 2021, 38 Suppl. S1, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- Rangroo, R.; Young, M.; Davis, A.; Pack, S.; Thakore, S.; Schepcoff, A.; Oyesanmi, O. The Severity of the Co-infection of Mycoplasma pneumoniae in COVID-19 Patients. Cureus 2022, 14, e24563. [Google Scholar] [CrossRef]
- Cai, F.; Shou, X.; Ye, Q. Epidemiological Study on Mycoplasma pneumoniae and Chlamydia pneumoniae Infection of Hospitalized Children in a Single Center During the COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 843463. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Haier, J.; Nasralla, M.; Nicolson, N.L.; Ngwenya, R.; De Meirleir, K. Mycoplasmal infections in Chronic Fatigue Syndrome, Fibromyalgia Syndrome and Gulf War Illness. J. Chron. Fatigue Syndr. 2000, 6, 23–39. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Paulus, B.; Wilson, I.; Chadwick, V.S. High prevalence of Mycoplasma pneumoniae in intestinal mucosal biopsies from patients with inflammatory bowel disease and controls. Dig. Dis. Sci. 2001, 46, 2529–2535. [Google Scholar] [CrossRef]
- Cortes, G.M.; Marcialis, M.A.; Bardanzellu, F.; Corrias, A.; Fanos, V.; Mussap, M. Inflammatory Bowel Disease and COVID-19: How Microbiomics and Metabolomics Depict Two Sides of the Same Coin. Front. Microbiol. 2022, 13, 856165. [Google Scholar] [CrossRef] [PubMed]
- Tsiodras, S.; Kelesidis, I.; Kelesidis, T.; Stamboulis, E.; Giamarellou, H. Central nervous system manifestations of Mycoplasma pneumoniae infections. J. Infect. 2005, 51, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Choo, Q.W.W.; Koean, R.A.G.; Chang, S.C.; Chng, W.J.; Chan, M.C.; Wang, W.; Er, J.Z.; Ding, J.L. Macrophages protect mycoplasma-infected chronic myeloid leukemia cells from natural killer cell killing. Immunol. Cell Biol. 2020, 98, 138–151, Epub 2020. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Montagnier, L. AIDS-associated mycoplasmas. Annu. Rev. Microbiol. 1994, 48, 687–712. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.J.; Burke, R.M.; Klugman, K.P. Estimating the prevalence of coinfection with influenza virus and the atypical bacteria Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1585–1589. [Google Scholar] [CrossRef][Green Version]
- Huang, S.; Li, J.Y.; Wu, J.; Meng, L.; Shou, C.C. Mycoplasma infections and different human carcinomas. World J. Gastroenterol. 2001, 7, 266–269. [Google Scholar] [CrossRef]
- Monte Serrano, J.; García-Gil, M.F.; Cruañes Monferrer, J.; Aldea Manrique, B.; Prieto-Torres, L.; García García, M.; Matovelle Ochoa, C.; Ara-Martín, M. COVID-19 and Mycoplasma pneumoniae: SARS-CoV-2 false positive or coinfection? Int. J. Dermatol. 2020, 59, 1282–1283. [Google Scholar] [CrossRef]
- Chaudhry, R.; Sreenath, K.; Vinayaraj, E.V.; Sahoo, B.; Vishnu Narayanan, M.R.; Kiran, K.V.P.S.; Batra, P.; Rathor, N.; Singh, S.; Mohan, A.; et al. Mycoplasma pneumoniae co-infection with SARS-CoV-2: A case report. Access Microbiol. 2021, 3, 000212. [Google Scholar] [CrossRef]
- Oliva, A.; Siccardi, G.; Migliarini, A.; Cancelli, F.; Carnevalini, M.; D’Andria, M.; Attilia, I.; Danese, V.C.; Cecchetti, V.; Romiti, R.; et al. Co-infection of SARS-CoV-2 with Chlamydia or Mycoplasma pneumoniae: A case series and review of the literature. Infection 2020, 48, 871–877. [Google Scholar] [CrossRef]
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728, table of contents. [Google Scholar] [CrossRef]
- Loens, K.; Ursi, D.; Goossens, H.; Ieven, M. Molecular diagnosis of Mycoplasma pneumoniae respiratory tract infections. J. Clin. Microbiol. 2003, 41, 4915–4923. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhu, B.; Wang, S.; Mo, H.; Wang, J.; Li, J.; Zhang, C.; Zeng, H.; Guan, L.; Shi, W.; et al. Association of targeted multiplex PCR with resequencing microarray for the detection of multiple respiratory pathogens. Front. Microbiol. 2015, 6, 532. [Google Scholar] [CrossRef] [PubMed]
- Halbedel, S.; Stülke, J. Tools for the genetic analysis of Mycoplasma. Int. J. Med. Microbiol. 2007, 297, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.; Steininger, P.; Irrgang, P.; Korn, K.; Tenbusch, M.; Diesch, K.; Achenbach, S.; Kremer, A.E.; Werblow, M.; Vetter, M.; et al. Diagnostic performance of four SARS-CoV-2 antibody assays in patients with COVID-19 or with bacterial and non-SARS-CoV-2 viral respiratory infections. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1983–1997. [Google Scholar] [CrossRef]
- Li, N.; Ma, X.; Zhou, J.; Deng, J.; Gu, C.; Fei, C.; Cao, L.; Zhang, Q.; Tao, F. Clinical application of metagenomic next-generation sequencing technology in the diagnosis and treatment of pulmonary infection pathogens: A prospective single-center study of 138 patients. J. Clin. Lab. Anal. 2022, 36, e24498. [Google Scholar] [CrossRef]
- Martire, B.; Foti, C.; Cassano, N.; Buquicchio, R.; Del Vecchio, G.C.; De Mattia, D. Persistent B-cell lymphopenia, multiorgan disease, and erythema multiforme caused by Mycoplasma pneumoniae infection. Pediatr. Dermatol. 2005, 22, 558–560. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019, (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Mirijello, A.; La Marca, A.; D’Errico, M.M.; Curci, S.; Vendemiale, G.; Grandone, E.; De Cosmo, S. Venous thromboembolism during mycoplasma pneumoniae infection: Case report and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10061–10068. [Google Scholar] [CrossRef]
- Porfidia, A.; Valeriani, E.; Pola, R.; Porreca, E.; Rutjes, A.W.S.; Di Nisio, M. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb. Res. 2020, 196, 67–74. [Google Scholar] [CrossRef]
- Ali, A.S.; ASattar, M.A.; Karim, S.; Kutbi, D.; Aljohani, H.; Bakhshwin, D.; Alsieni, M.; Alkreathy, H.M. Pharmacological basis for the potential role of Azithromycin and Doxycycline in management of COVID-19. Arab. J. Chem. 2021, 14, 102983. [Google Scholar] [CrossRef]
- Kouegnigan Rerambiah, L.; Ndong, J.C.; Medzegue, S.; Elisee-Ndam, M.; Djoba Siawaya, J.F. Genital Mycoplasma infections and their resistance phenotypes in an African setting. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Papazisi, L.; Gorton, T.S.; Geary, S.J. Identification of fibronectin-binding proteins in Mycoplasma gallisepticum strain R. Infect. Immun. 2006, 74, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, M.; Berry, I.J.; Jarocki, V.M.; Padula, M.P.; Dumke, R.; Djordjevic, S.P. Cell surface processing of the P1 adhesin of Mycoplasma pneumoniae identifies novel domains that bind host molecules. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Peterhans, E.; Frey, J. RGD motif of lipoprotein T, involved in adhesion of Mycoplasma conjunctivae to lamb synovial tissue cells. J. Bacteriol. 2010, 192, 3773–3779. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, F.; Chen, Y.; Plow, E.; Qin, J. Integrin mediates cell entry of the SARS-CoV-2 virus independent of cellular receptor ACE2. J. Biol. Chem. 2022, 298, 101710. [Google Scholar] [CrossRef]
- Nader, D.; Fletcher, N.; Curley, G.F.; Kerrigan, S.W. SARS-CoV-2 uses major endothelial integrin αvβ3 to cause vascular dysregulation in-vitro during COVID-19. PLoS ONE 2021, 16, e0253347. [Google Scholar] [CrossRef]
- Lemańska-Perek, A.; Krzyżanowska-Gołąb, D.; Dragan, B.; Tyszko, M.; Adamik, B. Fibronectin as a Marker of Disease Severity in Critically Ill COVID-19 Patients. Cells 2022, 11, 1566. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Kannan, T.R.; Baseman, J.B. The surface-exposed carboxyl region of Mycoplasma pneumoniae elongation factor Tu interacts with fibronectin. Infect. Immun. 2008, 76, 3116–3123. [Google Scholar] [CrossRef]
- Tarshis, M.; Morag, B.; Mayer, M. Mycoplasma cells stimulate in vitro activation of plasminogen by purified tissue-type plasminogen activator. FEMS Microbiol. Lett. 1993, 106, 201–204. [Google Scholar] [CrossRef][Green Version]
- Yavlovich, A.; Higazi, A.A.; Rottem, S. Plasminogen binding and activation by Mycoplasma fermentans. Infect. Immun. 2001, 69, 1977–1982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ji, H.L.; Zhao, R.; Matalon, S.; Matthay, M.A. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol. Rev. 2020, 100, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.A.; Guillén-Nieto, G.E.; Rodríguez-Rodríguez, N.; Mendoza-Mari, Y.; Bringas-Vega, M.L.; Berlanga-Saez, J.O.; García Del Barco Herrera, D.; Martinez-Jimenez, I.; Hernandez-Gutierrez, S.; Valdés-Sosa, P.A. Cellular Senescence as the Pathogenic Hub of Diabetes-Related Wound Chronicity. Front. Endocrinol. 2020, 11, 573032. [Google Scholar] [CrossRef]
- Dowsett, J.; Ferkingstad, E.; Rasmussen, L.J.H.; Thørner, L.W.; Magnússon, M.K.; Sugden, K.; Thorleifsson, G.; Frigge, M.; Burgdorf, K.S.; Ostrowski, S.R.; et al. Eleven genomic loci affect plasma levels of chronic inflammation marker soluble urokinase-type plasminogen activator receptor. Commun. Biol. 2021, 4, 655. [Google Scholar] [CrossRef] [PubMed]
- Arfi, Y.; Lartigue, C.; Sirand-Pugnet, P.; Blanchard, A. Beware of Mycoplasma Anti-immunoglobulin Strategies. mBio 2021, 12, e0197421. [Google Scholar] [CrossRef] [PubMed]
- Grover, R.K.; Zhu, X.; Nieusma, T.; Jones, T.; Boreo, I.; MacLeod, A.S.; Mark, A.; Niessen, S.; Kim, H.J.; Kong, L.; et al. A structurally distinct human mycoplasma protein that generically blocks antigen-antibody union. Science 2014, 343, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Nasralla, M.; Haier, J.; Pomfret, J. High frequency of systemic mycoplasmal infections in Gulf War veterans and civilians with Amyotrophic Lateral Sclerosis (ALS). J. Clin. Neurosci. 2002, 9, 525–529. [Google Scholar] [CrossRef]
- Lo, S.C.; Levin, L.; Ribas, J.; Chung, R.; Wang, R.Y.; Wear, D.; Shih, J.W. Lack of serological evidence for Mycoplasma fermentans infection in army Gulf War veterans: A large scale case-control study. Epidemiol. Infect. 2000, 125, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Donta, S.T.; Engel, C.C.; Collins, J.F.; Baseman, J.B.; Dever, L.L.; Taylor, T.; Boardman, K.D.; Kazis, L.E.; Martin, S.E.; Horney, R.A.; et al. Benefits and harms of doxycycline treatment for Gulf War veterans’ illnesses: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2004, 141, 85–94. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Nasralla, M.; Gan, R.; Haier, J.; De Meirleir, K. Evidence for bacterial (Mycoplasma, Chlamydia) and viral (HHV-6) co-infections in chronic fatigue syndrome patients. J. Chronic. Fatigue Syndr. 2003, 11, 7–20. [Google Scholar] [CrossRef]
- Nijs, J.; Nicolson, G.L.; De Becker, P.; Coomans, D.; De Meirleir, K. High prevalence of Mycoplasma infections among European chronic fatigue syndrome patients. Examination of four Mycoplasma species in blood of chronic fatigue syndrome patients. FEMS Immunol. Med. Microbiol. 2002, 34, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Endresen, G.K. Mycoplasma blood infection in chronic fatigue and fibromyalgia syndromes. Rheumatol. Int. 2003, 23, 211–215, Epub 2003. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.A.; Ou, T.Y.; Hung, W.H.; Sung, J.; Chen, W.; Lin, C.L.; Hung, Y.M.; Wei, J.C. Mycoplasma pneumonia Infection Is Associated With an Increased Risk of Systemic Lupus Erythematosus: A Nationwide, Retrospective Cohort Study. Front. Microbiol. 2022, 13, 815136. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, K.S.; Kundsin, R.B.; Walter, C.W.; Schur, P.H. Ureaplasma urealyticum and Mycoplasma hominis in women with systemic lupus erythematosus. Arthritis Rheum. 1992, 35, 429–433. [Google Scholar] [CrossRef]
- Azizoddin, D.R.; Gandhi, N.; Weinberg, S.; Sengupta, M.; Nicassio, P.M.; Jolly, M. Fatigue in systemic lupus: The role of disease activity and its correlates. Lupus 2019, 28, 163–173. [Google Scholar] [CrossRef]
- Iyama, K.; Zhang, S.; Lo, S.C. Effects of mycoplasmal LAMPs on receptor responses to steroid hormones in mammalian cells. Curr. Microbiol. 2001, 43, 163–169. [Google Scholar] [CrossRef]
- Fraser, C.M.; Gocayne, J.D.; White, O.; Adams, M.D.; Clayton, R.A.; Fleischmann, R.D.; Bult, C.J.; Kerlavage, A.R.; Sutton, G.; Kelley, J.M.; et al. The minimal gene complement of Mycoplasma genitalium. Science 1995, 270, 397–403. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Peterson, S.N.; Gill, S.R.; Cline, R.T.; White, O.; Fraser, C.M.; Smith, H.O.; Venter, J.C. Global transposon mutagenesis and a minimal Mycoplasma genome. Science 1999, 286, 2165–2169. [Google Scholar] [CrossRef]
- Almosara, J.O. Biotechnology: Genetically Engineered Pathogens. US Air Force Counterproliferation Center Future Warfare Series No. 53; 2010; Volume 53. Available online: https://media.defense.gov/2019/Apr/11/2002115517/-1/-1/0/53ALMOSARAMONO.PDF (accessed on 29 September 2022).
- Sharma, A.; Gupta, G.; Ahmad, T.; Krishan, K.; Kaur, B. Next generation agents (synthetic agents): Emerging threats and challenges in detection, protection, and decontamination. Handb. Biol. Warf. Prep. 2020, 217–256. [Google Scholar] [CrossRef]
- Busl, K.M.; Bleck, T.P. Treatment of neuroterrorism. Neurotherapeutics 2012, 9, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Malicoat, J.; Manivasagam, S.; Zuñiga, S.; Sola, I.; McCabe, D.; Rong, L.; Perlman, S.; Enjuanes, L.; Manicassamy, B. Development of a Single-Cycle Infectious SARS-CoV-2 Virus Replicon Particle System for Use in Biosafety Level 2 Laboratories. J. Virol. 2022, 96, e0183721. [Google Scholar] [CrossRef] [PubMed]
- Margarita, V.; Fiori, P.L.; Rappelli, P. Impact of Symbiosis Between Trichomonas vaginalis and Mycoplasma hominis on Vaginal Dysbiosis: A Mini Review. Front. Cell Infect. Microbiol. 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Montagnier, L.; Blanchard, A. Mycoplasmas as cofactors in infection due to the human immunodeficiency virus. Clin. Infect. Dis. 1993, 17 (Suppl. S1), S309–S315. [Google Scholar] [PubMed]
- Wagner, K.D.; Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells 2022, 11, 1966. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Juang, Y.P.; Chou, Y.T.; Lin, R.X.; Ma, H.H.; Chao, T.L.; Jan, J.T.; Chang, S.Y.; Liang, P.H. Design, synthesis and biological evaluations of niclosamide analogues against SARS-CoV-2. Eur. J. Med. Chem. 2022, 235, 114295. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature 2021, 594, 88–93. [Google Scholar] [CrossRef]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.M.; Advani, A.S.; et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef]
- Boada-Romero, E.; Martinez, J.; Heckmann, B.L.; Green, D.R. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 2020, 21, 398–414. [Google Scholar] [CrossRef]
- Heit, B.; Tasnim, T.; Le Lam, A. MER Tyrosine Kinase Mediates Efferocytosis Through a Novel β2 Integrin-Activating Signalling Pathway. J. Immunol. 2021, 206 (Suppl. S1), 97–104. [Google Scholar]
- Kelley, S.M.; Ravichandran, K.S. Putting the brakes on phagocytosis: “don’t-eat-me” signaling in physiology and disease. EMBO Rep. 2021, 22, e52564. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, D.; Roviello, G.N.; Montesarchio, D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharm. Ther. 2014, 141, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Avgousti, D.C.; Herrmann, C.; Kulej, K.; Pancholi, N.J.; Sekulic, N.; Petrescu, J.; Molden, R.C.; Blumenthal, D.; Paris, A.J.; Reyes, E.D.; et al. A core viral protein binds host nucleosomes to sequester immune danger signals. Nature 2016, 535, 173–177. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, I.H.; Kwak, M.S.; Rhee, W.J.; Kim, S.H.; Shin, J.S. HMGB1 orchestrates STING-mediated senescence via TRIM30α modulation in cancer cells. Cell Death Discov. 2021, 7, 28. [Google Scholar] [CrossRef]
- Tanaka, N.; Yonekura, H.; Yamagishi, S.; Fujimori, H.; Yamamoto, Y.; Yamamoto, H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J. Biol. Chem. 2000, 275, 25781–25790. [Google Scholar] [CrossRef]
- Liu, J.; Huang, K.; Cai, G.Y.; Chen, X.M.; Yang, J.R.; Lin, L.R.; Yang, J.; Huo, B.G.; Zhan, J.; He, Y.N. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal. 2014, 26, 110–121. [Google Scholar] [CrossRef]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of Advanced Glycation End Product (AGE) Formation and Accumulation. Handb. Exp. Pharmacol. 2021, 264, 395–423. [Google Scholar] [CrossRef]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef]
- Carlo-Stella, N.; Bozzini, S.; De Silvestri, A.; Sbarsi, I.; Pizzochero, C.; Lorusso, L.; Martinetti, M.; Cuccia, M. Molecular study of receptor for advanced glycation endproduct gene promoter and identification of specific HLA haplotypes possibly involved in chronic fatigue syndrome. Int. J. Immunopathol. Pharmacol. 2009, 22, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Hein, G.; Franke, S. Are advanced glycation end-product-modified proteins of pathogenetic importance in fibromyalgia? Rheumatology 2002, 41, 1163–1167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Alhasson, F.; Das, S.; Seth, R.; Dattaroy, D.; Chandrashekaran, V.; Ryan, C.N.; Chan, L.S.; Testerman, T.; Burch, J.; Hofseth, L.J.; et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS ONE 2017, 12, e0172914. [Google Scholar] [CrossRef]
- Fontes-Dantas, F.L.; Fernandes, G.G.; Gutman, E.G.; De Lima, E.V.; Antonio, L.S.; Hammerle, M.B.; Mota-Araujo, H.P.; Colodeti, L.C.; Araújo, S.M.B.; da Silva, T.N.; et al. SARS-CoV-2 spike protein induces long-term TLR4-mediated synapse and cognitive loss recapitulating Post-COVID syndrome. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Snelson, M.; Lucut, E.; Coughlan, M.T. The Role of AGE-RAGE Signalling as a Modulator of Gut Permeability in Diabetes. Int. J. Mol. Sci. 2022, 23, 1766. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Semple, B.; Piperi, C.; Othman, I.; Shaikh, M.F. Potential Neuroprotective Effect of the HMGB1 Inhibitor Glycyrrhizin in Neurological Disorders. ACS Chem. Neurosci. 2020, 11, 485–500. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, H.Y.; Ki, Y.J.; Kim, S.H.; Lim, K.J.; Jung, K.T. Gabexate mesilate ameliorates the neuropathic pain in a rat model by inhibition of proinflammatory cytokines and nitric oxide pathway via suppression of nuclear factor-κB. Korean J. Pain. 2020, 33, 30–39. [Google Scholar] [CrossRef]
- Gobbetti, T.; Cenac, N.; Motta, J.P.; Rolland, C.; Martin, L.; Andrade-Gordon, P.; Steinhoff, M.; Barocelli, E.; Vergnolle, N. Serine protease inhibition reduces post-ischemic granulocyte recruitment in mouse intestine. Am. J. Pathol. 2012, 180, 141–152. [Google Scholar] [CrossRef]
- Nishibori, M.; Mori, S.; Takahashi, H.K. Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J. Pharmacol. Sci. 2019, 140, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sgrignani, J.; Cecchinato, V.; Fassi, E.M.A.; D’Agostino, G.; Garofalo, M.; Danelon, G.; Pedotti, M.; Simonelli, L.; Varani, L.; Grazioso, G.; et al. Systematic Development of Peptide Inhibitors Targeting the CXCL12/HMGB1 Interaction. J. Med. Chem. 2021, 64, 13439–13450. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Isami, F.; Abe, Y.; Sakaguchi, T.; Higashimoto, Y.; Yamagishi, S.I. N-butanol extracts of Morinda citrifolia suppress advanced glycation end products (AGE)-induced inflammatory reactions in endothelial cells through its anti-oxidative properties. BMC Complement. Altern. Med. 2017, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.A.; Magalhães, D.A.; Sousa, S.G.; Ferreira, J.D.S.; Pereira, C.M.C.; Lima, J.; de Albuquerque, I.F.; Bezerra, N.L.S.D.; de Brito, T.V.; Monteiro, C.E.D.S.; et al. Polysaccharides derived from Morinda citrifolia Linn reduce inflammatory markers during experimental colitis. J. Ethnopharmacol. 2020, 248, 112303. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Shan, Y.; Wang, Y.; Li, Z.; Bi, Y.; Zhao, W.; Yin, Y.; Wang, T.; Li, S.; et al. MicroRNAs Involved in the Therapeutic Functions of Noni (Morinda citrifolia L.) Fruit Juice in the Treatment of Acute Gouty Arthritis in Mice Induced with Monosodium Urate. Foods 2021, 10, 1638. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Su, K.; Bo, L.; Jiang, C.; Deng, X.; Zhao, Y.Y.; Minshall, R.D.; Hu, G. TLR4 is required for macrophage efferocytosis during resolution of ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L787–L801. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Lu, X.; Zhen, S.; Huo, J. Toll-Like Receptor 4 Exacerbates Mycoplasma pneumoniaevia Promoting Transcription Factor EB-Mediated Autophagy. Contrast Media Mol. Imaging 2022, 2022, 3357694. [Google Scholar] [CrossRef]
- Anwar, M.A.; Panneerselvam, S.; Shah, M.; Choi, S. Insights into the species-specific TLR4 signaling mechanism in response to Rhodobacter sphaeroides lipid A detection. Sci. Rep. 2015, 5, 7657. [Google Scholar] [CrossRef]
- Tam, J.S.Y.; Coller, J.K.; Hughes, P.A.; Prestidge, C.A.; Bowen, J.M. Toll-like receptor 4 (TLR4) antagonists as potential therapeutics for intestinal inflammation. Indian J. Gastroenterol. 2021, 40, 5–21. [Google Scholar] [CrossRef]
- Ono, Y.; Maejima, Y.; Saito, M.; Sakamoto, K.; Horita, S.; Shimomura, K.; Inoue, S.; Kotani, J. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci. Rep. 2020, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Clatworthy, S.A.S.; Gamell, C.; Meggyesy, P.M.; Rigopoulos, A.T.; Haupt, S.; Haupt, Y.; Denoyer, D.; Adlard, P.A.; Bush, A.I.; et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018, 14, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.; Zhang, K.; Shen, L.; Deng, M. Ferroptosis in COVID-19-related liver injury: A potential mechanism and therapeutic target. Front. Cell Infect. Microbiol. 2022, 12, 922511. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Bullock, K.; Price, A.; Inderias, L.; Osorio, C. Ferrosenescence: The iron age of neurodegeneration? Mech. Ageing 2018, 174, 63–75. [Google Scholar] [CrossRef]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef]
- Leibundgut, G.; Witztum, J.L.; Tsimikas, S. Oxidation-specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr. Opin. Pharmacol. 2013, 13, 168–179. [Google Scholar] [CrossRef]
- Osthues, T.; Sisignano, M. Oxidized Lipids in Persistent Pain States. Front. Pharmacol. 2019, 10, 1147. [Google Scholar] [CrossRef]
- Liang, H.; Luo, D.; Liao, H.; Li, S. Coronavirus Usurps the Autophagy-Lysosome Pathway and Induces Membranes Rearrangement for Infection and Pathogenesis. Front. Microbiol. 2022, 13, 846543. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Zhang, P.; Wang, Z.; Ma, X.; Liu, C.; Vasilev, K.; Zhang, L.; Zhou, X.; Liu, L.; et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J. Adv. Res. 2022; in press. [Google Scholar] [CrossRef]
- Pedrera, L.; Espiritu, R.A.; Ros, U.; Weber, J.; Schmitt, A.; Stroh, J.; Hailfinger, S.; von Karstedt, S.; García-Sáez, A.J. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2021, 28, 1644–1657. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med. Sci. Monit. 2011, 17, SC11–SC15. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Kumar, D.S.; Ali, M.A.; Agarwal, S.; Khandpur, S. Nitric oxide, lipid peroxidation products, and antioxidants in primary fibromyalgia and correlation with disease severity. J. Med. Biochem. 2020, 39, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Wang, L.; Monk, J.M.; Bright, A.T.; Koslik, H.J.; Ritchie, J.B.; Golomb, B.A. Metabolic features of Gulf War illness. PLoS ONE 2019, 14, e0219531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, T.; Zhao, M.; Tao, X.; Zhang, B.; Sun, C.; Wang, P.; Wang, K.; Zhao, L. Sirtuin 2 Alleviates Chronic Neuropathic Pain by Suppressing Ferroptosis in Rats. Front. Pharmacol. 2022, 13, 827016. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef]
- Li, S.; Zhou, C.; Zhu, Y.; Chao, Z.; Sheng, Z.; Zhang, Y.; Zhao, Y. Ferrostatin-1 alleviates angiotensin II (Ang II)- induced inflammation and ferroptosis in astrocytes. Int. Immunopharmacol. 2021, 90, 107179. [Google Scholar] [CrossRef]
- von Mässenhausen, A.; Zamora Gonzalez, N.; Maremonti, F.; Belavgeni, A.; Tonnus, W.; Meyer, C.; Beer, K.; Hannani, M.T.; Lau, A.; Peitzsch, M.; et al. Dexamethasone sensitizes to ferroptosis by glucocorticoid receptor-induced dipeptidase-1 expression and glutathione depletion. Sci. Adv. 2022, 8, eabl8920. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ferreira de Mattos, G.; Ash, M.; Settineri, R.; Escribá, P.V. Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging. Membranes 2021, 11, 944. [Google Scholar] [CrossRef]
- Nicolson, G.L. Membrane Lipid Replacement—A functional approach to repairing cellular membranes, reducing symptoms, and restoring function. Funct. Food Sci. 2022, 2, 198–204. [Google Scholar]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Pinching, A.J. AIDS and CFS/ME: A tale of two syndromes. Clin. Med. 2003, 3, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Giron, L.B.; Peluso, M.J.; Ding, J.; Kenny, G.; Zilberstein, N.F.; Koshy, J.; Hong, K.Y.; Rasmussen, H.; Miller, G.; Bishehsari, F.; et al. Markers of Fungal Translocation Are Elevated During Post-Acute Sequelae of SARS-CoV-2 Infection and Induce NF-κB Triggered Inflammation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Michailidis, C.; Giannopoulos, G.; Vigklis, V.; Armenis, K.; Tsakris, A.; Gargalianos, P. Impaired phagocytosis among patients infected by the human immunodeficiency virus: Implication for a role of highly active anti-retroviral therapy. Clin. Exp. Immunol. 2012, 167, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, N.J.; Wang, Y.; Manickas-Hill, Z.; Carbone, C.; Dauphin, A.; Boribong, B.P.; Loiselle, M.; Davis, J.; Leonard, M.M.; Kuri-Cervantes, L. Innate lymphoid cells and COVID-19 severity in SARS-CoV-2 infection. Elife 2022, 11, e74681. [Google Scholar] [CrossRef] [PubMed]
- Kløverpris, H.N.; Kazer, S.W.; Mjösberg, J.; Mabuka, J.M.; Wellmann, A.; Ndhlovu, Z.; Yadon, M.C.; Nhamoyebonde, S.; Muenchhoff, M.; Simoni, Y.; et al. Innate Lymphoid Cells Are Depleted Irreversibly during Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity 2016, 44, 391–405. [Google Scholar] [CrossRef]
- Kong, X.; Feng, D.; Wang, H.; Hong, F.; Bertola, A.; Wang, F.S.; Gao, B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012, 56, 1150–1159. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Y.; Wang, D.; Li, C.; Qu, Y.; Guo, J.; Shi, T.; Bo, W.; Sun, Z.; Asakawa, T. The clinical value of cytokines in chronic fatigue syndrome. J. Transl. Med. 2019, 17, 213. [Google Scholar] [CrossRef]
- Wei, H.X.; Wang, B.; Li, B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Vogl, T.; Kalka, I.N.; Klompus, S.; Leviatan, S.; Weinberger, A.; Segal, E. Systemic antibody responses against human microbiota flagellins are overrepresented in chronic fatigue syndrome patients. Sci. Adv. 2022, 8, eabq2422. [Google Scholar] [CrossRef]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2022, 12, 628741. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani, F.; Maynard, R.D.; Sing, J.C.; Jahanbani, S.; Perrino, J.J.; Spacek, D.V.; Davis, R.W.; Snyder, M.P. Phenotypic characteristics of peripheral immune cells of Myalgic encephalomyelitis/chronic fatigue syndrome via transmission electron microscopy: A pilot study. PLoS ONE 2022, 17, e0272703. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).