Complications and Treatments in Adult X-Linked Hypophosphatemia
Abstract
1. Introduction
2. Case Presentation
3. Complications in Adult XLH Patients
3.1. Short Stature
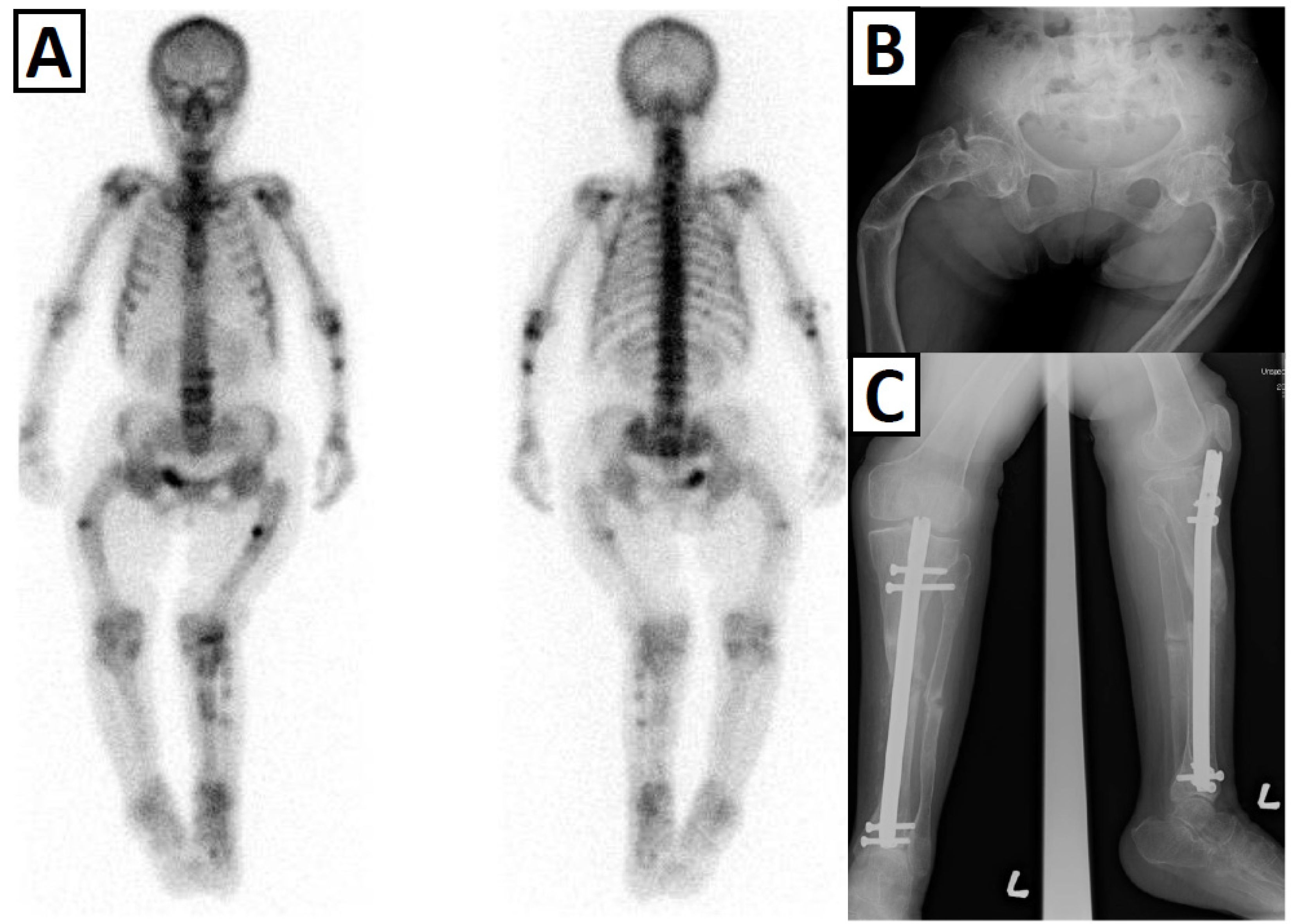
3.2. Lower Limb Deformity and Gait Difficulty
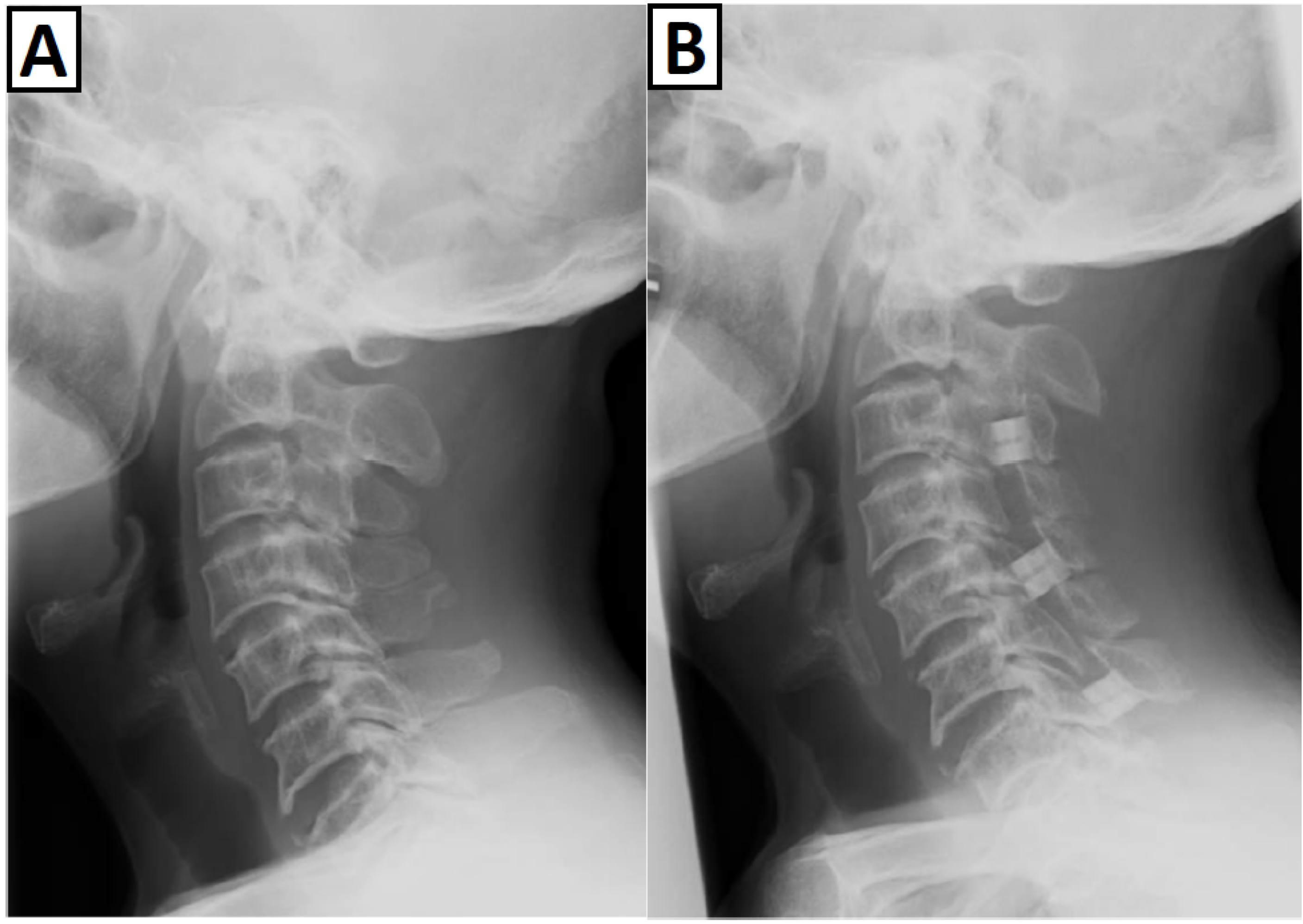
3.3. Spinal Complications
3.4. Bone Fragility
3.5. Hearing Loss
4. Conventional Treatment of Patients with XLH
5. Burosumab Treatment of Adult XLH Patients
5.1. Preclinical Findings
5.2. Clinical Findings in Adults with XLH
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- HYP-Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 1995, 11, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Albright, F.; Butler, A.; Bloomberg, E. Rickets resistant to vitamin D therapy. Am. J. Dis. Child. 1937, 54, 529–547. [Google Scholar] [CrossRef]
- Beck-Nielsen, S.S.; Brock-Jacobsen, B.; Gram, J.; Brixen, K.; Jensen, T.K. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur. J. Endocrinol. 2009, 160, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Fukumoto, S.; Ozono, K.; Namba, N.; Inoue, D.; Okazaki, R.; Yamauchi, M.; Sugimoto, T.; Minagawa, M.; Michigami, T.; et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: Prevalence, biochemical data and treatment. Endocr. J. 2015, 62, 811–816. [Google Scholar] [CrossRef]
- Rafaelsen, S.; Johansson, S.; Raeder, H.; Bjerknes, R. Hereditary hypophosphatemia in Norway: A retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur. J. Endocrinol. 2016, 174, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Mizutani, S.; Muto, T.; Yoneya, T.; Hino, R.; Takeda, S.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Yamashita, T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. USA 2001, 98, 6500–6505. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Shimada, T.; Muto, T.; Urakawa, I.; Yoneya, T.; Yamazaki, Y.; Okawa, K.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Yamashita, T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 2002, 143, 3179–3182. [Google Scholar] [CrossRef]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef]
- Shimada, T.; Urakawa, I.; Yamazaki, Y.; Hasegawa, H.; Hino, R.; Yoneya, T.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Yamashita, T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem. Biophys. Res. Commun. 2004, 314, 409–414. [Google Scholar] [CrossRef]
- Imanishi, Y.; Inaba, M.; Kawata, T.; Nishizawa, Y. Cinacalcet in hyperfunctioning parathyroid diseases. Ther. Apher. Dial. 2009, 13 (Suppl S1), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.B.; Zahradnik, R.; Larsson, T.; White, K.E.; Sugimoto, T.; Imanishi, Y.; Yamamoto, T.; Hampson, G.; Koshiyama, H.; Ljunggren, O.; et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 2003, 348, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Okazaki, R.; Shibata, M.; Hasegawa, Y.; Satoh, K.; Tajima, T.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Yamashita, T.; et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 2002, 87, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- ADHR-Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Riminucci, M.; Collins, M.T.; Fedarko, N.S.; Cherman, N.; Corsi, A.; White, K.E.; Waguespack, S.; Gupta, A.; Hannon, T.; Econs, M.J.; et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Investig. 2003, 112, 683–692. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imanishi, Y.; Koshiyama, H.; Miyauchi, A.; Wakasa, K.; Kawata, T.; Goto, H.; Ohashi, H.; Koyano, H.M.; Mochizuki, R.; et al. Expression of FGF23 is correlated with serum phosphate level in isolated fibrous dysplasia. Life Sci. 2006, 78, 2295–2301. [Google Scholar] [CrossRef]
- Wolf, M.; Koch, T.A.; Bregman, D.B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013, 28, 1793–1803. [Google Scholar] [CrossRef]
- Vilaca, T.; Velmurugan, N.; Smith, C.; Abrahamsen, B.; Eastell, R. Osteomalacia as a Complication of Intravenous Iron Infusion: A Systematic Review of Case Reports. J. Bone Miner Res. 2022, 37, 1188–1199. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Imel, E.A.; Holm, I.A.; Jan de Beur, S.M.; Insogna, K.L. A clinician’s guide to X-linked hypophosphatemia. J. Bone Miner. Res. 2011, 26, 1381–1388. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Whyte, M.P.; Imel, E.A.; Boot, A.M.; Hogler, W.; Linglart, A.; Padidela, R.; Van’t Hoff, W.; Mao, M.; Chen, C.Y.; et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N. Engl. J. Med. 2018, 378, 1987–1998. [Google Scholar] [CrossRef]
- Imel, E.A.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Simmons, J.H.; Padidela, R.; Namba, N.; Cheong, H.I.; et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: A randomised, active-controlled, open-label, phase 3 trial. Lancet 2019, 393, 2416–2427. [Google Scholar] [CrossRef]
- Lim, R.; Shailam, R.; Hulett, R.; Skrinar, A.; Nixon, A.; Williams, A.; Nixon, M.; Thacher, T.D. Validation of the Radiographic Global Impression of Change (RGI-C) score to assess healing of rickets in pediatric X-linked hypophosphatemia (XLH). Bone 2021, 148, 115964. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and diagnostic criteria for rickets and osteomalacia--proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J. Bone Miner. Metab. 2015, 33, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Schutt, S.M.; Schumacher, M.; Holterhus, P.M.; Felgenhauer, S.; Hiort, O. Effect of GH replacement therapy in two male siblings with combined X-linked hypophosphatemia and partial GH deficiency. Eur. J. Endocrinol. 2003, 149, 317–321. [Google Scholar] [CrossRef][Green Version]
- Beck-Nielsen, S.S.; Brusgaard, K.; Rasmussen, L.M.; Brixen, K.; Brock-Jacobsen, B.; Poulsen, M.R.; Vestergaard, P.; Ralston, S.H.; Albagha, O.M.; Poulsen, S.; et al. Phenotype presentation of hypophosphatemic rickets in adults. Calcif. Tissue Int. 2010, 87, 108–119. [Google Scholar] [CrossRef]
- Zivicnjak, M.; Schnabel, D.; Billing, H.; Staude, H.; Filler, G.; Querfeld, U.; Schumacher, M.; Pyper, A.; Schroder, C.; Bramswig, J.; et al. Age-related stature and linear body segments in children with X-linked hypophosphatemic rickets. Pediatr. Nephrol. 2011, 26, 223–231. [Google Scholar] [CrossRef]
- Weglage, J.; Funders, B.; Wilken, B.; Schubert, D.; Schmidt, E.; Burgard, P.; Ullrich, K. Psychological and social findings in adolescents with phenylketonuria. Eur. J. Pediatr. 1992, 151, 522–525. [Google Scholar] [CrossRef]
- Makitie, O.; Doria, A.; Kooh, S.W.; Cole, W.G.; Daneman, A.; Sochett, E. Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 2003, 88, 3591–3597. [Google Scholar] [CrossRef]
- Wilson, D.M.; Lee, P.D.; Morris, A.H.; Reiter, E.O.; Gertner, J.M.; Marcus, R.; Quarmby, V.E.; Rosenfeld, R.G. Growth hormone therapy in hypophosphatemic rickets. Am. J. Dis. Child. 1991, 145, 1165–1170. [Google Scholar] [CrossRef]
- Baroncelli, G.I.; Bertelloni, S.; Ceccarelli, C.; Saggese, G. Effect of growth hormone treatment on final height, phosphate metabolism, and bone mineral density in children with X-linked hypophosphatemic rickets. J. Pediatr. 2001, 138, 236–243. [Google Scholar] [CrossRef]
- Petje, G.; Meizer, R.; Radler, C.; Aigner, N.; Grill, F. Deformity correction in children with hereditary hypophosphatemic rickets. Clin. Orthop. Relat. Res. 2008, 466, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Wright, J.; Bockenhauer, D.; Van’t Hoff, W.; Eastwood, D.M. The orthopaedic management of lower limb deformity in hypophosphataemic rickets. J. Child. Orthop. 2017, 11, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kang, H.G.; Nishida, Y.; Evins, A.; Skrinar, A.; Cheong, H.I. Burden of disease of X-linked hypophosphatemia in Japanese and Korean patients: A cross-sectional survey. Endocr. J. 2022, 69, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Skrinar, A.; Dvorak-Ewell, M.; Evins, A.; Macica, C.; Linglart, A.; Imel, E.A.; Theodore-Oklota, C.; San Martin, J. The Lifelong Impact of X-Linked Hypophosphatemia: Results From a Burden of Disease Survey. J. Endocr. Soc. 2019, 3, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Seefried, L.; Smyth, M.; Keen, R.; Harvengt, P. Burden of disease associated with X-linked hypophosphataemia in adults: A systematic literature review. Osteoporos. Int. 2021, 32, 7–22. [Google Scholar] [CrossRef]
- Mindler, G.T.; Kranzl, A.; Stauffer, A.; Kocijan, R.; Ganger, R.; Radler, C.; Haeusler, G.; Raimann, A. Lower Limb Deformity and Gait Deviations Among Adolescents and Adults With X-Linked Hypophosphatemia. Front. Endocrinol. 2021, 12, 754084. [Google Scholar] [CrossRef]
- Veilleux, L.N.; Cheung, M.; Ben Amor, M.; Rauch, F. Abnormalities in muscle density and muscle function in hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 2012, 97, E1492–E1498. [Google Scholar] [CrossRef]
- Baia, L.C.; Heilberg, I.P.; Navis, G.; de Borst, M.H.; NIGRAM investigators. Phosphate and FGF-23 homeostasis after kidney transplantation. Nat. Rev. Nephrol. 2015, 11, 656–666. [Google Scholar] [CrossRef]
- Ambuhl, P.M.; Meier, D.; Wolf, B.; Dydak, U.; Boesiger, P.; Binswanger, U. Metabolic aspects of phosphate replacement therapy for hypophosphatemia after renal transplantation: Impact on muscular phosphate content, mineral metabolism, and acid/base homeostasis. Am. J. Kidney Dis. 1999, 34, 875–883. [Google Scholar] [CrossRef]
- Chesher, D.; Oddy, M.; Darbar, U.; Sayal, P.; Casey, A.; Ryan, A.; Sechi, A.; Simister, C.; Waters, A.; Wedatilake, Y.; et al. Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J. Inherit. Metab. Dis. 2018, 41, 865–876. [Google Scholar] [CrossRef]
- Kato, H.; Koga, M.; Kinoshita, Y.; Taniguchi, Y.; Kobayashi, H.; Fukumoto, S.; Nangaku, M.; Makita, N.; Ito, N. Incidence of Complications in 25 Adult Patients With X-linked Hypophosphatemia. J. Clin. Endocrinol. Metab. 2021, 106, e3682–e3692. [Google Scholar] [CrossRef] [PubMed]
- Berndt, M.; Ehrich, J.H.; Lazovic, D.; Zimmermann, J.; Hillmann, G.; Kayser, C.; Prokop, M.; Schirg, E.; Siegert, B.; Wolff, G.; et al. Clinical course of hypophosphatemic rickets in 23 adults. Clin. Nephrol. 1996, 45, 33–41. [Google Scholar]
- Reid, I.R.; Hardy, D.C.; Murphy, W.A.; Teitelbaum, S.L.; Bergfeld, M.A.; Whyte, M.P. X-linked hypophosphatemia: A clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine 1989, 68, 336–352. [Google Scholar] [CrossRef]
- Rosenthall, L. DEXA bone densitometry measurements in adults with X-linked hypophosphatemia. Clin. Nucl. Med. 1993, 18, 564–566. [Google Scholar] [CrossRef]
- Marie, P.J.; Glorieux, F.H. Bone histomorphometry in asymptomatic adults with hereditary hypophosphatemic vitamin D-resistant osteomalacia. Metab. Bone Dis. Relat. Res. 1982, 4, 249–253. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Liu, X.S.; Stein, E.M.; Zhou, B.; Zhang, C.A.; Nickolas, T.L.; Cohen, A.; Thomas, V.; McMahon, D.J.; Cosman, F.; Nieves, J.; et al. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J. Bone Miner. Res. 2012, 27, 263–272. [Google Scholar] [CrossRef]
- Colares Neto, G.P.; Pereira, R.M.; Alvarenga, J.C.; Takayama, L.; Funari, M.F.; Martin, R.M. Evaluation of bone mineral density and microarchitectural parameters by DXA and HR-pQCT in 37 children and adults with X-linked hypophosphatemic rickets. Osteoporos. Int. 2017, 28, 1685–1692. [Google Scholar] [CrossRef]
- Fanconi, A.; Fischer, J.A.; Prader, A. Serum parathyroid hormone concentrations in hypophosphataemic vitamin D resistant rickets. Helv Paediatr. Acta 1974, 29, 187–194. [Google Scholar]
- Ivanovic-Zuvic, D.; Santander, M.J.; Jimenez, M.; Novoa, I.; Winter, M.; Florenzano, P. Characterization of otologic involvement in patients with X-Linked Hypophosphatemia. Clin. Otolaryngol. 2021, 46, 1251–1256. [Google Scholar] [CrossRef]
- Glorieux, F.H.; Marie, P.J.; Pettifor, J.M.; Delvin, E.E. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N. Engl. J. Med. 1980, 303, 1023–1031. [Google Scholar] [CrossRef]
- Costa, T.; Marie, P.J.; Scriver, C.R.; Cole, D.E.; Reade, T.M.; Nogrady, B.; Glorieux, F.H.; Delvin, E.E. X-linked hypophosphatemia: Effect of calcitriol on renal handling of phosphate, serum phosphate, and bone mineralization. J. Clin. Endocrinol. Metab. 1981, 52, 463–472. [Google Scholar] [CrossRef]
- Harrell, R.M.; Lyles, K.W.; Harrelson, J.M.; Friedman, N.E.; Drezner, M.K. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia. Induction and maintenance with phosphorus and calcitriol. J. Clin. Investig. 1985, 75, 1858–1868. [Google Scholar] [CrossRef]
- Sullivan, W.; Carpenter, T.; Glorieux, F.; Travers, R.; Insogna, K. A prospective trial of phosphate and 1,25-dihydroxyvitamin D3 therapy in symptomatic adults with X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 1992, 75, 879–885. [Google Scholar]
- Jin, C.; Zhang, C.; Ni, X.; Zhao, Z.; Xu, L.; Wu, B.; Chi, Y.; Jiajue, R.; Jiang, Y.; Wang, O.; et al. The efficacy and safety of different doses of calcitriol combined with neutral phosphate in X-linked hypophosphatemia: A prospective study. Osteoporos. Int. 2022, 33, 1385–1395. [Google Scholar] [CrossRef]
- Verge, C.F.; Lam, A.; Simpson, J.M.; Cowell, C.T.; Howard, N.J.; Silink, M. Effects of therapy in X-linked hypophosphatemic rickets. N. Engl. J. Med. 1991, 325, 1843–1848. [Google Scholar] [CrossRef]
- DeLacey, S.; Liu, Z.; Broyles, A.; El-Azab, S.A.; Guandique, C.F.; James, B.C.; Imel, E.A. Hyperparathyroidism and parathyroidectomy in X-linked hypophosphatemia patients. Bone 2019, 127, 386–392. [Google Scholar] [CrossRef]
- Lecoq, A.L.; Chaumet-Riffaud, P.; Blanchard, A.; Dupeux, M.; Rothenbuhler, A.; Lambert, B.; Durand, E.; Boros, E.; Briot, K.; Silve, C.; et al. Hyperparathyroidism in Patients With X-Linked Hypophosphatemia. J. Bone Miner. Res. 2020, 35, 1263–1273. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Tamada, T.; Kasai, N.; Urakawa, I.; Aono, Y.; Hasegawa, H.; Fujita, T.; Kuroki, R.; Yamashita, T.; Fukumoto, S.; et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 2008, 23, 1509–1518. [Google Scholar] [CrossRef]
- Aono, Y.; Yamazaki, Y.; Yasutake, J.; Kawata, T.; Hasegawa, H.; Urakawa, I.; Fujita, T.; Wada, M.; Yamashita, T.; Fukumoto, S.; et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J. Bone Miner. Res. 2009, 24, 1879–1888. [Google Scholar] [CrossRef]
- Aono, Y.; Hasegawa, H.; Yamazaki, Y.; Shimada, T.; Fujita, T.; Yamashita, T.; Fukumoto, S. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J. Bone Miner. Res. 2011, 26, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Padidela, R.; Whyte, M.P.; Glorieux, F.H.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Portale, A.A.; Simmons, J.H.; Namba, N.; Cheong, H.I.; et al. Patient-Reported Outcomes from a Randomized, Active-Controlled, Open-Label, Phase 3 Trial of Burosumab Versus Conventional Therapy in Children with X-Linked Hypophosphatemia. Calcif. Tissue Int. 2021, 108, 622–633. [Google Scholar] [CrossRef]
- Imanishi, Y.; Ito, N.; Rhee, Y.; Takeuchi, Y.; Shin, C.S.; Takahashi, Y.; Onuma, H.; Kojima, M.; Kanematsu, M.; Kanda, H.; et al. Interim Analysis of a Phase 2 Open-Label Trial Assessing Burosumab Efficacy and Safety in Patients With Tumor-Induced Osteomalacia. J. Bone Miner. Res. 2021, 36, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Jan de Beur, S.M.; Miller, P.D.; Weber, T.J.; Peacock, M.; Insogna, K.; Kumar, R.; Rauch, F.; Luca, D.; Cimms, T.; Roberts, M.S.; et al. Burosumab for the Treatment of Tumor-Induced Osteomalacia. J. Bone Miner. Res. 2021, 36, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Imel, E.A.; Ruppe, M.D.; Weber, T.J.; Klausner, M.A.; Wooddell, M.M.; Kawakami, T.; Ito, T.; Zhang, X.; Humphrey, J.; et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Investig. 2014, 124, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Imel, E.A.; Ruppe, M.D.; Weber, T.J.; Klausner, M.A.; Ito, T.; Vergeire, M.; Humphrey, J.; Glorieux, F.H.; Portale, A.A.; et al. Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J. Clin. Pharmacol. 2016, 56, 176–185. [Google Scholar] [CrossRef]
- Zhang, X.; Peyret, T.; Gosselin, N.H.; Marier, J.F.; Imel, E.A.; Carpenter, T.O. Population pharmacokinetic and pharmacodynamic analyses from a 4-month intradose escalation and its subsequent 12-month dose titration studies for a human monoclonal anti-FGF23 antibody (KRN23) in adults with X-linked hypophosphatemia. J. Clin. Pharmacol. 2016, 56, 429–438. [Google Scholar] [CrossRef]
- Imel, E.A.; Zhang, X.; Ruppe, M.D.; Weber, T.J.; Klausner, M.A.; Ito, T.; Vergeire, M.; Humphrey, J.S.; Glorieux, F.H.; Portale, A.A.; et al. Prolonged Correction of Serum Phosphorus in Adults With X-Linked Hypophosphatemia Using Monthly Doses of KRN23. J. Clin. Endocrinol. Metab. 2015, 100, 2565–2573. [Google Scholar] [CrossRef]
- Ruppe, M.D.; Zhang, X.; Imel, E.A.; Weber, T.J.; Klausner, M.A.; Ito, T.; Vergeire, M.; Humphrey, J.S.; Glorieux, F.H.; Portale, A.A.; et al. Effect of four monthly doses of a human monoclonal anti-FGF23 antibody (KRN23) on quality of life in X-linked hypophosphatemia. Bone Rep. 2016, 5, 158–162. [Google Scholar] [CrossRef]
- Insogna, K.L.; Briot, K.; Imel, E.A.; Kamenicky, P.; Ruppe, M.D.; Portale, A.A.; Weber, T.; Pitukcheewanont, P.; Cheong, H.I.; Jan de Beur, S.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial Evaluating the Efficacy of Burosumab, an Anti-FGF23 Antibody, in Adults With X-Linked Hypophosphatemia: Week 24 Primary Analysis. J. Bone Miner. Res. 2018, 33, 1383–1393. [Google Scholar] [CrossRef]
- Portale, A.A.; Carpenter, T.O.; Brandi, M.L.; Briot, K.; Cheong, H.I.; Cohen-Solal, M.; Crowley, R.; Jan De Beur, S.; Eastell, R.; Imanishi, Y.; et al. Continued Beneficial Effects of Burosumab in Adults with X-Linked Hypophosphatemia: Results from a 24-Week Treatment Continuation Period After a 24-Week Double-Blind Placebo-Controlled Period. Calcif. Tissue Int. 2019, 105, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Briot, K.; Portale, A.A.; Brandi, M.L.; Carpenter, T.O.; Cheong, H.I.; Cohen-Solal, M.; Crowley, R.K.; Eastell, R.; Imanishi, Y.; Ing, S.; et al. Burosumab treatment in adults with X-linked hypophosphataemia: 96-week patient-reported outcomes and ambulatory function from a randomised phase 3 trial and open-label extension. RMD Open 2021, 7, e001714. [Google Scholar] [CrossRef]
- Insogna, K.L.; Rauch, F.; Kamenicky, P.; Ito, N.; Kubota, T.; Nakamura, A.; Zhang, L.; Mealiffe, M.; San Martin, J.; Portale, A.A. Burosumab Improved Histomorphometric Measures of Osteomalacia in Adults with X-Linked Hypophosphatemia: A Phase 3, Single-Arm, International Trial. J. Bone Miner. Res. 2019, 34, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Fratzl-Zelman, N.; Hartmann, M.A.; Gamsjaeger, S.; Rokidi, S.; Paschalis, E.P.; Blouin, S.; Zwerina, J. Bone matrix mineralization and response to burosumab in adult patients with X -linked hypophosphatemia: Results from the phase 3, single-arm international trial. J. Bone Miner. Res. 2022 in press. [CrossRef] [PubMed]
- Linglart, A.; Biosse-Duplan, M.; Briot, K.; Chaussain, C.; Esterle, L.; Guillaume-Czitrom, S.; Kamenicky, P.; Nevoux, J.; Prie, D.; Rothenbuhler, A.; et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr. Connect. 2014, 3, R13–R30. [Google Scholar] [CrossRef] [PubMed]
| Poor adherence to medication |
| Gastrointestinal side effects |
| Secondary hyperparathyroidism |
| Ectopic calcification |
| Renal insufficiency |
| Insufficient effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imanishi, Y.; Shoji, T.; Emoto, M. Complications and Treatments in Adult X-Linked Hypophosphatemia. Endocrines 2022, 3, 560-569. https://doi.org/10.3390/endocrines3030047
Imanishi Y, Shoji T, Emoto M. Complications and Treatments in Adult X-Linked Hypophosphatemia. Endocrines. 2022; 3(3):560-569. https://doi.org/10.3390/endocrines3030047
Chicago/Turabian StyleImanishi, Yasuo, Tetsuo Shoji, and Masanori Emoto. 2022. "Complications and Treatments in Adult X-Linked Hypophosphatemia" Endocrines 3, no. 3: 560-569. https://doi.org/10.3390/endocrines3030047
APA StyleImanishi, Y., Shoji, T., & Emoto, M. (2022). Complications and Treatments in Adult X-Linked Hypophosphatemia. Endocrines, 3(3), 560-569. https://doi.org/10.3390/endocrines3030047







