Abstract
Since phosphate is indispensable for skeletal mineralization, chronic hypophosphatemia causes rickets and osteomalacia. Fibroblast growth factor 23 (FGF23), which is mainly produced by osteocytes in bone, functions as the central regulator of phosphate metabolism by increasing the renal excretion of phosphate and suppressing the production of 1,25-dihydroxyvitamin D. The excessive action of FGF23 results in hypophosphatemic diseases, which include a number of genetic disorders such as X-linked hypophosphatemic rickets (XLH) and tumor-induced osteomalacia (TIO). Phosphate-regulating gene homologous to endopeptidase on the X chromosome (PHEX), dentin matrix protein 1 (DMP1), ectonucleotide pyrophosphatase phosphodiesterase-1, and family with sequence similarity 20c, the inactivating variants of which are responsible for FGF23-related hereditary rickets/osteomalacia, are highly expressed in osteocytes, similar to FGF23, suggesting that they are local negative regulators of FGF23. Autosomal dominant hypophosphatemic rickets (ADHR) is caused by cleavage-resistant variants of FGF23, and iron deficiency increases serum levels of FGF23 and the manifestation of symptoms in ADHR. Enhanced FGF receptor (FGFR) signaling in osteocytes is suggested to be involved in the overproduction of FGF23 in XLH and autosomal recessive hypophosphatemic rickets type 1, which are caused by the inactivation of PHEX and DMP1, respectively. TIO is caused by the overproduction of FGF23 by phosphaturic tumors, which are often positive for FGFR. FGF23-related hypophosphatemia may also be associated with McCune-Albright syndrome, linear sebaceous nevus syndrome, and the intravenous administration of iron. This review summarizes current knowledge on the pathogenesis of FGF23-related hypophosphatemic diseases.
1. Introduction
Phosphorus is an essential nutrient that mediates the majority of biological processes, including the integrity of cell membranes, the maintenance and inheritance of genetic information, energy metabolism, and the regulation of protein function by phosphorylation []. In vertebrates, phosphorus also contributes to skeletal mineralization as a constituent of hydroxyapatite (calcium-phosphate crystals). In the human adult body, approximately 90% of total phosphorus is stored in bone, while the remainder is present in the soft tissues and less than 1% in extracellular fluid []. In serum, the majority of phosphorus exists as free ions of inorganic phosphate (Pi), such as HPO42− and H2PO4−, at a ratio of 4:1 at physiological pH []. Serum levels of Pi are influenced by age, dietary intake, serum pH, and so on.
Since phosphate is indispensable for the formation of hydroxyapatite, its chronic deficiency or wasting leads to impaired skeletal mineralization, namely, rickets in children and osteomalacia in adults []. At the beginning of this century, fibroblast growth factor 23 (FGF23) was identified as the molecule responsible for autosomal dominant hypophosphatemic rickets (ADHR) and tumor-induced hypophosphatemic osteomalacia (TIO) [,]. Since then, our understanding of the mechanisms underlying phosphate metabolism and the pathogenesis of various related diseases has increased. This review discusses current concepts on the role of FGF23 in phosphate homeostasis and the pathogenesis of FGF23-related hypophosphatemic diseases.
2. Phosphorus Homeostasis
In mammals, the phosphate balance in postnatal life is maintained by its intestinal absorption, renal excretion, and accumulation in and release from bone and soft tissue []. The insufficient absorption, excess renal excretion, or excessive shift of Pi to bone or soft tissue may cause hypophosphatemia []. Since phosphate is indispensable for skeletal mineralization, chronic hypophosphatemia leads to rickets and osteomalacia.
Pi absorption in the intestines is mediated by two processes: a passive, paracellular diffusion process and an active, transcellular transport process. The latter is mediated by the type IIb Na+/Pi co-transporter (NaPi-IIb), which is encoded by the SLC34A2 gene in humans []. The expression of NaPi-IIb in the intestines is up-regulated by the low dietary intake of Pi and 1,25-dihydroxyvitamin D (1,25(OH)2D), an active metabolite of vitamin D [,]. A dietary deficiency of phosphate is rare because the majority of foods contain large amounts of phosphate. However, the insufficient action of vitamin D decreases the absorption of Pi in the intestines.
In the kidneys, the majority of Pi filtered in the glomeruli is mainly reabsorbed by type IIa and IIc Na+/Pi co-transporters (NaPi-IIa and NaPi-IIc) localized at the brush border membrane (BBM) of the proximal tubules [,,]. NaPi-IIa and NaPi-IIc are encoded by the SLC34A1 and SLC34A3 genes, respectively, in humans. Inactivating variants in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria (HHRH) []. HHRH is characterized by renal Pi wasting, hypophosphatemia, increased levels of serum 1,25(OH)2D, and secondary hypocalciuria. On the other hand, inactivating variants in the SLC34A1 gene have been shown to cause Fanconi renotubular syndrome 2, infantile hypercalciuria 2, and nephrolithiasis/osteoporosis associated with hypophosphatemia [].
Endocrine factors including 1,25(OH)2D, parathyroid hormone (PTH), and FGF23 play critical roles in phosphate homeostasis. As described above, 1,25(OH)2D increases the intestinal absorption of Pi by up-regulating NaPi-IIb []. PTH increases the renal excretion of Pi by decreasing the amounts of NaPi-IIa and NaPi-IIc that localize in the BBM [,,]. FGF23 functions as the central regulator of phosphate metabolism, as described in the following section.
3. Roles of FGF23 in Mineral Metabolism
FGF23 is a 32-kDa protein that consists of 251 amino acids including a 24-amino acid signal peptide at its N terminus []. It is mainly produced by osteoblast lineage cells, particularly osteocytes, which terminally differentiate from osteoblasts and are embedded within the bone matrix []. In adult bone, osteocytes make up more than 90% to 95% of all bone cells. Although the location and inaccessibility of osteocytes in the mineralized bone matrix delayed our understanding of their cellular functions, studies conducted in the last few decades have revealed their roles in bone homeostasis. Osteocytes are essential for controlling the bone mass in postnatal life by sensing mechanical strain and regulating the formation and resorption of bone []. They also produce sclerostin, an inhibitor of Wnt/β-catenin signaling. Mechanical strain reduces the production of sclerostin by osteocytes, which in turn enhances Wnt/β-catenin signaling and increases bone formation []. In addition, mouse studies suggested that the receptor activator of nuclear factor kappa B ligand produced by osteocytes controls the postnatal bone mass []. The expression of FGF23 by osteocytes indicates that they also play critical roles in phosphate homeostasis.
FGF23 belongs to the FGF19 subfamily together with FGF19 and FGF21, based on their unique features among FGFs []. Members of the FGF19 family exert their effects on distant target organs and tissues in an endocrine manner, and their low binding affinity to heparin/heparan sulfate has been suggested to confer endocrine functions due to less binding to heparan sulfate surrounding their producing cells and their entry into the circulation []. Members of the FGF19 subfamily require the single-pass transmembrane protein Klotho, αKlotho for FGF23, and βKlotho for FGF19 and FGF21, for their signal transduction through an FGF receptor (FGFR) [,,]. A previous study demonstrated that the N-terminal domain of FGF23 bound to FGFR while its C-terminal domain bound to αKlotho [].
Since FGF23 requires αKlotho together with FGFR to exert its effects, organs and tissues expressing both αKlotho and FGFR, such as the kidneys, parathyroid glands, placenta, and choroid plexus, may be the physiological targets of FGF23 [,]. In the kidneys, which are the main target, FGF23 increases urinary Pi excretion by suppressing the expression of NaPi-IIa and NaPi-IIc. In addition, FGF23 decreases the production of 1,25(OH)2D by suppressing the renal expression of Cyp27b1 encoding 25-hydroxyvitamin D 1α-hydroxylase and increasing that of Cyp24a1 encoding 25-hydroxyvitamin D-24-hydroxylase, which leads to a reduction in the absorption of Pi in the intestines [] (Figure 1).
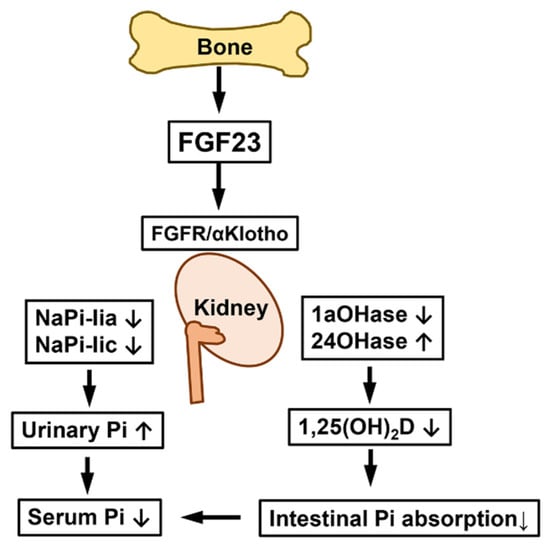
Figure 1.
Effects of FGF23 on kidneys. FGF23 produced in bone binds to FGFR and αKlotho in the kidneys and suppresses the renal expression of NaPi-IIa and NaPi-IIc Na+/Pi co-transporters, which increases urinary Pi excretion and reduces serum Pi levels. In addition, FGF23 decreases the production of 1,25(OH)2D by the down-regulation of 25-hydroxyvitamin D-1α-hydroxylase (1αOHase) and up-regulation of 25-hydroxyvitamin D-24-hydroxylase (24OHase), which reduces the intestinal absorption of Pi and further lowers serum Pi levels.
The findings of animal studies have suggested that FGF23 suppresses the production and secretion of PTH in both αKlotho-dependent and -independent manners [,]. Since 1,25(OH)2D and PTH have been shown to increase the expression of FGF23 [,,,], these factors counter-regulate with FGF23 to maintain phosphate homeostasis. The findings of mouse studies have indicated that maternal FGF23 exerts its effects on the placenta to increase the expression of Cyp24a1; however, it does not alter placental Pi transport [].
FGF23 is inactivated by cleavage between Arg179 and Ser180 via a subtilisin-like proprotein convertase, which recognizes the motif R176XXR179/S180 []. FGF23 is O-glycosylated at Thr178 by the enzyme UDP-N-acetyl-α-d-galacosamine:polypeptide N-acetylgalactosaminyltransferase 3 (GalNAc-T3), which is suggested to prevent cleavage by the subtilisin-like proprotein convertase []. Inactivating mutations in the GALNT3 gene encoding GalNAc-T3 result in the cleavage of intact FGF23 before secretion, and the resultant deficiency of intact FGF23 causes hyperphosphatemic familial tumoral calcinosis (HFTC), a rare autosomal recessive disease characterized by hyperphosphatemia, normal or elevated levels of serum 1,25(OH)2D, and ectopic calcification [,]. Inactivating mutations in the FGF23 gene itself and the KLOTHO gene encoding αKlotho were also found to be responsible for HFTC [,,].
Both Fgf23-knockout mice and Klotho-deficient mice exhibit hyperphosphatemia with increased renal Pi reabsorption and elevated serum 1,25(OH)2D levels, which resemble the features in patients with HFTC [,]. These mutant mice also show severe growth retardation with abnormal skeletal phenotype and short lifespan. Interestingly, Fgf23-knockout mice display a marked accumulation of osteoid despite hyperphosphatemia []. Although mechanisms for this counterintuitive observation remain unclear, it may suggest the effects of FGF23 to be independent of phosphate metabolism.
4. Pathogenesis of FGF23-Related Hypophosphatemic Diseases
4.1. FGF23-Related Hypophosphatemic Diseases
Since the FGF23-αKlotho axis plays a central role in phosphate homeostasis, its disruption causes hyperphosphatemic conditions, as described in the previous section. On the other hand, the excessive action of FGF23 underlies various hypophosphatemic diseases, which are characterized by urinary phosphate wasting, hypophosphatemia, and inappropriately low levels of serum 1,25(OH)2D [,]. Phosphate is indispensable for skeletal mineralization; therefore, chronic hypophosphatemia due to excessive FGF23 leads to rickets in children and osteomalacia in adults. The impaired production of 1,25(OH)2D contributes to the resistance of FGF23-related hypophosphatemic rickets/osteomalacia to native vitamin D. FGF23-related hypophosphatemic rickets/osteomalacia include various conditions such as genetic diseases (Table 1 and Table 2). The following sections will describe the pathogenesis of each condition.

Table 1.
FGF23-related hypophosphatemic diseases and their causes.

Table 2.
Clinical features of FGF23-related hypophosphatemic diseases.
4.2. Autosomal Dominant Hypophosphatemic Rickets (ADHR)
ADHR (OMIM #193100) is caused by missense variants in the FGF23 gene. Since the responsible variants occur at Arg176 or Arg179 within the RXXR/S motif recognized by subtilisin-like proprotein convertase, the resultant mutant FGF23 protein is resistant to cleavage-mediated inactivation []. However, this disease shows incomplete penetrance. Patients with ADHR variants do not always have high serum levels of intact FGF23, and the disease may occur with an early or delayed onset and variable expressivity []. Late-onset ADHR primarily manifests in post-pubertal women who are prone to iron deficiency []. Serum iron levels were previously shown to negatively correlate with serum levels of both the C-terminal fragment of FGF23 and intact FGF23 in ADHR patients; however, serum iron levels also negatively correlated with C-terminal FGF23 levels in healthy control subjects, whereas no relationship was observed with intact FGF23 levels []. Farrow et al. generated FGF23-knock-in mice carrying the R176Q ADHR point mutation (ADHR mice) and found that a low-iron diet increased bone FGF23 mRNA levels and the serum levels of both intact and C-terminal FGF23 with hypophosphatemia in ADHR mice. On the other hand, wild-type mice fed the low-iron diet showed normal serum levels of intact FGF23 and phosphate, but an elevated level of the C-terminal fragment of FGF23 []. Furthermore, the chelation of iron up-regulated the expression of Fgf23 in a cultured osteoblastic cell line, which involved hypoxia-inducible factor 1α. Collectively, these findings suggest that iron deficiency increases the expression of Fgf23 in bone and also that the FGF23 protein is cleaved in iron deficiency to maintain normal serum levels of FGF23 and normophosphatemia in control subjects, whereas the cleavage resistance of mutant FGF23 leads to the accumulation of intact FGF23 and hypophosphatemia in ADHR subjects [].
4.3. X-Linked Hypophosphatemic Rickets (XLH)
XLH (OMIM #307800) is the most common form of hereditary hypophosphatemic rickets. Patients with XLH have elevated serum levels of FGF23, which result in urinary Pi wasting, hypophosphatemia, and inappropriately low levels of 1,25(OH)2D []. XLH was initially called vitamin D-resistant rickets because of a poor response to treatment with native vitamin D at dosages that cure vitamin D-deficient rickets. XLH is caused by inactivating variants in the phosphate-regulating gene homologous to endopeptidase on the X chromosome (PHEX) located at Xp22.1, showing X-linked dominant inheritance []. Similar to FGF23, PHEX is expressed in osteoblast lineage cells and is more highly expressed in osteocytes [,]. Although its structure suggests that the product of PHEX functions as a cell surface-bound, zinc-dependent protease, its physiological substrates remain elusive. In hypophosphatemic Hyp mice, which harbor a large deletion in the Phex gene and are widely used as a model for XLH, the expression of Fgf23 in osteocytes was found to be increased [,]; however, FGF23 did not serve as a substrate for PHEX []. Therefore, the regulation of FGF23 by PHEX may be indirect and involve other molecule(s).
Since PHEX is highly expressed in osteocytes, in an attempt to clarify abnormalities in Phex-deficient osteocytes, we previously compared the gene expression profiles of osteoblasts and osteocytes in Hyp mice and wild-type littermates []. As osteoblasts mature into osteocytes, the expression of dentin matrix protein 1 (Dmp1) and family with sequence similarity 20c (Fam20c), which are responsible for autosomal recessive hypophosphatemic type 1 (ARHR1) and Raine syndrome (RNS), respectively, increased in both Hyp and wild-type cells, and these genes were up-regulated in Hyp cells, similar to Fgf23 []. These findings indicated the critical roles of osteocytes in phosphate homeostasis and also suggested complex abnormalities in Phex-deficient osteocytes. We found that the expression of the genes encoding canonical FGF ligands (Fgf1 and Fgf2), their receptors (Fgfr1-3), and early growth response 1, which is a target for FGFR activation, was also up-regulated in Hyp osteocytes, indicating enhanced FGFR signaling []. Furthermore, Martin et al. suggested enhanced FGFR signaling in Hyp bone [], and Xiao et al. demonstrated that the conditional deletion of Fgfr1 in osteocytes and mature osteoblasts partially restored the overproduction of FGF23 and ameliorated hypophosphatemia and rickets []. The regulation of FGF23 production by FGFR signaling is also supported by osteoglophonic dysplasia, which is a rare skeletal dysplasia caused by activating mutations in FGFR1 that is frequently associated with elevated serum FGF23 levels and hypophosphatemia [].
Enhanced FGFR signaling in Phex-deficient osteocytes is of interest based on previous findings suggesting that FGFR plays a critical role in the transduction of signaling evoked by increased extracellular Pi [,,]. In various cell types, treatment with high extracellular Pi activated FGFR for the regulation of gene expression. In an osteoblastic cell line, treatment with an FGFR inhibitor abolished the up-regulation of Dmp1 by increased extracellular Pi []. In HEK293 cells, the knockdown of FGFR1 diminished the Pi-induced phosphorylation of ERK1/2 []. More recently, the activation of FGFR1 by extracellular Pi was shown to increase the expression of Galnt3 in bone, leading to an elevated serum level of FGF23 in mice []. Collectively, these findings suggest that FGFR1 is involved in the sensing of Pi availability. In consideration of this role of FGFR and enhanced FGFR signaling in the osteocytes of Hyp mice, abnormal Pi sensing may be involved in the pathogenesis of XLH.
Matrix extracellular phosphoglycoprotein (MEPE) is a member of the SIBLING (small integrin-binding ligand, N-linked glycoproteins) family, and was initially cloned from the tumor of a patient with TIO []. A genome-wide association study proposed MEPE as a factor influencing bone mineral density in humans []. MEPE contains an acidic serine-aspartate rich MEPE-associated motif (ASARM) consisting of 23 residues at the C terminus. ASARM peptides released from MEPE by cathepsin-mediated cleavage have been shown to inhibit mineralization. Rowe et al. demonstrated that PHEX bound to the ASARM motif in MEPE and the released ASARM peptide and its serum levels were elevated in Hyp mice [,]. The ASARM motif is also present in other SIBLING proteins, such as DMP1 and osteopontin, and PHEX may bind to these proteins at these motifs []. A previous study implicated ASARM peptides released from these SIBLING proteins in defective mineralization in XLH [].
Growth retardation is often observed in patients with XLH: however, the underlying mechanisms have not yet been elucidated in detail. A study published by Fuente et al. demonstrated marked alterations in the structure, dynamics, and maturation of growth plate cartilage in growth-retarded young Hyp mice []. In the growth plates of Hyp mice, both proliferation and apoptosis rates of chondrocytes were reduced, and the hypertrophy and maturation of chondrocytes were severely disturbed. The spatial organization of the chondro-osseous junction and the primary spongiosa trabeculae were markedly deformed. These alterations in the growth plates might be the mechanisms for the growth retardation in Hyp mice. The authors also found an enhanced activation of the extracellular signal-regulated kinase (ERK)1/2 signaling pathway in the Hyp growth plates, implying an involvement of FGF23 in these abnormalities []. Reduction in caspase-mediated apoptosis of hypertrophic chondrocytes was also reported in rachitic mice with low-phosphate diet-induced hypophosphatemia as well as in Hyp mice, which suggests that hypophosphatemia impairs apoptosis of hypertrophic chondrocytes, leading to rickets [].
Although chondrocytes do not express αKlotho, which is required for FGF23 to activate its downstream signaling pathways at physiological concentrations, soluble forms of αKlotho are present in serum and cerebrospinal fluid [] and have been implicated in the regulation of FGF23 signaling in cells without the transmembrane form of αKlotho []. We previously demonstrated that FGF23 suppressed the linear growth of mouse metatarsal cartilage in cultures in the presence of soluble αKlotho by decreasing the proliferation of chondrocytes, which suggests that suppressed chondrocyte proliferation by FGF23 plays a causative role in the growth retardation associated with XLH [].
Since the placenta expresses FGFR1 and αKlotho, high levels of FGF23 in pregnant women with XLH may affect their fetuses. We previously investigated this issue using pregnant Hyp female mice []. Hyp and wild-type female mice were mated with wild-type male mice, and the pregnant mothers and their male fetuses were subjected to analyses. FGF23 levels were higher in Hyp mothers than in wild-type mothers. Hyp fetuses and wild-type fetuses were obtained from mating between Hyp females and wild-type males. FGF23 levels in Hyp fetuses were approximately 20-fold higher than in their mothers, while wild-type fetuses from Hyp mothers had low levels of FGF23, as did fetuses from wild-type mothers, suggesting that FGF23 does not cross the placenta []. The expression of Cyp24a1 was higher in the placentas of fetuses from Hyp mothers than in those of fetuses from wild-type mothers, which resulted in decreased levels of plasma 25-hydroxyvitamin D in fetuses from Hyp mothers. Therefore, increased levels of circulating FGF23 in Hyp mothers may exert direct effects on the placenta during pregnancy and alter fetal vitamin D metabolism via the regulation of Cyp24a1 expression []. Further studies are needed to clarify whether similar phenomena occur with pregnancy in human patients with XLH.
The enthesis is a tissue that forms at the site of insertion of a tendon to bone and consists of a bony eminence, mineralized fibrocartilage, unmineralized fibrocartilage, and a tendon. It optimizes the transfer of mechanical force from muscle to bone, which is required for efficient movements []. Enthesopathy is a pathological change at the insertion of tendons and ligaments. Mineralizing enthesopathy is one of the complications of XLH and other types of FGF23-related hypophosphatemia and accounts for a high morbidity rate in adult patients []. Karaplis et al. previously reported that a transgenic mouse model overexpressing a secreted form of the human FGF23[p.R176Q] variant, which is resistant to cleavage, displayed mineralizing enthesopathy of the Achilles and planar facial insertions, suggesting the involvement of FGF23 in the development of mineralizing enthesopathy []. More recently, Liu et al. investigated the cellular and molecular mechanisms involved in the development of mineralizing enthesopathy in Hyp mice and reported that Achilles tendon entheses of Hyp mice showed the expansion of hypertrophic-appearing chondrogenic cells. In comparison with the entheses of wild-type mice, Hyp entheses exhibited the expansion of cells expressing the chondrogenic marker gene Sox9 and enhanced bone morphogenetic protein and Indian hedgehog signaling pathways, both of which play critical roles in chondrocyte differentiation []. Although oral phosphate salts and active vitamin D metabolites are administered as conventional medical treatments for XLH to correct their deficiencies, it does not prevent or ameliorate enthesopathies []. Burosumab, a humanized monoclonal neutralizing antibody to FGF23, has recently become available as a new treatment for XLH []. In Japan, burosumab has been approved for the treatment of all types of FGF23-related hypophosphatemic rickets/osteomalacia. In pediatric patients with XLH, improvements in the severity of rickets and biochemical parameters were greater in patients treated with burosumab than in those who continued conventional therapy []. Further studies are needed to clarify the effects of burosumab on the prevention and treatment of enthesopathies.
4.4. Autosomal Recessive Hypophosphatemic Rickets Type 1 (ARHR1)
ARHR1 (OMIM #241520) is caused by inactivating variants of the DMP1 gene [,]. DMP1 is an extracellular matrix protein belonging to the SIBLING family and is highly expressed in osteocytes as well as in dentin. Patients with ARHR1 manifest elevated FGF23 levels, hypophosphatemia, inappropriately low 1,25(OH)2D levels, and skeletal hypomineralization, similar to patients with XLH. Dmp1-null mice reproduced the phenotype of ARHR1 and exhibited defective osteocyte maturation and the up-regulated expression of Fgf23 in osteocytes [,]. Although the pathogenesis of ARHR1 remains largely unknown, the findings of studies using Phex-deficient Hyp mice and Dmp1-null mice suggest that the overproduction of FGF23 is attributable to enhanced FGFR signaling in bone in both mouse models [].
4.5. Autosomal Recessive Hypophosphatemic Rickets Type 2 (ARHR2)
ARHR2 (OMIM #613312) also belongs to FGF23-related hypophosphatemic rickets and is caused by inactivating variants in the ectonucleotide pyrophosphatase phosphodiesterase-1 (ENPP1) gene [,]. ENPP1 encodes an enzyme that produces pyrophosphate (PPi), a potent inhibitor of mineralization, and inactivating variants in ENPP1 are also responsible for hypermineralization disorders, such as generalized arterial calcification in infancy []. The ectoenzyme tissue non-specific alkaline phosphatase (TNSALP) facilitates skeletal mineralization by degrading PPi to produce Pi. Although PPi may regulate the production of FGF23, patients with hypophosphatasia, which is caused by inactivating variants in TNSALP, had normal levels of FGF23 despite elevated extracellular levels of PPi []. Therefore, the mechanisms by which ENPP1 deficiency results in the overproduction of FGF23 remain unclear. Since inactivating variants in ENPP1 cause conditions characterized by ectopic calcification and FGF23-related hypophosphatemia, a close relationship may exist between ectopic calcification and the overproduction of FGF23.
4.6. Raine Syndrome (RNS)
FAM20C, also known as DMP4, encodes a kinase that phosphorylates various secreted proteins. The proteins phosphorylated by FAM20C include FGF23 and members of the SIBLING family, such as DMP1, osteopontin, and MEPE [,]. Inactivating variants in the FAM20C gene are responsible for RNS (OMIM #259775). RNS is an autosomal recessive disease that is characterized by craniofacial malformation, osteosclerotic bone dysplasia, and a poor prognosis []. Surviving patients with mild RNS manifest hypophosphatemia due to elevated levels of FGF23 and dental anomalies [,]. Fam20c-null mice exhibited elevated levels of serum FGF23, hypophosphatemia, and dental anomalies []. These mice also showed low expression levels of Dmp1 in osteocytes, which suggested that the down-regulated expression of DMP1 plays a causal role in the overproduction of FGF23 in RNS []. However, the overexpression of Dmp1 failed to rescue the defects in Fam20c-null mice []. A previous study reported that FAM20C phosphorylated FGF23 on Ser180, which inhibited the O-glycosylation of FGF23 on Thr178 by GalNAc-T3 and accelerated cleavage []. Therefore, inactivating variants in FAM20C may increase FGF23 levels by inhibiting its cleavage.
4.7. Tumor-Induced Osteomalacia (TIO)
TIO is a rare paraneoplastic syndrome characterized by urinary phosphate wasting, hypophosphatemia, and osteomalacia. Responsible tumors are generally benign, slow-growing phosphaturic mesenchymal tumors (PMT) []. The overproduction of FGF23 by tumors was previously shown to enhance the renal excretion of Pi and induce hypophosphatemia, low 1,25(OH)2D levels, and osteomalacia, which were cured by the surgical removal of the responsible tumor [,]. Lee et al. identified the fusion genes Fibronectin 1 (FN1)-FGFR1 and FN1-FGF1 in subgroups of PMT and showed that immunoreactivity for FGFR1 was positive in 82% of PMT [,]. These findings suggest the involvement of the FGF/FGFR signaling pathway in the development of PMT.
4.8. Other Causes of FGF23-Related Hypophosphatemia
McCune-Albright syndrome (MAS, OMIM #174800) is characterized by polyostotic fibrous dysplasia, café-au-lait skin pigmentation, and precocious puberty, and is caused by a somatic activating variant in GNAS1 encoding the subunit of the stimulatory G protein. MAS is clinically heterogeneous and may manifest various endocrinological abnormalities. Some patients with MAS exhibit hypophosphatemia, which results from the overproduction of FGF23 by abnormal skeletal progenitor cells in the bone lesions of fibrous dysplasia []. Serum levels of FGF23 in MAS patients correlate with disease activity [], and significant hypophosphatemia only occurs in patients with a severe disease burden. A previous study suggested that the ratio of the C-terminal fragment of FGF23 to intact FGF23 was elevated by accelerated cleavage in the bone lesions of fibrous dysplasia [].
Linear sebaceous nevus syndrome, also called Schimmelpenning-Feuerstein-Mims (SFM) syndrome (OMIM #163200), is characterized by congenital linear nevus sebaceous and abnormalities in neuroectodermal organs and is caused by somatic variants in RAS genes, including KRAS, HRAS, and NRAS, which are detectable in skin lesions [,]. Hypophosphatemia due to elevated levels of FGF23 is rarely associated with SFM syndrome. Lim et al. suggested that the source of FGF23 in SFM syndrome was bone lesions carrying RAS variants rather than skin lesions [].
Osteoglophonic dysplasia (OMIM #166250) is a rare autosomal dominant disease characterized by rhizomelic dwarfism, non-ossifying bone lesions, craniosynostosis, and face abnormalities, and is caused by activating variants in the FGFR1 gene. As discussed earlier, this disease may be associated with FGF23-related hypophosphatemia, indicating the involvement of FGFR1 in the regulation of FGF23 production [].
Jansen’s metaphyseal chondrodysplasia (OMIM #156400) is an autosomal dominant disease caused by an activating variant in the PTH type 1 receptor (PTH1R) gene []. Previous studies reported that FGF23-related hypophosphatemia may be associated with Jansen’s metaphyseal chondrodysplasia []. This finding suggests that PTH signaling stimulates FGF23 production, which is also supported by the findings of several in vivo and in vitro studies [,,].
FGF23-related hypophosphatemic rickets/osteomalacia may also be associated with the intravenous administration of saccharated ferric oxide or iron polymaltose [,]. The mechanisms by which these drugs cause the overproduction of FGF23 remain unclear; however, their discontinuance rapidly restores elevated FGF23 levels and hypophosphatemia.
5. Conclusions
FGF23-related hypophosphatemia is characterized by urinary Pi wasting, hypophosphatemia, and inappropriately low levels of 1,25(OH)2D, and includes various types of hereditary rickets/osteomalacia, such as XLH, and acquired diseases, including TIO. The molecules responsible for hereditary rickets/osteomalacia are highly expressed by osteocytes, indicating that these cells play a central role in phosphate homeostasis. Since inactivating variants of PHEX, DMP1, ENPP1, and FAM20C lead to the overproduction of FGF23, these molecules appear to function as negative regulators of FGF23. Although the mechanisms underlying the overproduction of FGF23 remain unclear in most FGF23-related hypophosphatemic diseases, enhanced FGFR signaling may be involved in the overproduction of FGF23 in XLH and ARHR1 as well as in TIO. Since FGFR1 is suggested to be involved in Pi sensing, abnormalities in Pi sensing may play a role in the pathogenesis of these diseases.
Author Contributions
Conceptualization, T.M.; writing—original draft preparation, T.N. and T.M.; writing—review and editing, T.N. and T.M.; supervision, T.M.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
The preparation of this review was partly supported by a grant from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 21K07835) to T.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a Signaling Molecule and Its Sensing Mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef]
- Mitchell, H.; Hamilton, T.; Steggerda, F.; Bean, H. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J. Biol. Chem. 1945, 158, 625–637. [Google Scholar] [CrossRef]
- Peters, J.P.; Wakeman, A.M.; Lee, C. Total Acid-Base Eqiilibrium of plasma in health and disease: XI. Hypochloremia and Total Salt Deficiency in Nephritis. J. Clin. Investig. 1929, 6, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Michigami, T.; Ozono, K. Roles of Phosphate in Skeleton. Front. Endocrinol. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- ADHR-CONSORTIUM. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Mizutani, S.; Muto, T.; Yoneya, T.; Hino, R.; Takeda, S.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Yamashita, T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. USA 2001, 98, 6500–6505. [Google Scholar] [CrossRef] [PubMed]
- Gaasbeek, A.; Meinders, A.E. Hypophosphatemia: An update on its etiology and treatment. Am. J. Med. 2005, 118, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009, 20, 2348–2358. [Google Scholar] [CrossRef]
- Hattenhauer, O.; Traebert, M.; Murer, H.; Biber, J. Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am. J. Physiol. 1999, 277, G756–G762. [Google Scholar] [CrossRef]
- Capuano, P.; Radanovic, T.; Wagner, C.A.; Bacic, D.; Kato, S.; Uchiyama, Y.; St-Arnoud, R.; Murer, H.; Biber, J. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am. J. Physiol. Cell Physiol. 2005, 288, C429–C434. [Google Scholar] [CrossRef]
- Hernando, N.; Gagnon, K.; Lederer, E. Phosphate Transport in Epithelial and Nonepithelial Tissue. Physiol. Rev. 2021, 101, 1–35. [Google Scholar] [CrossRef]
- Beck, L.; Karaplis, A.C.; Amizuka, N.; Hewson, A.S.; Ozawa, H.; Tenenhouse, H.S. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA 1998, 95, 5372–5377. [Google Scholar] [CrossRef]
- Segawa, H.; Kaneko, I.; Takahashi, A.; Kuwahata, M.; Ito, M.; Ohkido, I.; Tatsumi, S.; Miyamoto, K. Growth-related renal type II Na/Pi cotransporter. J. Biol. Chem. 2002, 277, 19665–19672. [Google Scholar] [CrossRef]
- Bergwitz, C.; Roslin, N.M.; Tieder, M.; Loredo-Osti, J.C.; Bastepe, M.; Abu-Zahra, H.; Frappier, D.; Burkett, K.; Carpenter, T.O.; Anderson, D.; et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 2006, 78, 179–192. [Google Scholar] [CrossRef]
- Bacic, D.; Lehir, M.; Biber, J.; Kaissling, B.; Murer, H.; Wagner, C.A. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006, 69, 495–503. [Google Scholar] [CrossRef]
- Picard, N.; Capuano, P.; Stange, G.; Mihailova, M.; Kaissling, B.; Murer, H.; Biber, J.; Wagner, C.A. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflugers. Arch. 2010, 460, 677–687. [Google Scholar] [CrossRef]
- Segawa, H.; Yamanaka, S.; Onitsuka, A.; Tomoe, Y.; Kuwahata, M.; Ito, M.; Taketani, Y.; Miyamoto, K. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am. J. Physiol. Renal Physiol. 2007, 292, F395–F403. [Google Scholar] [CrossRef]
- Miyagawa, K.; Yamazaki, M.; Kawai, M.; Nishino, J.; Koshimizu, T.; Ohata, Y.; Tachikawa, K.; Mikuni-Takagaki, Y.; Kogo, M.; Ozono, K.; et al. Dysregulated gene expression in the primary osteoblasts and osteocytes isolated from hypophosphatemic Hyp mice. PLoS ONE 2014, 9, e93840. [Google Scholar]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.; Beenken, A.; Ibrahimi, O.A.; Kalinina, J.; Olsen, S.K.; Eliseenkova, A.V.; Xu, C.; Neubert, T.A.; Zhang, F.; Linhardt, R.J.; et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell. Biol. 2007, 27, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Stubbs, J.R.; Liu, S.; Tang, W.; Zhou, J.; Wang, Y.; Yao, X.; Quarles, L.D. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 2007, 18, 2116–2124. [Google Scholar] [CrossRef]
- Ben-Dov, I.Z.; Galitzer, H.; Lavi-Moshayoff, V.; Goetz, R.; Kuro-o, M.; Mohammadi, M.; Sirkis, R.; Naveh-Many, T.; Silver, J. The parathyroid is a target organ for FGF23 in rats. J. Clin. Investig. 2007, 117, 4003–4008. [Google Scholar] [CrossRef]
- Olauson, H.; Lindberg, K.; Amin, R.; Jia, T.; Wernerson, A.; Andersson, G.; Larsson, T.E. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J. Am. Soc. Nephrol. 2012, 23, 1641–1651. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Zhou, J.; Stubbs, J.R.; Luo, Q.; Pi, M.; Quarles, L.D. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006, 17, 1305–1315. [Google Scholar] [CrossRef]
- Haussler, M.R.; Livingston, S.; Sabir, Z.L.; Haussler, C.A.; Jurutka, P.W. Vitamin D Receptor Mediates a Myriad of Biological Actions Dependent on Its 1,25-Dihydroxyvitamin D Ligand: Distinct Regulatory Themes Revealed by Induction of Klotho and Fibroblast Growth Factor-23. JBMR Plus 2021, 5, e10432. [Google Scholar] [CrossRef]
- Kawata, T.; Imanishi, Y.; Kobayashi, K.; Miki, T.; Arnold, A.; Inaba, M.; Nishizawa, Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 2007, 18, 2683–2688. [Google Scholar] [CrossRef]
- Rhee, Y.; Bivi, N.; Farrow, E.; Lezcano, V.; Plotkin, L.I.; White, K.E.; Bellido, T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 2011, 49, 636–643. [Google Scholar] [CrossRef]
- Ohata, Y.; Yamazaki, M.; Kawai, M.; Tsugawa, N.; Tachikawa, K.; Koinuma, T.; Miyagawa, K.; Kimoto, A.; Nakayama, M.; Namba, N.; et al. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J. Bone Miner. Res. 2014, 29, 1627–1638. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wiley, S.E.; Xiao, J.; Gonzalez, D.J.; Nidumanda Appaiah, H.; Koller, A.; Nizet, V.; White, K.E.; Dixon, J.E. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 5520–5525. [Google Scholar] [CrossRef]
- Kato, K.; Jeanneau, C.; Tarp, M.A.; Benet-Pages, A.; Lorenz-Depiereux, B.; Bennett, E.P.; Mandel, U.; Strom, T.M.; Clausen, H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 2006, 281, 18370–18377. [Google Scholar] [CrossRef]
- Topaz, O.; Shurman, D.L.; Bergman, R.; Indelman, M.; Ratajczak, P.; Mizrachi, M.; Khamaysi, Z.; Behar, D.; Petronius, D.; Friedman, V.; et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 2004, 36, 579–581. [Google Scholar] [CrossRef]
- Ichikawa, S.; Baujat, G.; Seyahi, A.; Garoufali, A.G.; Imel, E.A.; Padgett, L.R.; Austin, A.M.; Sorenson, A.H.; Pejin, Z.; Topouchian, V.; et al. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations. Am. J. Med. Genet. A 2010, 152A, 896–903. [Google Scholar] [CrossRef]
- Benet-Pages, A.; Orlik, P.; Strom, T.M.; Lorenz-Depiereux, B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 2005, 14, 385–390. [Google Scholar] [CrossRef]
- Araya, K.; Fukumoto, S.; Backenroth, R.; Takeuchi, Y.; Nakayama, K.; Ito, N.; Yoshii, N.; Yamazaki, Y.; Yamashita, T.; Silver, J.; et al. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J. Clin. Endocrinol. Metab. 2005, 90, 5523–5527. [Google Scholar] [CrossRef]
- Ichikawa, S.; Imel, E.A.; Kreiter, M.L.; Yu, X.; Mackenzie, D.S.; Sorenson, A.H.; Goetz, R.; Mohammadi, M.; White, K.E.; Econs, M.J. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Investig. 2007, 117, 2684–2691. [Google Scholar] [CrossRef]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and diagnostic criteria for rickets and osteomalacia-proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J. Bone Miner. Metab. 2015, 33, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S. FGF23-related hypophosphatemic rickets/osteomalacia: Diagnosis and new treatment. J. Mol. Endocrinol. 2021, 66, R57–R65. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A.; Hui, S.L.; Econs, M.J. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J. Bone Miner. Res. 2007, 22, 520–526. [Google Scholar] [CrossRef]
- Imel, E.A.; Peacock, M.; Gray, A.K.; Padgett, L.R.; Hui, S.L.; Econs, M.J. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J. Clin. Endocrinol. Metab. 2011, 96, 3541–3549. [Google Scholar] [CrossRef]
- Farrow, E.G.; Yu, X.; Summers, L.J.; Davis, S.I.; Fleet, J.C.; Allen, M.R.; Robling, A.G.; Stayrook, K.R.; Jideonwo, V.; Magers, M.J.; et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl. Acad. Sci. USA 2011, 108, E1146–E1155. [Google Scholar] [CrossRef]
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Levtchenko, E.; et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455. [Google Scholar] [CrossRef]
- HYP-CONSORTIUM. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat. Genet. 1995, 11, 130–136. [Google Scholar] [CrossRef]
- Beck, L.; Soumounou, Y.; Martel, J.; Krishnamurthy, G.; Gauthier, C.; Goodyer, C.G.; Tenenhouse, H.S. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J. Clin. Investig. 1997, 99, 1200–1209. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Tang, W.; Jiang, X.; Rowe, D.W.; Quarles, L.D. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E38–E49. [Google Scholar] [CrossRef]
- Benet-Pages, A.; Lorenz-Depiereux, B.; Zischka, H.; White, K.E.; Econs, M.J.; Strom, T.M. FGF23 is processed by proprotein convertases but not by PHEX. Bone 2004, 35, 455–462. [Google Scholar] [CrossRef]
- Martin, A.; Liu, S.; David, V.; Li, H.; Karydis, A.; Feng, J.Q.; Quarles, L.D. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011, 25, 2551–2562. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, J.; Cao, L.; Liang, Y.; Han, X.; Quarles, L.D. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS ONE 2014, 9, e104154. [Google Scholar] [CrossRef][Green Version]
- White, K.E.; Cabral, J.M.; Davis, S.I.; Fishburn, T.; Evans, W.E.; Ichikawa, S.; Fields, J.; Yu, X.; Shaw, N.J.; McLellan, N.J.; et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am. J. Hum. Genet. 2005, 76, 361–367. [Google Scholar] [CrossRef]
- Yamazaki, M.; Ozono, K.; Okada, T.; Tachikawa, K.; Kondou, H.; Ohata, Y.; Michigami, T. Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J. Cell. Biochem. 2010, 111, 1210–1221. [Google Scholar] [CrossRef]
- Nishino, J.; Yamazaki, M.; Kawai, M.; Tachikawa, K.; Yamamoto, K.; Miyagawa, K.; Kogo, M.; Ozono, K.; Michigami, T. Extracellular Phosphate Induces the Expression of Dentin Matrix Protein 1 Through the FGF Receptor in Osteoblasts. J. Cell. Biochem. 2017, 118, 1151–1163. [Google Scholar] [CrossRef]
- Takashi, Y.; Kosako, H.; Sawatsubashi, S.; Kinoshita, Y.; Ito, N.; Tsoumpra, M.K.; Nangaku, M.; Abe, M.; Matsuhisa, M.; Kato, S.; et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc. Natl. Acad. Sci. USA 2019, 116, 11418–11427. [Google Scholar] [CrossRef]
- Rowe, P.S. The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled. Cell Biochem. Funct. 2012, 30, 355–375. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Kiel, D.P. Clinical review: Genome-wide association studies of skeletal phenotypes: What we have learned and where we are headed. J. Clin. Endocrinol. Metab. 2012, 97, E1958–E1977. [Google Scholar] [CrossRef]
- Bresler, D.; Bruder, J.; Mohnike, K.; Fraser, W.D.; Rowe, P.S. Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): Implications for phosphaturia and rickets. J. Endocrinol. 2004, 183, R1–R9. [Google Scholar] [CrossRef]
- Rowe, P.S.; Garrett, I.R.; Schwarz, P.M.; Carnes, D.L.; Lafer, E.M.; Mundy, G.R.; Gutierrez, G.E. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: A model for impaired mineralization in X-linked rickets (HYP). Bone 2005, 36, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; David, V.; Laurence, J.S.; Schwarz, P.M.; Lafer, E.M.; Hedge, A.M.; Rowe, P.S. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology 2008, 149, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Fuente, R.; Gil-Pena, H.; Claramunt-Taberner, D.; Hernandez-Frias, O.; Fernandez-Iglesias, A.; Hermida-Prado, F.; Anes-Gonzalez, G.; Rubio-Aliaga, I.; Lopez, J.M.; Santos, F. Marked alterations in the structure, dynamics and maturation of growth plate likely explain growth retardation and bone deformities of young Hyp mice. Bone 2018, 116, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; Carpenter, T.O.; Demay, M.B. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 9637–9642. [Google Scholar] [CrossRef]
- Imura, A.; Iwano, A.; Tohyama, O.; Tsuji, Y.; Nozaki, K.; Hashimoto, N.; Fujimori, T.; Nabeshima, Y. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004, 565, 143–147. [Google Scholar] [CrossRef]
- Shalhoub, V.; Ward, S.C.; Sun, B.; Stevens, J.; Renshaw, L.; Hawkins, N.; Richards, W.G. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif. Tissue Int. 2011, 89, 140–150. [Google Scholar] [CrossRef]
- Kawai, M.; Kinoshita, S.; Kimoto, A.; Hasegawa, Y.; Miyagawa, K.; Yamazaki, M.; Ohata, Y.; Ozono, K.; Michigami, T. FGF23 suppresses chondrocyte proliferation in the presence of soluble alpha-Klotho both in vitro and in vivo. J. Biol. Chem. 2013, 288, 2414–2427. [Google Scholar] [CrossRef]
- Zelzer, E.; Blitz, E.; Killian, M.L.; Thomopoulos, S. Tendon-to-bone attachment: From development to maturity. Birth Defects Res. Part C Embryo Today 2014, 102, 101–112. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Imel, E.A.; Holm, I.A.; Jan de Beur, S.M.; Insogna, K.L. A clinician’s guide to X-linked hypophosphatemia. J. Bone Miner. Res. 2011, 26, 1381–1388. [Google Scholar] [CrossRef]
- Karaplis, A.C.; Bai, X.; Falet, J.P.; Macica, C.M. Mineralizing enthesopathy is a common feature of renal phosphate-wasting disorders attributed to FGF23 and is exacerbated by standard therapy in hyp mice. Endocrinology 2012, 153, 5906–5917. [Google Scholar] [CrossRef]
- Liu, E.S.; Martins, J.S.; Zhang, W.; Demay, M.B. Molecular analysis of enthesopathy in a mouse model of hypophosphatemic rickets. Development 2018, 145, dev163519. [Google Scholar] [CrossRef]
- Imel, E.A.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Simmons, J.H.; Padidela, R.; Namba, N.; Cheong, H.I.; et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: A randomised, active-controlled, open-label, phase 3 trial. Lancet 2019, 393, 2416–2427. [Google Scholar] [CrossRef]
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Bastepe, M.; Benet-Pages, A.; Amyere, M.; Wagenstaller, J.; Muller-Barth, U.; Badenhoop, K.; Kaiser, S.M.; Rittmaster, R.S.; Shlossberg, A.H.; et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 2006, 38, 1248–1250. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Schnabel, D.; Tiosano, D.; Hausler, G.; Strom, T.M. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 2010, 86, 267–272. [Google Scholar] [CrossRef]
- Levy-Litan, V.; Hershkovitz, E.; Avizov, L.; Leventhal, N.; Bercovich, D.; Chalifa-Caspi, V.; Manor, E.; Buriakovsky, S.; Hadad, Y.; Goding, J.; et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am. J. Hum. Genet. 2010, 86, 273–278. [Google Scholar] [CrossRef]
- Rutsch, F.; Ruf, N.; Vaingankar, S.; Toliat, M.R.; Suk, A.; Hohne, W.; Schauer, G.; Lehmann, M.; Roscioli, T.; Schnabel, D.; et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat. Genet. 2003, 34, 379–381. [Google Scholar] [CrossRef]
- Linglart, A.; Biosse-Duplan, M. Hypophosphatasia. Curr. Osteoporos. Rep. 2016, 14, 95–105. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wen, J.; Wiley, S.E.; Worby, C.A.; Kinch, L.N.; Xiao, J.; Grishin, N.V.; Dixon, J.E. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science 2012, 336, 1150–1153. [Google Scholar] [CrossRef]
- Simpson, M.A.; Hsu, R.; Keir, L.S.; Hao, J.; Sivapalan, G.; Ernst, L.M.; Zackai, E.H.; Al-Gazali, L.I.; Hulskamp, G.; Kingston, H.M.; et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am. J. Hum. Genet. 2007, 81, 906–912. [Google Scholar] [CrossRef]
- Rafaelsen, S.H.; Raeder, H.; Fagerheim, A.K.; Knappskog, P.; Carpenter, T.O.; Johansson, S.; Bjerknes, R. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J. Bone Miner. Res. 2013, 28, 1378–1385. [Google Scholar] [CrossRef]
- Takeyari, S.; Yamamoto, T.; Kinoshita, Y.; Fukumoto, S.; Glorieux, F.H.; Michigami, T.; Hasegawa, K.; Kitaoka, T.; Kubota, T.; Imanishi, Y.; et al. Hypophosphatemic osteomalacia and bone sclerosis caused by a novel homozygous mutation of the FAM20C gene in an elderly man with a mild variant of Raine syndrome. Bone 2014, 67, 56–62. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Li, C.; Gao, T.; Liu, Y.; Rangiani, A.; Sun, Y.; Hao, J.; George, A.; Lu, Y.; et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet. 2012, 8, e1002708. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Yuan, B.; Lu, Y.; Feng, J.Q.; Qin, C. Overexpression of Dmp1 fails to rescue the bone and dentin defects in Fam20C knockout mice. Connect. Tissue Res. 2014, 55, 299–303. [Google Scholar] [CrossRef][Green Version]
- Rendina, D.; Abate, V.; Cacace, G.; Elia, L.; De Filippo, G.; Del Vecchio, S.; Galletti, F.; Cuocolo, A.; Strazzullo, P. Tumor induced osteomalacia: A systematic review and individual patient’s data analysis. J. Clin. Endocrinol. Metab. 2022, dgac253. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Suzuki, H.; Ogura, S.; Imai, R.; Yamazaki, Y.; Yamashita, T.; Miyamoto, Y.; Okazaki, H.; Nakamura, K.; Nakahara, K.; et al. Venous sampling for fibroblast growth factor-23 confirms preoperative diagnosis of tumor-induced osteomalacia. J. Clin. Endocrinol. Metab. 2004, 89, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Jeng, Y.M.; Su, S.Y.; Wu, C.T.; Tsai, K.S.; Lee, C.H.; Lin, C.Y.; Carter, J.M.; Huang, J.W.; Chen, S.H.; et al. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J. Pathol. 2015, 235, 539–545. [Google Scholar] [CrossRef]
- Lee, J.C.; Su, S.Y.; Changou, C.A.; Yang, R.S.; Tsai, K.S.; Collins, M.T.; Orwoll, E.S.; Lin, C.Y.; Chen, S.H.; Shih, S.R.; et al. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod. Pathol. 2016, 29, 1335–1346. [Google Scholar] [CrossRef]
- Riminucci, M.; Collins, M.T.; Fedarko, N.S.; Cherman, N.; Corsi, A.; White, K.E.; Waguespack, S.; Gupta, A.; Hannon, T.; Econs, M.J.; et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Investig. 2003, 112, 683–692. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Wiench, M.; Dumitrescu, C.; Connolly, B.M.; Bugge, T.H.; Patel, H.V.; Gafni, R.I.; Cherman, N.; Cho, M.; Hager, G.L.; et al. Mechanism of FGF23 processing in fibrous dysplasia. J. Bone Miner. Res. 2012, 27, 1132–1141. [Google Scholar] [CrossRef]
- Groesser, L.; Herschberger, E.; Ruetten, A.; Ruivenkamp, C.; Lopriore, E.; Zutt, M.; Langmann, T.; Singer, S.; Klingseisen, L.; Schneider-Brachert, W.; et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat. Genet. 2012, 44, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Ovejero, D.; Sugarman, J.S.; Deklotz, C.M.; Maruri, A.; Eichenfield, L.F.; Kelley, P.K.; Juppner, H.; Gottschalk, M.; Tifft, C.J.; et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum. Mol. Genet. 2014, 23, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zweifler, L.E.; Ao, M.; Yadav, M.; Kuss, P.; Narisawa, S.; Kolli, T.N.; Wimer, H.F.; Farquharson, C.; Somerman, M.J.; Millan, J.L.; et al. Role of PHOSPHO1 in Periodontal Development and Function. J. Dent. Res. 2016, 95, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Kruse, K.; Jüppner, H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science 1995, 268, 98–100. [Google Scholar] [CrossRef]
- Brown, W.W.; Jüppner, H.; Langman, C.B.; Price, H.; Farrow, E.G.; White, K.E.; McCormick, K.L. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J. Clin. Endocrinol. Metab. 2009, 94, 17–20. [Google Scholar] [CrossRef]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 2010, 299, F882–F889. [Google Scholar] [CrossRef]
- Shimizu, Y.; Tada, Y.; Yamauchi, M.; Okamoto, T.; Suzuki, H.; Ito, N.; Fukumoto, S.; Sugimoto, T.; Fujita, T. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: Another form of FGF23-related hypophosphatemia. Bone 2009, 45, 814–816. [Google Scholar] [CrossRef]
- Schouten, B.J.; Hunt, P.J.; Livesey, J.H.; Frampton, C.M.; Soule, S.G. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: A prospective study. J. Clin. Endocrinol. Metab. 2009, 94, 2332–2337. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).