Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options
Abstract
1. Introduction
2. MODY Subtypes and Associated Genetic Mutations
2.1. HNF1A MODY (MODY 3)
2.2. GCK MODY (MODY 2)
2.3. HNF4A MODY (MODY 1)
2.4. HNF1B MODY (MODY 5)
2.5. PDX1/IPF1-MODY (MODY 4)
2.6. NEUROD1 MODY (MODY 6)
2.7. KLF11 MODY (MODY 7)
2.8. CEL MODY (MODY 8)
2.9. PAX4 MODY (MODY 9)
2.10. INS MODY (MODY 10)
2.11. BLK MODY (MODY 11)
2.12. ABCC8 MODY (MODY 12)
2.13. KCNJ11 MODY (MODY 13)
2.14. APPL1 MODY (MODY 14)
3. Clinical Presentation
3.1. HNF1a (MODY 3)
3.2. GCK (MODY 2)
3.3. HNF4a (MODY 1)
3.4. HNF1b (MODY 5)
3.5. PDX1 (MODY 4)
3.6. NEUROD1 (MODY 6)
3.7. KLF11 (MODY 7)
3.8. CEL (MODY 8)
3.9. PAX4 (MODY 9)
3.10. INS (MODY 10)
3.11. BLK (MODY 11)
3.12. ABCC8 (MODY 12) and KCNJ11 (MODY 13)
3.13. APPL1 (MODY 14)
4. Indications for Testing
4.1. General Considerations
4.2. Patients with Fasting Hyperglycemia or IGT
4.3. Patients with Positive Diabetes Autoantibodies
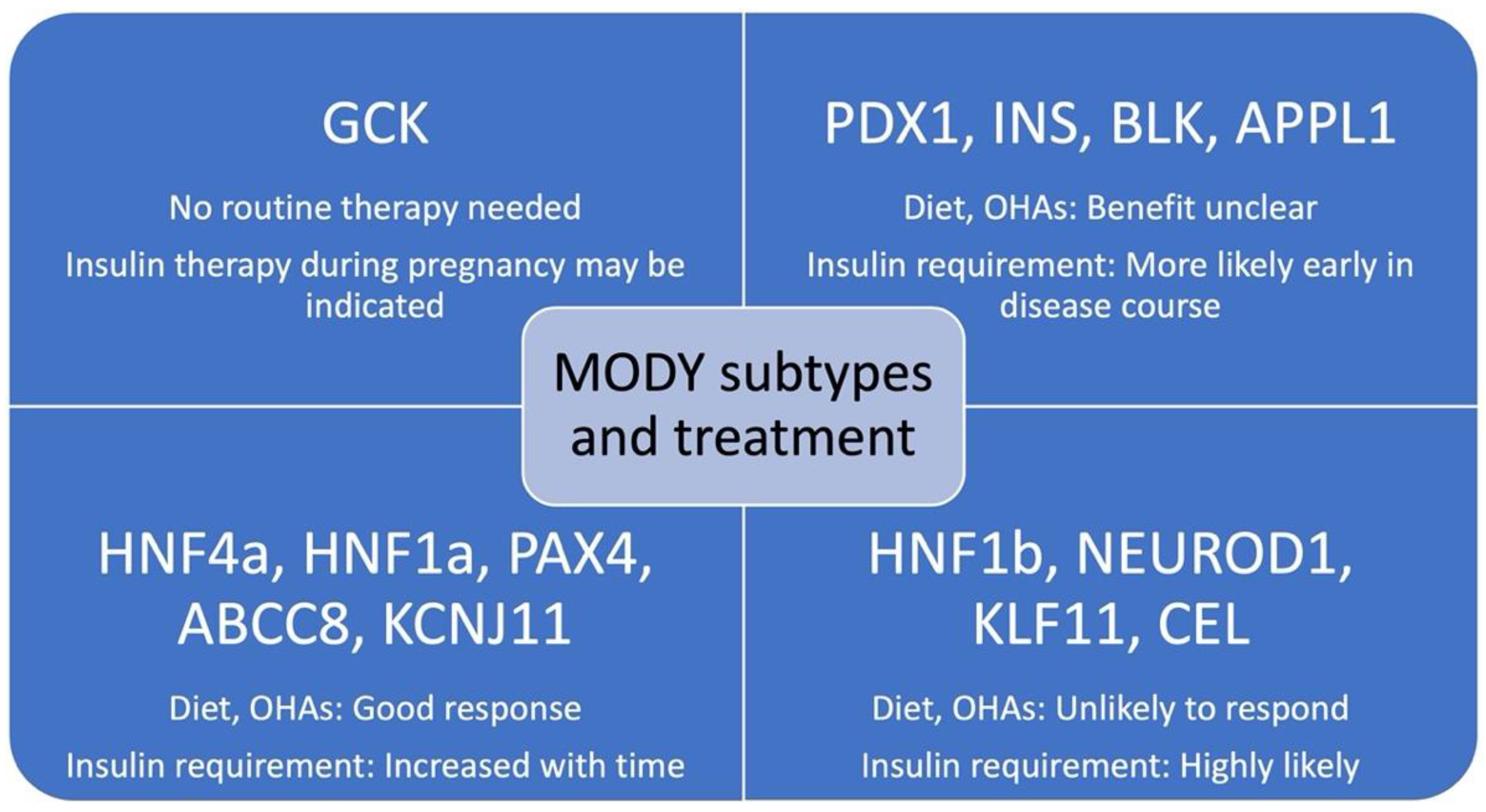
5. Treatment
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020. Available online: Cdc.gov/diabetes/data/statistics-report/index.html (accessed on 26 November 2021).
- Van Name, M.A.; Cheng, P.; Gal, R.L.; Kollman, C.; Lynch, J.; Nelson, B.; Tamborlane, W.V. Pediatric Diabetes Consortium. Children and adolescents with type 1 and type 2 diabetes mellitus in the Pediatric Diabetes Consortium Registries: Comparing clinical characteristics and glycaemic control. Diabet. Med. 2020, 37, 863–867. [Google Scholar] [CrossRef]
- Nkonge, K.M.; Nkonge, D.K.; Nkonge, T.N. The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin. Diabetes Endocrinol. 2020, 6, 20. [Google Scholar] [CrossRef]
- Fajans, S.S.; Bell, G.I.; Polonsky, K.S. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N. Engl. J. Med. 2001, 345, 971–980. [Google Scholar] [CrossRef]
- Valkovicova, T.; Skopkova, M.; Stanik, J.; Gasperikova, D. Novel insights into genetics and clinics of the HNF1A-MODY. Endocr. Regul. 2019, 53, 110–134. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.A.; Garg, A. Monogenic Forms of Diabetes. In Diabetes in America, 3rd ed.; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Knowler, W.C., Barrett-Connor, E., Becker, D.J., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018; pp. 1–27. [Google Scholar]
- Pontoglio, M.; Prie, D.; Cheret, C.; Doyen, A.; Leroy, C.; Froguel, P.; Velho, G.; Yaniv, M.; Friedlander, G. HNF1α controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000, 1, 359–365. [Google Scholar] [CrossRef]
- Lau, H.H.; Ng, N.H.J.; Loo, L.S.W.; Jasmen, J.B.; Teo, A.K.K. The molecular functions of hepatocyte nuclear factors—In and beyond the liver. J. Hepatol. 2018, 68, 1033–1048. [Google Scholar] [CrossRef]
- Godart, F.; Bellanne-Chantelot, C.; Clauin, S.; Gragnoli, C.; Abderrahmani, A.; Blanche, H.; Boutin, P.; Chevre, J.C.; Froguel, P.; Bailleul, B. Identification of seven novel nucleotide variants in the hepatocyte nuclear factor-1α (TCF1) promoter region in MODY patients. Hum. Mutat. 2000, 15, 173–180. [Google Scholar] [CrossRef]
- Lausen, J.; Thomas, H.; Lemm, I.; Bulman, M.; Borgschulze, M.; Lingott, A.; Hattersley, A.T.; Ryffel, G.U. Naturally occurring mutations in the human HNF4alpha gene impair the function of the transcription factor to a varying degree. Nucleic Acids Res. 2000, 28, 430–437. [Google Scholar] [CrossRef][Green Version]
- Colclough, K.; Bellanne-Chantelot, C.; Saint-Martin, C.; Flanagan, S.E.; Ellard, S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum. Mutat. 2013, 34, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Vaxillaire, M.; Abderrahmani, A.; Boutin, P.; Bailleul, B.; Froguel, P.; Yaniv, M.; Pontoglio, M. Anatomy of a homeoprotein revealed by the analysis of human MODY3 mutations. J. Biol. Chem. 1999, 274, 35639–35646. [Google Scholar] [CrossRef] [PubMed]
- Harries, L.W.; Ellard, S.; Stride, A.; Morgan, N.G.; Hattersley, A.T. Isomers of the TCF1 gene encoding hepatocyte nuclear factor-1 alpha show differential expression in the pancreas and define the relationship between mutation position and clinical phenotype in monogenic diabetes. Hum. Mol. Genet. 2006, 15, 2216–2224. [Google Scholar] [CrossRef]
- Bellanné-Chantelot, C.; Carette, C.; Riveline, J.P.; Valéro, R.; Gautier, J.F.; Larger, E.; Reznik, Y.; Ducluzeau, P.H.; Sola, A.; Hartemann-Heurtier, A.; et al. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes 2008, 57, 503–508. [Google Scholar] [CrossRef]
- Awa, W.L.; Thon, A.; Raile, K.; Grulich-Henn, J.; Meissner, T.; Schober, E.; Holl, R.W.; DPV-Wiss. Study Group. Genetic and clinical characteristics of patients with HNF1A gene variations from the German-Austrian DPV database. Eur. J. Endocrinol. 2011, 164, 513–520. [Google Scholar] [CrossRef]
- Stride, A.; Vaxillaire, M.; Tuomi, T.; Barbetti, F.; Njølstad, P.R.; Hansen, T.; Costa, A.; Conget, I.; Pedersen, O.; Søvik, O.; et al. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia 2002, 45, 427–435. [Google Scholar] [CrossRef]
- Klupa, T.; Warram, J.H.; Antonellis, A.; Pezzolesi, M.; Nam, M.; Malecki, M.T.; Doria, A.; Rich, S.S.; Krolewski, A.S. Determinants of the development of diabetes (maturity-onset diabetes of the young-3) in carriers of HNF-1alpha mutations: Evidence for parent-of-origin effect. Diabetes Care 2002, 25, 2292–2301. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Hasan, G.M.; Hassan, M.I. Investigating the role of transcription factors of pancreas development in pancreatic cancer. Pancreatology 2018, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Vesterhus, M.; Haldorsen, I.S.; Raeder, H.; Molven, A.; Njølstad, P.R. Reduced pancreatic volume in hepatocyte nuclear factor 1A-maturity-onset diabetes of the young. J. Clin. Endocrinol. Metab. 2008, 93, 3505–3509. [Google Scholar] [CrossRef]
- Pearson, E.R.; Starkey, B.J.; Powell, R.J.; Gribble, F.M.; Clark, P.M.; Hattersley, A.T. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003, 362, 1275–1281. [Google Scholar] [CrossRef]
- Małachowska, B.; Borowiec, M.; Antosik, K.; Michalak, A.; Baranowska-Jaźwiecka, A.; Deja, G.; Jarosz-Chobot, P.; Brandt, A.; Myśliwiec, M.; Stelmach, M.; et al. Monogenic diabetes prevalence among Polish children-Summary of 11 years-long nationwide genetic screening program. Pediatr. Diabetes 2018, 19, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Aloi, C.; Salina, A.; Minuto, N.; Tallone, R.; Lugani, F.; Mascagni, A.; Mazza, O.; Cassanello, M.; Maghnie, M.; d’Annunzio, G. Glucokinase mutations in pediatric patients with impaired fasting glucose. Acta Diabetol. 2017, 54, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Osbak, K.K.; Colclough, K.; Saint-Martin, C.; Beer, N.L.; Bellanné-Chantelot, C.; Ellard, S.; Gloyn, A.L. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat. 2009, 30, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Froguel, P.; Zouali, H.; Vionnet, N.; Velho, G.; Vaxillaire, M.; Sun, F.; Lesage, S.; Stoffel, M.; Takeda, J.; Passa, P. Familial hyperglycemia due to mutations in glucokinase—definition of a subtype of diabetes mellitus. N. Engl. J. Med. 1993, 328, 697–702. [Google Scholar] [CrossRef]
- Njølstad, P.R.; Søvik, O.; Cuesta-Muñoz, A.; Bjørkhaug, L.; Massa, O.; Barbetti, F.; Undlien, D.E.; Shiota, C.; Magnuson, M.A.; Molven, A.; et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N. Engl. J. Med. 2001, 344, 1588–1592. [Google Scholar] [CrossRef]
- Hughes, A.E.; De Franco, E.; Globa, E.; Zelinska, N.; Hilgard, D.; Sifianou, P.; Hattersley, A.T.; Flanagan, S.E. Identification of GCK-maturity-onset diabetes of the young in cases of neonatal hyperglycemia: A case series and review of clinical features. Pediatr. Diabetes 2021, 22, 876–881. [Google Scholar] [CrossRef]
- Ellard, S.; Thomas, K.; Edghill, E.L.; Owens, M.; Ambye, L.; Cropper, J.; Little, J.; Strachan, M.; Stride, A.; Ersoy, B.; et al. Partial and whole gene deletion mutations of the GCK and HNF1A genes in maturity-onset diabetes of the young. Diabetologia 2007, 50, 2313–2317. [Google Scholar] [CrossRef]
- Garin, I.; Rica, I.; Estalella, I.; Oyarzabal, M.; Rodríguez-Rigual, M.; San Pedro, J.I.; Pérez-Nanclares, G.; Fernández-Rebollo, E.; Busturia, M.A.; Castaño, L.; et al. Haploinsufficiency at GCK gene is not a frequent event in MODY2 patients. Clin. Endocrinol. (Oxf.) 2008, 68, 873–878. [Google Scholar] [CrossRef]
- Gloyn, A.L.; Odili, S.; Buettger, C.; Njolstad, P.R.; Shiota, C.; Magnuson, M.A.; Matschinsky, F.M. Glucokinase and the regulation of blood sugar: A mathematical model predicts the threshold for glucose stimulated insulin release for GCK gene mutations that cause hyper- and hypoglycemia. In Glucokinase and Glycemic Diseases: From the Basics to Novel Therapies; Magnuson, M., Matschinsky, F.M., Eds.; Karger: Basel, Switzerland, 2004; pp. 92–109. [Google Scholar] [CrossRef]
- Dussoix, P.; Vaxillaire, M.; Iynedjian, P.B.; Tiercy, J.M.; Ruiz, J.; Spinas, G.A.; Berger, W.; Zahnd, G.; Froguel, P.; Philippe, J. Diagnostic heterogeneity of diabetes in lean young adults: Classification based on immunological and genetic parameters. Diabetes 1997, 46, 622–631. [Google Scholar] [CrossRef]
- Saker, P.J.; Hattersley, A.T.; Barrow, B.; Hammersley, M.S.; McLellan, J.A.; Lo, Y.M.; Olds, R.J.; Gillmer, M.D.; Holman, R.R.; Turner, R.C. High prevalence of a missense mutation of the glucokinase gene in gestational diabetic patients due to a founder-effect in a local population. Diabetologia 1996, 39, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Estalella, I.; Rica, I.; Perez de Nanclares, G.; Bilbao, J.R.; Vazquez, J.A.; San Pedro, J.I.; Busturia, M.A.; Castaño, L.; Spanish MODY Group. Mutations in GCK and HNF-1alpha explain the majority of cases with clinical diagnosis of MODY in Spain. Clin. Endocrinol. (Oxf.) 2007, 67, 538–546. [Google Scholar] [CrossRef]
- Sagen, J.V.; Bjørkhaug, L.; Molnes, J.; Raeder, H.; Grevle, L.; Søvik, O.; Molven, A.; Njølstad, P.R. Diagnostic screening of MODY2/GCK mutations in the Norwegian MODY Registry. Pediatr. Diabetes 2008, 9, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.M.; Hicks, S.; Shepherd, M.H.; Colclough, K.; Hattersley, A.T.; Ellard, S. Maturity-onset diabetes of the young (MODY): How many cases are we missing? Diabetologia 2010, 53, 2504. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Furuta, H.; Oda, N.; Kaisaki, P.J.; Menzel, S.; Cox, N.J.; Fajans, S.S.; Signorini, S.; Stoffel, M.; Bell, G.I. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature 1996, 384, 458–460. [Google Scholar] [CrossRef]
- Harries, L.W.; Locke, J.M.; Shields, B.; Hanley, N.A.; Hanley, K.P.; Steele, A.; Njølstad, P.R.; Ellard, S.; Hattersley, A.T. The diabetic phenotype in HNF4A mutation carriers is moderated by the expression of HNF4A isoforms from the P1 promoter during fetal development. Diabetes 2008, 57, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, Y.; Iwasaki, N.; Hara, M.; Furuta, H.; Hinokio, Y.; Cockburn, B.N.; Lindner, T.; Yamagata, K.; Ogata, M.; Tomogana, O.; et al. Mutation in hepatocyte nuclear factor-1 beta gene (TCF-2) associated with MODY. Nat. Genet. 1997, 17, 384–385. [Google Scholar] [CrossRef]
- Clissold, R.L.; Hamilton, A.J.; Hattersley, A.T.; Ellard, S.; Bingham, C. HNF1B-associated renal and extrarenal disease: An expanding clinical spectrum. Nat. Rev. Nephrol. 2015, 11, 101–112. [Google Scholar] [CrossRef] [PubMed]
- El-Khairi, R.; Vallier, L. The role of hepatocyte nuclear factor 1beta in disease and development. Diabetes Obes. Metab. 2016, 18, 23–32. [Google Scholar] [CrossRef]
- Harries, L.W.; Brown, J.E.; Gloyn, A.L. Species-specific differences in the expression of the HNF1A, HNF1B and HNF4A genes. PLoS ONE 2009, 4, e785. [Google Scholar] [CrossRef]
- Bellanne-Chantelot, C.; Clauin, S.; Chauveau, D.; Colin, P.; Daumont, M.; Douillard, C.; Dubois-Laforgue, D.; Dusselier, L.; Gautier, J.F.; Jadoul, M.; et al. Large genomic rearrangements in the hetapocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity onset diabetes of the young (MODY)5. Diabetes 2005, 54, 3126–3132. [Google Scholar] [CrossRef]
- Edghill, E.L.; Oram, R.A.; Owens, M.; Stals, K.L.; Harrier, L.W.; Hattersley, A.T.; Ellard, S.; Bingham, C. Hepatocyte nuclear factor-1beta gene deletions—a common cause of renal disease. Nephrol. Dial. Transpl. 2008, 23, 627–635. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, J.H.; Han, K.H.; Ahn, Y.H.; Kang, H.G.; Ha, I.S.; Cheong, H.I. Genotype and phenotype analyses in pediatric patients with HNF1B mutations. J. Clin. Med. 2020, 9, 2320. [Google Scholar] [CrossRef]
- Laffargue, F.; Bourthoumieu, S.; Llanas, B.; Baudouin, V.; Lahoche, A.; Morin, D.; Bessanay, L.; De Parscau, L.; Cloarec, S.; Delrue, M.A.; et al. Toward a new point of view on the phenotype of patients with 17q12 microdeletion syndrome. Arch. Dis. Child. 2015, 100, 259–264. [Google Scholar] [CrossRef]
- Mitchel, M.W.; Moreno-De-Luca, D.; Myers, S.M.; Finucane, B.; Ledbetter, D.H.; Martin, C.L. 17q12 recurrent deletion syndrome. In Genereviews®; Pagon, R.A., Adam, M.P., Ardinger, H.H., Eds.; University of Washington: Seattle, WA, USA, 2016. Available online: ncbi.nlm.nih.gov/books/NBK1116 (accessed on 25 November 2021).
- Dubois-Laforgue, D.; Cornu, E.; Saint-Martin, C.; Coste, J.; Bellanné-Chantelot, C.; Timsit, J.; Monogenic Diabetes Study Group of the Société Francophone du Diabète. Diabetes, Associated Clinical Spectrum, Long-term Prognosis, and Genotype/Phenotype Correlations in 201 Adult Patients with Hepatocyte Nuclear Factor 1B (HNF1B) Molecular Defects. Diabetes Care 2017, 40, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Stoffers, D.A.; Stanojevic, V.; Habener, J.F. Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J. Clin. Invest. 1998, 102, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.G.; Shah, N.N.; Briston, S.J.; Shepherd, R.M.; Khoo, C.P.; Dunne, M.J.; Moore, H.D.; Cosgrove, K.E.; Andrews, P.W. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS ONE 2008, 3, e1783. [Google Scholar] [CrossRef]
- Wang, X.; Sterr, M.; Burtscher, I.; Böttcher, A.; Beckenbauer, J.; Siehler, J.; Meitinger, T.; Häring, H.U.; Staiger, H.; Cernilogar, F.M.; et al. Point mutations in the PDX1 transactivation domain impair human β-cell development and function. Mol. Metab. 2019, 24, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, J.; Carlsson, L.; Edlund, T.; Edlund, H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994, 371, 606–609. [Google Scholar] [CrossRef]
- Brissova, M.; Shiota, M.; Nicholson, W.E.; Gannon, M.; Knobel, S.M.; Piston, D.W.; Wright, C.V.E.; Powers, A.C. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 2002, 277, 11225–11232. [Google Scholar] [CrossRef]
- Malecki, M.T.; Jhala, U.S.; Antonellis, A.; Fields, L.; Doria, A.; Orban, T.; Saad, M.; Warram, J.H.; Montminy, M.; Krolewski, A.S. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat. Genet. 1999, 23, 323–328. [Google Scholar] [CrossRef]
- Tamimi, R.; Steingrimsson, E.; Copeland, N.G.; Dyer-Montgomery, K.; Lee, J.E.; Hernandez, R.; Jenkins, N.A.; Tapscott, S.J. The NEUROD gene maps to human chromosome 2q32 and mouse chromosome 2. Genomics 1996, 34, 418–421. [Google Scholar] [CrossRef]
- Naya, F.J.; Huang, H.P.; Qiu, Y.; Mutoh, H.; DeMayo, F.J.; Leiter, A.B.; Tsai, M.J. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997, 11, 2323–2334. [Google Scholar] [CrossRef]
- Kanatsuka, A.; Tokuyama, Y.; Nozaki, O.; Matsui, K.; Egashira, T. Beta-cell dysfunction in late-onset diabetic subjects carrying homozygous mutation in transcription factors NeuroD1 and Pax4. Metabolism 2002, 51, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Zapico, M.E.; van Velkinburgh, J.C.; Gutiérrez-Aguilar, R.; Neve, B.; Froguel, P.; Urrutia, R.; Stein, R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J. Biol. Chem. 2009, 284, 36482–36490. [Google Scholar] [CrossRef] [PubMed]
- Neve, B.; Fernandez-Zapico, M.E.; Ashkenazi-Katalan, V.; Dina, C.; Hamid, Y.H.; Joly, E.; Vaillant, E.; Benmezroua, Y.; Durand, E.; Bakaher, N.; et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc. Natl. Acad. Sci. USA 2005, 102, 4807–4812. [Google Scholar] [CrossRef]
- Buttar, N.S.; DeMars, C.J.; Lomberk, G.; Rizvi, S.; Bonilla-Velez, J.; Achra, S.; Rashtak, S.; Wang, K.K.; Fernandez-Zapico, M.E.; Urrutia, R. Distinct role of Kruppel-like factor 11 in the regulation of prostaglandin E2 biosynthesis. J. Biol. Chem. 2010, 285, 11433–11444. [Google Scholar] [CrossRef]
- Cao, S.; Fernandez-Zapico, M.E.; Jin, D.; Puri, V.; Cook, T.A.; Lerman, L.O.; Zhu, X.Y.; Urrutia, R.; Shah, V. KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. J. Biol. Chem. 2005, 280, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Johnson, S.; Lu, D.; Wang, Z.; Lomberk, G.; Albert, P.R.; Stockmeier, C.A.; Meyer, J.H.; Urrutia, R.; Miczek, K.A.; et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J. Biol. Chem. 2012, 287, 24195–24206. [Google Scholar] [CrossRef]
- Ou, X.M.; Chen, K.; Shih, J.C. Dual functions of transcription factors, transforming growth factor-β-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine oxidase (MAO) B gene. J. Biol. Chem. 2004, 279, 21021–21028. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sakaguchi, M.; Medina, R.J.; Niida, A.; Sakaguchi, Y.; Miyazaki, M.; Kataoka, K.; Huh, N.H. Transcriptional regulation of a brown adipocyte-specific gene, UCP1, by KLF11 and KLF15. Biochem. Biophys. Res. Commun. 2010, 400, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K.; Narumi, S.; Ogata, T.; Yokota, I.; Sugihara, S.; Kaname, T.; Horikawa, Y.; Matsubara, Y.; Fukami, M.; Kawamura, T.; et al. KLF11 variant in a family clinically diagnosed with early childhood-onset type 1B diabetes. Pediatr. Diabetes 2019, 20, 712–719. [Google Scholar] [CrossRef]
- Tanahashi, T.; Shinohara, K.; Keshavarz, P.; Yamaguchi, Y.; Miyawaki, K.; Kunika, K.; Moritani, M.; Nakamura, N.; Yoshikawa, T.; Shiota, H.; et al. The association of genetic variants in Krüppel-like factor 11 and Type 2 diabetes in the Japanese population. Diabet Med. 2008, 25, 19–26. [Google Scholar] [CrossRef]
- Ma, L.; Hanson, R.L.; Que, L.N.; Mack, J.L.; Franks, P.W.; Infante, A.M.; Kobes, S.; Bogardus, C.; Baier, L.J. Association analysis of Krüppel-like factor 11 variants with type 2 diabetes in Pima Indians. J. Clin. Endocrinol. Metab. 2008, 93, 3644–3649. [Google Scholar] [CrossRef]
- Torsvik, J.; Johansson, S.; Johansen, A.; Ek, J.; Minton, J.; Raeder, H.; Ellard, S.; Hattersley, A.; Pedersen, O.; Hansen, T.; et al. Mutations in the VNTR of the carboxyl-ester lipase gene (CEL) are a rare cause of monogenic diabetes. Hum. Genet. 2010, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D. Bile salt-dependent lipase: Its pathophysiological implications. Biochim. Biophys. Acta 2001, 1533, 1–28. [Google Scholar] [CrossRef]
- Blackberg, L.; Angquist, K.A.; Hernell, O. Bile-salt-stimulated lipase in human milk: Evidence for its synthesis in the lactating mammary gland. FEBS Lett. 1987, 217, 37–41. [Google Scholar] [CrossRef]
- Lombardo, D.; Guy, O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim. Biophys. Acta 1980, 611, 147–155. [Google Scholar] [CrossRef]
- Dalva, M.; Lavik, I.K.; El Jellas, K.; Gravdal, A.; Lugea, A.; Pandol, S.J.; Njølstad, P.R.; Waldron, R.T.; Fjeld, K.; Johansson, B.B.; et al. Pathogenic Carboxyl Ester Lipase (CEL) Variants Interact with the Normal CEL Protein in Pancreatic Cells. Cells 2020, 9, 244. [Google Scholar] [CrossRef]
- Raeder, H.; Johansson, S.; Holm, P.I.; Haldorsen, I.S.; Mas, E.; Sbarra, V.; Nermoen, I.; Eide, S.A.; Grevle, L.; Bjorkhaug, L.; et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat. Genet. 2006, 38, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ræder, H.; Vesterhus, M.; El Ouaamari, A.; Paulo, J.A.; McAllister, F.E.; Liew, C.W.; Hu, J.; Kawamori, D.; Molven, A.; Gygi, S.P.; et al. Absence of diabetes and pancreatic exocrine dysfunction in a transgenic model of carboxyl-ester lipase-MODY (maturity-onset diabetes of the young). PLoS ONE 2013, 8, e60229. [Google Scholar] [CrossRef]
- Plengvidhya, N.; Kooptiwut, S.; Songtawee, N.; Doi, A.; Furuta, H.; Nishi, M.; Nanjo, K.; Tantibhedhyangkul, W.; Boonyasrisawat, W.; Yenchitsomanus, P.T.; et al. PAX4 mutations in Thais with maturity onset diabetes of the young. J. Clin. Endocrinol. Metab. 2007, 92, 2821–2826. [Google Scholar] [CrossRef]
- Wang, J.; Elghazi, L.; Parker, S.E.; Kizilocak, H.; Asano, M.; Sussel, L.; Sosa-Pineda, B. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev. Biol. 2004, 266, 178–189. [Google Scholar] [CrossRef]
- Sosa-Pineda, B.; Chowdhury, K.; Torres, M.; Oliver, G.; Gruss, P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 1997, 386, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Biason-Lauber, A.; Boehm, B.; Lang-Muritano, M.; Gauthier, B.R.; Brun, T.; Wollheim, C.B.; Schoenle, E.J. Association of childhood type 1 diabetes mellitus with a variant of PAX4: Possible link to beta-cell regenerative capacity. Diabetologia 2005, 48, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Molven, A.; Ringdal, M.; Nordbø, A.M.; Raeder, H.; Støy, J.; Lipkind, G.M.; Steiner, D.F.; Philipson, L.H.; Bergmann, I.; Aarskog, D.; et al. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes 2008, 57, 1131–1135. [Google Scholar] [CrossRef]
- Boesgaard, T.W.; Pruhova, S.; Andersson, E.A.; Cinek, O.; Obermannova, B.; Lauenborg, J.; Damm, P.; Bergholdt, R.; Pociot, F.; Pisinger, C.; et al. Further evidence that mutations in INS can be a rare cause of Maturity-Onset Diabetes of the Young (MODY). BMC Med. Genet. 2010, 1211, 42. [Google Scholar] [CrossRef]
- Edghill, E.L.; Flanagan, S.E.; Patch, A.M.; Boustred, C.; Parrish, A.; Shields, B.; Shepherd, M.H.; Hussain, K.; Kapoor, R.R.; Malecki, M.; et al. Insulin mutation screening in 1044 patients with diabetes: Mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes 2008, 57, 1034–1042. [Google Scholar] [CrossRef]
- Borowiec, M.; Liew, C.W.; Thompson, R.; Boonyasrisawat, W.; Hu, J.; Mlynarski, W.M.; El Khattabi, I.; Kim, S.H.; Marselli, L.; Rich, S.S.; et al. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 14460–14465. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, A.; Yengo, L.; Philippe, J.; Dechaume, A.; Ezzidi, I.; Vaillant, E.; Gjesing, A.P.; Andersson, E.A.; Czernichow, S.; Hercberg, S.; et al. Reassessment of the putative role of BLK-p.A71T loss-of-function mutation in MODY and type 2 diabetes. Diabetologia 2013, 56, 492–496. [Google Scholar] [CrossRef]
- Bowman, P.; Flanagan, S.E.; Edghill, E.L.; Damhuis, A.; Shepherd, M.H.; Paisey, R. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia 2012, 55, 123–127. [Google Scholar] [CrossRef]
- De Franco, E.; Saint-Martin, C.; Brusgaard, K.; Knight Johnson, A.E.; Aguilar-Bryan, L.; Bowman, P.; Arnoux, J.B.; Larsen, A.R.; Sanyoura, M.; Greeley, S.A.W.; et al. Update of variants identified in the pancreatic β-cell KATP channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum. Mutat. 2020, 41, 884–905. [Google Scholar] [CrossRef]
- Huopio, H.; Otonkoski, T.; Vauhkonen, I.; Reimann, F.; Ashcroft, F.M.; Laakso, M. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet 2003, 361, 301–307. [Google Scholar] [CrossRef]
- Bonnefond, A.; Philippe, J.; Durand, E.; Dechaume, A.; Huyvaert, M.; Montagne, L.; Marre, M.; Balkau, B.; Fajardi, I.; Vambergue, A.; et al. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. PLoS ONE 2012, 7, e37423. [Google Scholar] [CrossRef]
- Yorifuji, T.; Nagashima, K.; Kurokawa, K.; Kawai, M.; Oishi, M.; Hosokawa, M.; Yamada, Y.; Inagaki, N.; Nakahata, T. The C42R mutation in the Kir6.2 (KCNJ11) gene as a cause of transient neonatal diabetes, childhood diabetes, or later-onset, apparently type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2005, 90, 3174–3178. [Google Scholar] [CrossRef]
- Ivanoshchuk, D.E.; Shakhtshneider, E.V.; Rymar, O.D.; Ovsyannikova, A.K.; Mikhailova, S.V.; Orlov, P.S.; Yi, R.; Mi, V. Analysis of APPL1 gene polymorphisms in patients with a phenotype of maturity onset diabetes of the young. J. Pers. Med. 2020, 10, E100. [Google Scholar] [CrossRef]
- Prudente, S.; Jungtrakoon, P.; Marucci, A.; Ludovico, O.; Buranasupkajorn, P.; Mazza, T.; Hastings, T.; Milano, T.; Morini, E.; Mercuri, L.; et al. Loss-of-function mutations in APPL1 in familial diabetes mellitus. Am. J. Hum. Genet. 2015, 97, 177–185. [Google Scholar] [CrossRef]
- Deepa, S.S.; Dong, L.Q. APPL1: Role in adiponectin signaling and beyond. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E22–E36. [Google Scholar] [CrossRef]
- Ryu, J.; Galan, A.K.; Xin, X.; Dong, F.; Abdul-Ghani, M.A.; Zhou, L.; Wang, C.; Li, C.; Holmes, B.M.; Sloane, L.B.; et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 2014, 7, 1227–1238. [Google Scholar] [CrossRef]
- Cheng, K.K.; Lam, K.S.; Wu, D.; Wang, Y.; Sweeney, G.; Hoo, R.L.; Zhang, J.; Hu, A. APPL1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 8919–8924. [Google Scholar] [CrossRef] [PubMed]
- Schenck, A.; Goto-Silva, L.; Collinet, C.; Rhinn, M.; Giner, A.; Habermann, B.; Brand, M.; Zerial, M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 2008, 133, 486–497. [Google Scholar] [CrossRef]
- Pearson, E.R.; Boj, S.F.; Steele, A.M.; Barrett, T.; Stals, K.; Shield, J.P.; Ellard, S.; Ferrer, J.; Hattersley, A.T. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007, 4, e118. [Google Scholar] [CrossRef] [PubMed]
- Gragnoli, C.; Stanojevic, V.; Gorini, A.; Von Preussenthal, G.M.; Thomas, M.K.; Habener, J.F. IPF-1/MODY4 gene missense mutation in an Italian family with type 2 and gestational diabetes. Metabolism 2005, 54, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Thorolfsdottir, E.T.; Talseth, B.; Steingrimsson, E.; Thorsson, A.V.; Helgason, T.; Hreidarsson, A.B.; Arngrimsson, R. MODY in Iceland is associated with mutations in HNF-1alpha and a novel mutation in NeuroD1. Diabetologia 2001, 44, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Ræder, H.; McAllister, F.E.; Tjora, E.; Bhatt, S.; Haldorsen, I.; Hu, J.; Willems, S.M.; Vesterhus, M.; El Ouaamari, A.; Liu, M.; et al. Carboxyl-ester lipase maturity-onset diabetes of the young is associated with development of pancreatic cysts and upregulated MAPK signaling in secretin-stimulated duodenal fluid. Diabetes 2014, 63, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Smith, S.B.; Le May, C.; Leal, S.M.; Gautier, J.F.; Molokhia, M.; Riveline, J.P.; Rajan, A.S.; Kevorkian, J.P.; Zhang, S.; et al. PAX4 gene variations predispose to ketosis-prone diabetes. Hum. Mol. Genet. 2004, 13, 3151–3159. [Google Scholar] [CrossRef]
- Riveline, J.P.; Rousseau, E.; Reznik, Y.; Fetita, S.; Philippe, J.; Dechaume, A.; Hartemann, A.; Polak, M.; Petit, C.; Charpentier, G.; et al. Clinical and metabolic features of adult-onset diabetes caused by ABCC8 mutations. Diabetes Care 2012, 35, 248–251. [Google Scholar] [CrossRef]
- Naylor, R.; Philipson, L.H. Who should have genetic testing for maturity-onset diabetes of the young? Clin. Endocrinol. (Oxf.) 2011, 75, 422–426. [Google Scholar] [CrossRef]
- Chakera, A.J.; Spyer, G.; Vincent, N.; Ellard, S.; Hattersley, A.T.; Dunne, F.P. The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: The Atlantic Diabetes in Pregnancy cohort. Diabetes Care 2014, 37, 1230–1236. [Google Scholar] [CrossRef]
- Shields, B.M.; McDonald, T.J.; Ellard, S.; Campbell, M.J.; Hyde, C.; Hattersley, A.T. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia 2012, 55, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ping, F.; Wang, T.; Liu, Y.; Wang, X.; Yu, J.; Deng, M.; Liu, J.; Zhang, Q.; Yu, M.; et al. A Clinical Prediction Model to Distinguish Maturity-Onset Diabetes of the Young from Type 1 and Type 2 Diabetes in the Chinese Population. Endocr. Pr. 2021, 27, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, M.; Acquaviva, F.; Barbetti, F.; Caredda, E.; Cocozza, S.; Delvecchio, M.; Mozzillo, E.; Pirozzi, D.; Prisco, F.; Rabbone, I.; et al. Identification of candidate children for maturity-onset diabetes of the young type 2 (MODY2) gene testing: A seven-item clinical flowchart (7-iF). PLoS ONE 2013, 8, e79933. [Google Scholar] [CrossRef]
- Peixoto-Barbosa, R.; Reis, A.F.; Giuffrida, F.M.A. Update on clinical screening of maturity-onset diabetes of the young (MODY). Diabetol. Metab. Syndr. 2020, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, B.; Hysenaj, G.; Atay, Z.; Guran, T.; Abalı, S.; Turan, S.; Bereket, A.; Ellard, S. GCK gene mutations are a common cause of childhood-onset MODY (maturity-onset diabetes of the young) in Turkey. Clin. Endocrinol. (Oxf.) 2016, 85, 393–399. [Google Scholar] [CrossRef]
- Gloyn, A.L.; van de Bunt, M.; Stratton, I.M.; Lonie, L.; Tucker, L.; Ellard, S.; Holman, R.R. Prevalence of GCK mutations in individuals screened for fasting hyperglycaemia. Diabetologia 2009, 52, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Leslie, R.D.; Atkinson, M.A.; Notkins, A.L. Autoantigens IA-2 and GAD in Type I (insulin-dependent) diabetes. Diabetologia 1999, 42, 3–14. [Google Scholar] [CrossRef][Green Version]
- McDonald, T.J.; Colclough, K.; Brown, R.; Shields, B.; Shepherd, M.; Bingley, P.; Williams, A.; Hattersley, A.T.; Ellard, S. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet. Med. 2011, 28, 1028–1033. [Google Scholar] [CrossRef]
- Carlsson, A.; Shepherd, M.; Ellard, S.; Weedon, M.; Lernmark, Å.; Forsander, G.; Colclough, K.; Brahimi, Q.; Valtonen-Andre, C.; Ivarsson, S.A.; et al. Absence of Islet Autoantibodies and Modestly Raised Glucose Values at Diabetes Diagnosis Should Lead to Testing for MODY: Lessons From a 5-Year Pediatric Swedish National Cohort Study. Diabetes Care 2020, 43, 82–89. [Google Scholar] [CrossRef]
- Urbanová, J.; Rypáčková, B.; Kučera, P.; Anděl, M.; Heneberg, P. Should the negativity for islet cell autoantibodies be used in a prescreening for genetic testing in maturity-onset diabetes of the young? The case of autoimmunity-associated destruction of pancreatic β-cells in a family of HNF1A-MODY subjects. Int. Arch. Allergy Immunol. 2013, 161, 279–284. [Google Scholar] [CrossRef]
- Bowden, S.A.; Hoffman, R.P. Triple diabetes: Coexistence of type 1 diabetes mellitus and a novel mutation in the gene responsible for MODY3 in an overweight adolescent. Pediatr. Diabetes 2008, 9, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, J.; Rypáčková, B.; Procházková, Z.; Kučera, P.; Cerná, M.; Anděl, M.; Heneberg, P. Positivity for islet cell autoantibodies in patients with monogenic diabetes is associated with later diabetes onset and higher HbA1c level. Diabet. Med. 2014, 31, 466–471. [Google Scholar] [CrossRef]
- Lindgren, C.M.; Widén, E.; Tuomi, T.; Li, H.; Almgren, P.; Kanninen, T.; Melander, O.; Weng, J.; Lehto, M.; Groop, L.C. Contribution of known and unknown susceptibility genes to early-onset diabetes in scandinavia: Evidence for heterogeneity. Diabetes 2002, 51, 1609–1617. [Google Scholar] [CrossRef][Green Version]
- American Diabetes Association. 13. Children and adolescents: Standards of Medical Care in Diabetes 2021. Diabetes Care 2021, 44, S180–S199. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Acerini, C.L.; Codner, E.; Craig, M.E.; Hofer, S.E.; Pillay, K.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatric. Diabetes 2018, 19, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Meyerovitch, J.; Zlotnik, M.; Yackobovitch-Gavan, M.; Phillip, M.; Shalitin, S. Real-Life Glycemic Control in Children with Type 2 Diabetes: A Population-Based Study. J. Pediatr. 2017, 188, 173–180. [Google Scholar] [CrossRef] [PubMed]
- TODAY Study Group; Zeitler, P.; Hirst, K.; Pyle, L.; Linder, B.; Copeland, K.; Arslanian, S.; Cuttler, L.; Nathan, D.M.; Tollefsen, S.; et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N. Engl. J. Med. 2012, 366, 2247–2256. [Google Scholar] [CrossRef]
- Steele, A.M.; Shields, B.M.; Wensley, K.J.; Colclough, K.; Ellard, S.; Hattersley, A.T. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA 2014, 311, 279–286. [Google Scholar] [CrossRef]
- Dickens, L.T.; Letourneau, L.R.; Sanyoura, M.; Greeley, S.A.W.; Philipson, L.H.; Naylor, R.N. Management and pregnancy outcomes of women with GCK-MODY enrolled in the US Monogenic Diabetes Registry. Acta Diabetol. 2019, 56, 405–411. [Google Scholar] [CrossRef]
- Broome, D.T.; Pantalone, K.M.; Kashyap, S.R.; Philipson, L.H. Approach to the Patient with MODY-Monogenic Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 237–250. [Google Scholar] [CrossRef]
- Delvecchio, M.; Mozzillo, E.; Salzano, G.; Iafusco, D.; Frontino, G.; Patera, P.I.; Rabbone, I.; Cherubini, V.; Grasso, V.; Tinto, N.; et al. Monogenic diabetes accounts for 6.3% of cases referred to 15 Italian pediatric diabetes centers during 2007 to 2012. J. Clin. Endocrinol. Metab. 2017, 102, 1826–1834. [Google Scholar] [CrossRef]
- Hattersley, A.T.; Greeley, S.A.W.; Polak, M.; Rubio-Cabezas, O.; Njolstad, P.R.; Mlynarski, W.; Castano, L.; Carlsson, A.; Raile, K.; Chi, D.V.; et al. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 47–63. [Google Scholar] [CrossRef]
- Pearson, E.; Liddell, W.; Shepherd, M.; Corrall, R.; Hattersley, A. Sensitivity to sulphonylureas in patients with HNF-1a gene mutations: Evidence for pharmacogenetics in diabetes. Diabet. Med. 2000, 17, 543–545. [Google Scholar] [CrossRef]
- Broome, D.T.; Tekin, Z.; Pantalone, K.M.; Mehta, A.E. Novel use of GLP-1 receptor agonist therapy in HNF4A-MODY. Diabet. Care 2020, 43, e65. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.; Shields, B.; Ellard, S.; Rubio-Cabezas, O.; Hattersley, A.T. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet. Med. 2009, 26, 437–441. [Google Scholar] [CrossRef]
- Carrillo, E.; Lomas, A.; Pines, P.J.; Lamas, C. Long-lasting response to oral therapy in a young male with monogenic diabetes as part of HNF1B-related disease. Endocrinol. Diabetes Metab. Case Rep. 2017, 17, 0052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delvecchio, M.; Pastore, C.; Giordano, P. Treatment Options for MODY Patients: A Systematic Review of Literature. Diabetes 2020, 11, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Fajans, S.S.; Bell, G.I. MODY: History, genetics, pathophysiology, and clinical decision making. Diabetes Care 2011, 34, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
| Gene | Subtype | Chromosome | Effect on Beta Cell Function |
|---|---|---|---|
| HNF1a | MODY 3 | 12q24.2 | Insufficient glucose mediated insulin secretion |
| GCK | MODY 2 | 7p15–p13 | Higher glucose threshold for insulin release, preserved beta cell secretion capacity |
| HNF4 | MODY 1 | 20q13.2 | Insufficient glucose-mediated insulin secretion; progressive beta cell dysfunction |
| HNF1b | MODY 5 | 17q12 | Beta cell dysfunction, possible pancreatic agenesis/atrophy |
| PDX | MODY 4 | 13q12.2 | Impaired beta cell differentiation, impaired glucose-mediated insulin secretion |
| NEUROD1 | MODY 6 | 2q32 | Beta cell dysfunction; insulinopenia or insulin resistance |
| KLF11 | MODY 7 | 2q25 | Impaired insulin biosynthesis, impaired glucose-mediated insulin secretion |
| CEL | MODY 8 | 9q34 | Beta cell dysfunction from protein misfolding and aggregation; pancreatic atrophy |
| PAX4 | MODY 9 | 7q32 | Impaired beta cell maturation; impaired glucose-mediated insulin secretion |
| INS | MODY 10 | 11p15.5 | Beta cell apoptosis, progressive decrease in beta cell mass |
| BLK | MODY 11 | 8p23 | Decreased beta cell mass; impaired glucose-mediated insulin secretion |
| ABCC8 | MODY 12 | 11.15 | Impaired insulin secretion |
| KCJN11 | MODY 13 | 11p15 | Impaired insulin secretion |
| APPL1 | MODY 14 | 3p14.3 | Reduced beta cell survival; impaired glucose-mediated insulin secretion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antal, Z. Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options. Endocrines 2021, 2, 485-501. https://doi.org/10.3390/endocrines2040043
Antal Z. Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options. Endocrines. 2021; 2(4):485-501. https://doi.org/10.3390/endocrines2040043
Chicago/Turabian StyleAntal, Zoltan. 2021. "Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options" Endocrines 2, no. 4: 485-501. https://doi.org/10.3390/endocrines2040043
APA StyleAntal, Z. (2021). Maturity-Onset Diabetes of the Young (MODY): Genetic Causes, Clinical Characteristics, Considerations for Testing, and Treatment Options. Endocrines, 2(4), 485-501. https://doi.org/10.3390/endocrines2040043






