Endocrinological and Nutritional Implications of Anorexia of Aging
Abstract
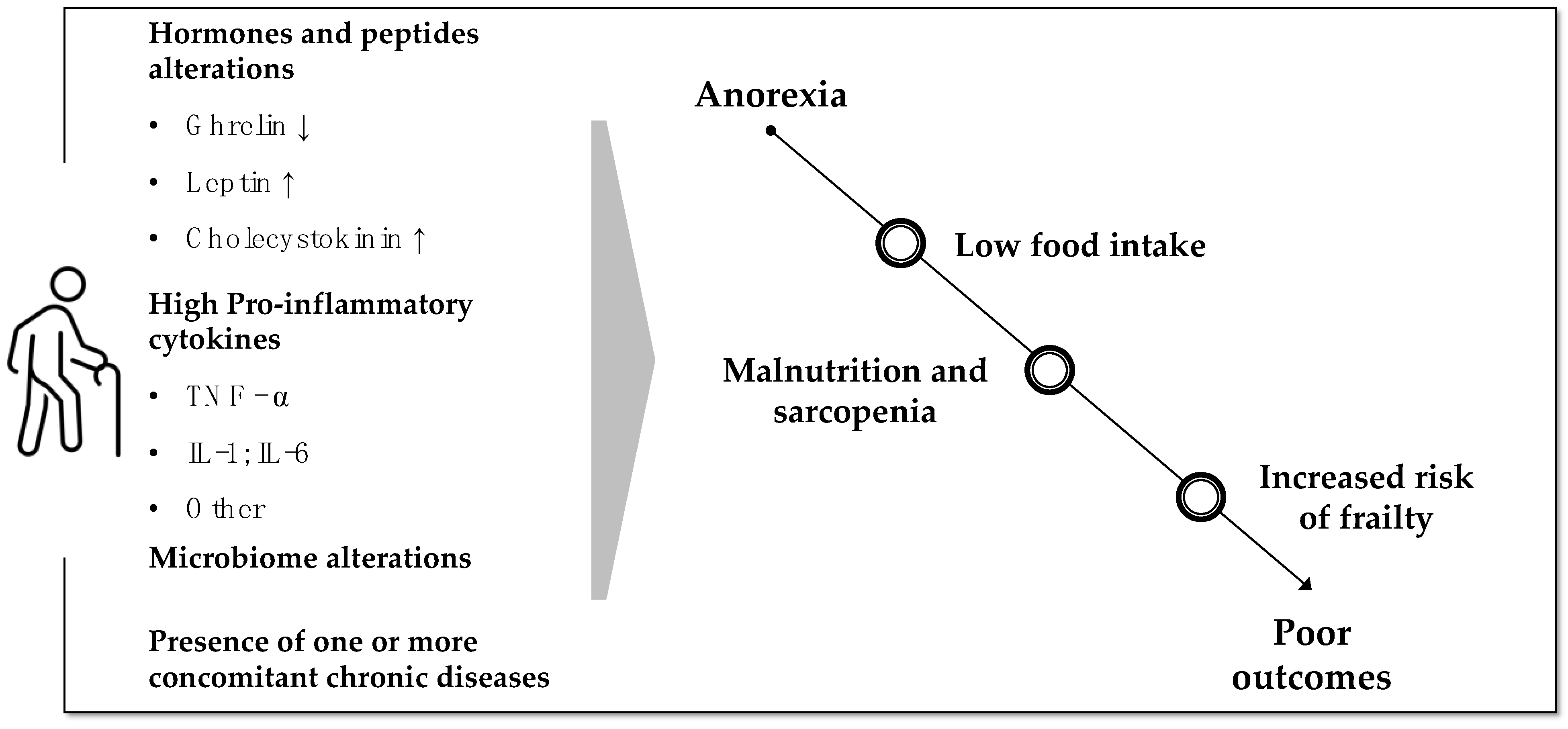
:1. Introduction—Prevalence and Impact of Anorexia of Aging
2. Endocrinological and Metabolic Changes Promoting Anorexia of Aging
2.1. Ghrelin
2.2. Leptin
2.3. Cholecystokinin
2.4. Inflammation
2.5. Microbiome
2.6. Thyroid Function and Other Factors
3. Differences and Overlap between Anorexia of Aging and Disease-Associated Anorexia in the Elderly
4. Can We Consider Anorexia of Aging a Component of Frailty?
5. Diagnosis of Anorexia and Malnutrition in the Elderly and Guidelines for Nutritional Interventions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BWL | Body weight loss |
| GI | Gastrointestinal |
| GLIM | Global Leadership Initiative on Malnutrition |
| MNA-SF | Mini Nutritional Assessment-Short Form |
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Dominguez, L.J.; Barbagallo, M.; Savina, C.; Castellaneta, E.; Cucinotta, D.; Fiorito, A.; Inelmen, E.M.; Sergi, G.; Enzi, G.; et al. Senile anorexia in different geriatric settings in Italy. J. Nutr. Health Aging 2011, 15, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Lattanzio, F.; Russo, A.; Tosato, M.; Barillaro, C.; Bernabei, R.; Onder, G. Effects of anorexia on mortality among older adults receiving home care: An observation study. J. Nutr. Health Aging 2012, 16, 79–83. [Google Scholar] [CrossRef]
- Tsutsumimoto, K.; Doi, T.; Nakakubo, S.; Kim, M.; Kurita, S.; Ishii, H.; Shimada, H. Association between anorexia of ageing and sarcopenia among Japanese older adults. J. Cachexia Sarcopenia Muscle 2020, 11, 1250–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieske, L.; Janssen, G.; Babel, N.; Westhoff, T.H.; Wirth, R.; Pourhassan, M. Inflammation, Appetite and Food Intake in Older Hospitalized Patients. Nutrients 2019, 11, 1986. [Google Scholar] [CrossRef] [Green Version]
- Molfino, A.; Iannace, A.; Colaiacomo, M.C.; Farcomeni, A.; Emiliani, A.; Gualdi, G.; Laviano, A.; Fanelli, F.R. Cancer anorexia: Hypothalamic activity and its association with inflammation and appetite-regulating peptides in lung cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Aging-related anorexia and its association with disability and frailty. J. Cachexia Sarcopenia Muscle 2018, 9, 834–843. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Gil-Guerrero, L.; Iniesta, R. The anorexia of ageing: Physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 2013, 74, 293–302. [Google Scholar] [CrossRef]
- Sanger, G.J.; Furness, J.B. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 38–48. [Google Scholar] [CrossRef]
- Molfino, A.; Laviano, A.; Rossi Fanelli, F. Contribution of anorexia to tissue wasting in cachexia. Curr. Opin. Support. Palliat. Care 2010, 4, 249–253. [Google Scholar] [CrossRef]
- Howick, K.; Griffin, B.T.; Cryan, J.F.; Schellekens, H. From Belly to Brain: Targeting the Ghrelin Receptor in Appetite and Food Intake Regulation. Int. J. Mol. Sci. 2017, 18, 273. [Google Scholar] [CrossRef] [Green Version]
- Hickson, M.; Moss, C.; Dhillo, W.S.; Bottin, J.; Frost, G. Increased peptide YY blood concentrations, not decreased acyl-ghrelin, are associated with reduced hunger and food intake in healthy older women: Preliminary evidence. Appetite 2016, 105, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Miyake, M.; Hori, S.; Itami, Y.; Oda, Y.; Owari, T.; Fujii, T.; Ohnishi, S.; Morizawa, Y.; Gotoh, D.; Nakai, Y.; et al. Supplementary Oral Anamorelin Mitigates Anorexia and Skeletal Muscle Atrophy Induced by Gemcitabine Plus Cisplatin Systemic Chemotherapy in a Mouse Model. Cancers 2020, 12, 1942. [Google Scholar] [CrossRef]
- Temel, J.S.; Abernethy, A.P.; Currow, D.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef]
- Di Francesco, V.; Fantin, F.; Omizzolo, F.; Residori, L.; Bissoli, L.; Bosello, O.; Zamboni, M. The anorexia of aging. Dig. Dis. 2007, 25, 129–137. [Google Scholar] [CrossRef]
- Di Francesco, V.; Zamboni, M.; Zoico, E.; Mazzali, G.; Dioli, A.; Omizzolo, F.; Bissoli, L.; Fantin, F.; Rizzotti, P.; Solerte, S.B.; et al. Unbalanced serum leptin and ghrelin dynamics prolong postprandial satiety and inhibit hunger in healthy elderly: Another reason for the “anorexia of aging”. Am. J. Clin. Nutr. 2006, 83, 1149–1152. [Google Scholar] [CrossRef] [Green Version]
- Milos, G.; Antel, J.; Kaufmann, L.-K.; Barth, N.; Koller, A.; Tan, S.; Wiesing, U.; Hinney, A.; Libuda, L.; Wabitsch, M.; et al. Short-term metreleptin treatment of patients with anorexia nervosa: Rapid on-set of beneficial cognitive, emotional, and behavioral effects. Transl. Psychiatry 2020, 10, 303. [Google Scholar] [CrossRef]
- MacIntosh, C.G.; Morley, J.E.; Wishart, J.; Morris, H.; Jansen, J.B.M.J.; Horowitz, M.; Chapman, I.M. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: Evidence for increased CCK activity as a cause of the anorexia of aging. J. Clin. Endocrinol. Metab. 2001, 86, 5830–5837. [Google Scholar] [CrossRef]
- Khalil, T.; Walker, J.P.; Wiener, I.; Fagan, C.J.; Townsend, C.M., Jr.; Greeley, G.H., Jr.; Thompson, J.C. Effect of aging on gallbladder contraction and release of cholecystokinin-33 in humans. Surgery 1985, 98, 423–429. [Google Scholar]
- Sturm, K.; MacIntosh, C.G.; Parker, B.A.; Wishart, J.; Horowitz, M.; Chapman, I.M. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J. Clin. Endocrinol. Metab. 2003, 88, 3747–3755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Rossi-Fanelli, F.; Laviano, A. The interaction between pro-inflammatory cytokines and the nervous system. Nat. Rev. Cancer 2009, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.S.; Schuster, M.W. Geriatric cachexia: The role of cytokines. Am. J. Clin. Nutr. 1999, 70, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Dent, E.; Hoogendijk, E.O.; Wright, O.R.L. New insights into the anorexia of ageing: From prevention to treatment. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 44–51. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Cox, N.J.; Bowyer, R.C.E.; Ni Lochlainn, M.; Wells, P.M.; Roberts, H.C.; Steves, C.J. The composition of the gut microbiome differs among community dwelling older people with good and poor appetite. J. Cachexia Sarcopenia Muscle 2021, 12, 368–377. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Emiliani, A.; Ramaccini, C.; Lahaye, E.; Takagi, K.; Fetissov, S.O. Plasma enterobacterial ClpB levels and ClpB-and α-MSH-reactive immunoglobulins in lung cancer patients with and without anorexia. Nutrition 2020, 78, 110952. [Google Scholar] [CrossRef]
- Breton, J.; Legrand, R.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 2016, 49, 805–808. [Google Scholar] [CrossRef]
- Breton, J.; Tennoune, N.; Lucas, N.; Francois, M.; Legrand, R.; Jacquemot, J.; Goichon, A.; Guérin, C.; Peltier, J.; Pestel-Caron, M.; et al. Gut Commensal E. coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. Cell Metab. 2016, 23, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Duntas, L.H.; Yen, P.M. Diagnosis and treatment of hypothyroidism in the elderly. Endocrine 2019, 66, 63–69. [Google Scholar] [CrossRef]
- Van Hulsteijn, L.T.; Pasquali, R.; Casanueva, F.; Haluzik, M.; Ledoux, S.; Monteiro, M.P.; Salvador, J.; Santini, F.; Toplak, H.; Dekkers, O.M. Prevalence of endocrine disorders in obese patients: Systematic review and meta-analysis. Eur. J. Endocrinol. 2020, 182, 11–21. [Google Scholar] [CrossRef]
- Amin, A.; Dhillo, W.S.; Murphy, K.G. The central effects of thyroid hormones on appetite. J. Thyroid Res. 2011, 2011, 306510. [Google Scholar] [CrossRef] [Green Version]
- Kamwa, V.; Welch, C.; Hassan-Smith, Z.K. The endocrinology of sarcopenia and frailty. Minerva Endocrinol. 2020. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of ageing: A key component in the pathogenesis of both sarcopenia and cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Rizzo, V.; Pediconi, F.; Catalano, C.; Emiliani, A.; Belli, R.; Ramaccini, C.; Parisi, C.; et al. Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients. Cancers 2020, 13, 99. [Google Scholar] [CrossRef]
- Olson, B.; Zhu, X.; Norgard, M.A.; Levasseur, P.R.; Butler, J.T.; Buenafe, A.; Burfeind, K.G.; Michaelis, K.A.; Pelz, K.R.; Mendez, H.; et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat. Commun. 2021, 12, 2057. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Clegg, A.; Hassan-Smith, Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018, 6, 743–752. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.D.; Newman, A.B.; Hirsch, C.; Gottdiener, J.S.; Seeman, T.; Tracy, R.P.; Kop, W.J.; Burke, G.L.; et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Wei, K.; Nyunt, M.S.; Gao, Q.; Wee, S.L.; Yap, K.B.; Ng, T.P. Association of Frailty and Malnutrition With Long-term Functional and Mortality Outcomes Among Community-Dwelling Older Adults: Results From the Singapore Longitudinal Aging Study 1. JAMA Netw. Open 2018, 1, e180650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Barillaro, C.; Capoluongo, E.D.; Bernabei, R.; Onder, G. Association of anorexia with sarcopenia in a community-dwelling elderly population: Results from the ilSIRENTE study. Eur. J. Nutr. 2013, 52, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front. Endocrinol. 2019, 10, 255. [Google Scholar] [CrossRef]
- Dawson, A.; Dennison, E. Measuring the musculoskeletal aging phenotype. Maturitas 2016, 93, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Mannucci, P.M.; Nobili, A.; Pasina, L.; REPOSI Collaborators. Polypharmacy in older people: Lessons from 10 years of experience with the REPOSI register. Intern. Emerg. Med. 2018, 13, 1191–1200. [Google Scholar] [CrossRef] [Green Version]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Molfino, A.; Kaysen, G.A.; Chertow, G.M.; Doyle, J.; Delgado, C.; Dwyer, T.; Laviano, A.; Fanelli, F.R.; Johansen, K.L. Validating Appetite Assessment Tools Among Patients Receiving Hemodialysis. J. Ren. Nutr. 2016, 26, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Molfino, A.; de van der Schueren, M.A.E.; Sánchez-Lara, K.; Milke, P.; Amabile, M.I.; Imbimbo, G.; Di Lazzaro, L.; Cavuto, S.; Ronzani, G.; Snegovoy, A.; et al. Cancer-associated anorexia: Validity and performance overtime of different appetite tools among patients at their first cancer diagnosis. Clin. Nutr. 2021, 40, 4037–4042. [Google Scholar] [CrossRef]
- Cox, N.J.; Ibrahim, K.; Sayer, A.A.; Robinson, S.M.; Roberts, H.C. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; Bunn, D.; Jimoh, F.O.; Fairweather-Tait, S.J. Water-loss dehydration and aging. Mech. Ageing Dev. 2014, 136–137, 50–58. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfino, A.; Imbimbo, G.; Muscaritoli, M. Endocrinological and Nutritional Implications of Anorexia of Aging. Endocrines 2021, 2, 439-448. https://doi.org/10.3390/endocrines2040039
Molfino A, Imbimbo G, Muscaritoli M. Endocrinological and Nutritional Implications of Anorexia of Aging. Endocrines. 2021; 2(4):439-448. https://doi.org/10.3390/endocrines2040039
Chicago/Turabian StyleMolfino, Alessio, Giovanni Imbimbo, and Maurizio Muscaritoli. 2021. "Endocrinological and Nutritional Implications of Anorexia of Aging" Endocrines 2, no. 4: 439-448. https://doi.org/10.3390/endocrines2040039
APA StyleMolfino, A., Imbimbo, G., & Muscaritoli, M. (2021). Endocrinological and Nutritional Implications of Anorexia of Aging. Endocrines, 2(4), 439-448. https://doi.org/10.3390/endocrines2040039





