Abstract
Early diagnosis and long-term management of endometriosis is important in adolescent girls considering their potential for future pregnancy and need for preventing disease progression. However, symptoms and clinical findings of adolescent endometriosis may differ from those of typical adult endometriosis, making diagnosis difficult. In adolescents, menstrual pain may present as acyclic and unresponsive to commonly used medication. Typical imaging findings in adult endometriosis, such as ovarian endometriotic cysts and fibrotic scars, are less common in adolescents. Peritoneal lesions, characteristic of early-stage endometriosis, are commonly found in this age group. It should be noted that endometriosis may also be found in adolescents before menarche, because of premenarcheal endometriosis or congenital uterine anomaly and outflow obstruction; the latter requiring surgical correction. Although surgery is reported to be effective for pain, postsurgical recurrence rate is high, and the effect of hormonal treatment is controversial. The optimal timing for surgical intervention also remains to be determined. Here, we aim to identify the unique characteristics of endometriosis in adolescents to achieve early diagnosis and optimal management for this group of patients.
1. Introduction
Endometriosis is a chronic condition that affects women throughout various stages of their lives by causing pain, infertility, and malignant progression. Most women with endometriosis are diagnosed after their mid-twenties. However, two-thirds of women over twenty years of age who were diagnosed with endometriosis have reported having symptoms such as dysmenorrhea or chronic pelvic pain since they were adolescents. Endometriosis is also considered a progressive disease. Therefore, early diagnosis and long-term management are especially important in younger patients to prevent disease progression and preserve fertility [1]. However, endometriosis in adolescents possesses several unique characteristics, which vary from those in adults and should be considered to achieve early diagnosis (Table 1). Endometriosis is under-recognized in adolescents, and many adolescents are hesitant to seek medical attention [2]. Their diagnoses are complicated due to its atypical symptom presentation. Careful pelvic ultrasound and laparoscopic studies are necessary to detect the subtle signs of endometriosis in adolescents. Moreover, medical and surgical management should be tailored to fit the developmental stage and future plans of adolescents. This review aims to identify the unique characteristics of adolescent endometriosis to obtain early diagnosis and optimal management for this group of patients.

Table 1.
Characteristics of adolescent endometriosis.
2. Prevalence and Social Context
The precise age range of “adolescence” is not clearly defined. Some reports limit the age range to teenagers, while others include women until their mid-twenties. However, most reports seem to refer to women under the age of 22 years [3]. This is generally the age at which most women graduate from school and become members of society. Therefore, we defined adolescents as women under the age of 22 in this report.
Noninvasive studies such as pelvic ultrasound play a critical role in the early diagnosis of endometriosis, especially in adolescents. In a study of 270 women aged 12–20 years who underwent ultrasound pelvic examination (transvaginal or transrectal), at least one endometriosis feature was identified in 13.3%. Ovarian endometriomas were found in 11%, adenomyosis in 5.2%, and deep infiltrating endometriosis in 3.7%. Among adolescents with dysmenorrhea, the detection of pelvic endometriosis with ultrasound increased to 21% [4].
Laparoscopically diagnosed endometriosis among adolescents is reported to range from 19% to 43%, depending on reports [17,21]. In 2013, Jansen et al. reviewed 15 studies including 880 adolescents aged 10–21 years with dysmenorrhea or pelvic pain. Endometriosis was diagnosed in 62% of adolescent women who underwent laparoscopic examinations for pain. The rate of diagnosis was 49% in those experiencing chronic pelvic pain (204 out of 420 women), 70% in those with dysmenorrhea (102 out of 146 women), and 75% in those with chronic pelvic pain resistant to medical therapy (237 out of 314 women) [3].
Despite the relatively high prevalence of endometriosis among adolescents, menstrual stigma and lack of knowledge may hamper adolescents to seek medical attention. Gupta et al. examined how symptoms suggestive of endometriosis among adolescents are perceived at the peer and community levels. Menstruation was often associated with weakness and considered taboo, impeding conversation about menstruation. This leads to a lack of knowledge among adolescents about the variety of menstruation experiences and symptoms suggestive of endometriosis. Lack of training among school personnel in identifying adolescents susceptive of endometriosis was also considered to contribute to help-seeking. Their findings highlighted a need for education/access to information as well as de-stigmatization campaigns [2].
3. Clinical Findings
Dysmenorrhea and chronic pelvic pain are typical symptoms of endometriosis, and acyclic pain has been reported in adolescents more often than in adults who complain frequently of cyclical pain, implying dysmenorrhea. In adults, the pain commonly precedes the onset of the menstruation and increases during menstruation [3,22]. In adolescents, Laufer et al. reported that 62.5% had both acyclic and cyclic pain and 28.1% had acyclic pain, whereas only 9.4% of adolescents had the typical cyclic pain with menstruation [22]. Although non-steroidal anti-inflammatory drugs (NSAIDs) and combined oral contraceptives (COC) are frequently prescribed as empiric therapy, young patients may frequently report dysmenorrhea resistant to these medications. In a systematic review, the prevalence of endometriosis was higher in girls with chronic pelvic pain resistant to COC and/or NSAIDs (75%; 237/314) than in girls with chronic pelvic pain not resistant to medication (49%; 204/420) [3]. Endometriosis should be highly suspected when treatment with these medications fails [1,23]. A retrospective cohort study of 900 women who received surgical treatment for endometriosis compared the difference in characteristics between 55 adolescent girls between 13 and 21 years and 845 women aged beyond 22 years. The study showed that adolescents had more risk factors for endometriosis than adults, such as early menarche, first-degree relatives with endometriosis, history of asthma, and congenital anomalies [24].
Ultrasound pelvic examination is noninvasive, and therefore, most accessible for diagnosing endometriosis in adolescents. However, adolescents may have an earlier stage of the disease with smaller endometriotic lesions, which could be difficult to detect using ultrasonography. For small endometriomas, persistence beyond at least three menstrual cycles serves as an indicator [4]. Indirect sonographic signs, or “soft markers,” could help in detecting superficial tissue invasions. These “soft markers,” including site-specific tenderness and reduced ovarian mobility, are correlated with endometriosis and adhesions detected during laparoscopy [5,6]. Adhesions may be suspected if the ovaries or the uterus move together with adjacent structures, such as the bladder, intestines, pouch of Douglas, and parietal peritoneum, as the sonographer palpates the abdomen [6]. Obliteration at the pouch of Douglas may be detected using the sliding sign, where the cervix is gently pressed with the transvaginal sonography probe and/or the uterus is palpated abdominally by hand, to assess whether the rectosigmoid slides independently against the posterior uterine wall [25]. Small deep infiltrating endometriosis nodules may be easier to identify by the “tenderness-guided” transvaginal sonography and using additional ultrasound gel in the probe cover, instead of the generally used 4 mL of gel [26].
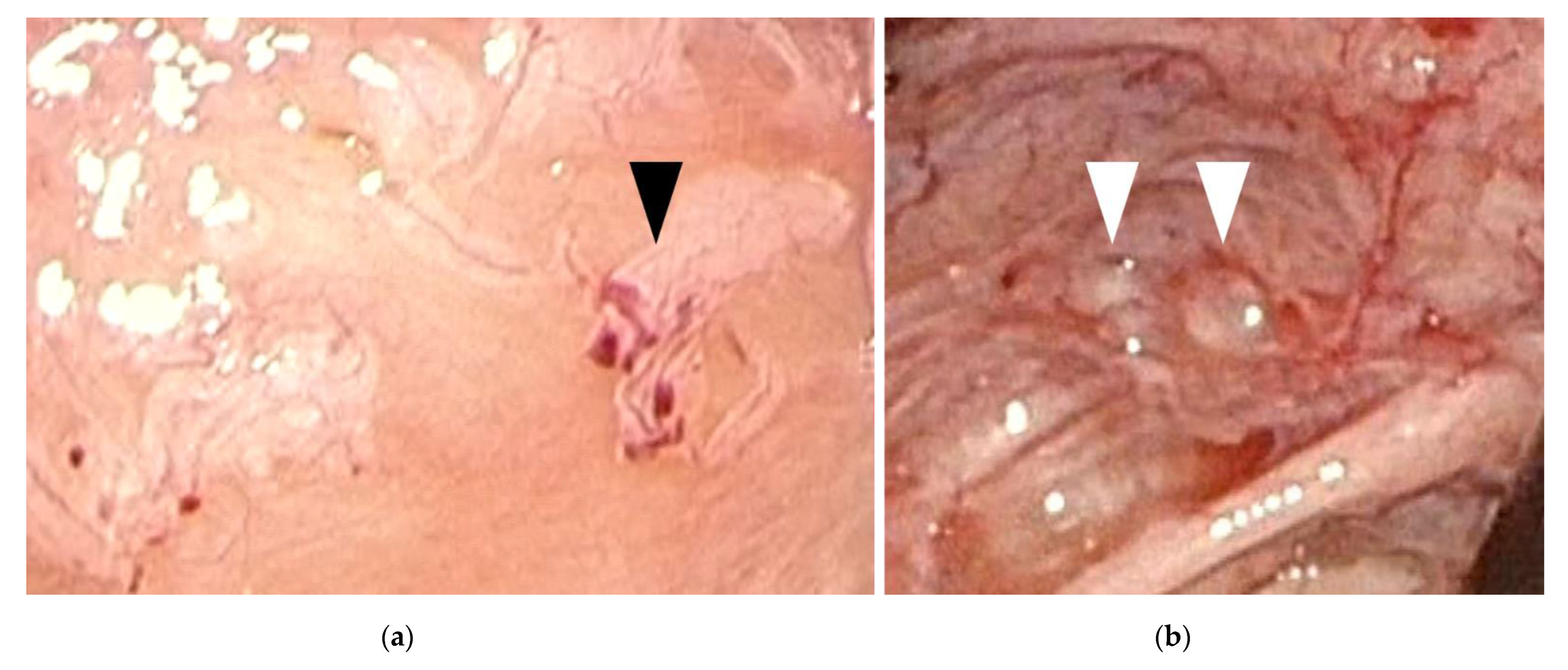
The findings of laparoscopic surgery are key factors in diagnosing endometriosis. Matalliotakis et al. reported that, during laparoscopic examinations, 45 out of 55 adolescent young girls (81.8%) were diagnosed with endometriosis stage I–II based on the revised American Society for Reproductive Medicine classification, while the remaining 10 (18.2%) were diagnosed as stage III–IV [24]. Audebert et al. reported similar results [27]. In general, ovarian endometriomas are frequently observed in women aged >20 years. “Powder-burn” lesions consisting of a mixture of black lesions and white scars, and “blueberry spots” referring to blue-black nodules, are typical peritoneal lesions that reflect chronic hemorrhage and fibrosis. In contrast, ovarian endometriomas are not frequently found in adolescents. Peritoneal lesions, especially red and clear peritoneal lesions (Figure 1a) and vesicular lesions (Figure 1b), which are characteristic of early-stage endometriosis, are the majority of lesions found in adolescents [7,28,29]. These superficial peritoneal lesions are reported to be highly inflammatory. Moreover, it has been shown that clear and red lesions are the most painful [30]. Early-stage lesions are difficult to detect even under laparoscopy. These microvascularizations and the filmy, free-floating adhesions on the peritoneal and ovarian surfaces collapse under pneumoperitoneum pressure. Laufer et al. reported that the conformation of these subtle lesions becomes easier to identify by filling the pelvis with normal saline, thereby distending the peritoneum and preventing collapse of lesions. Submersing the laparoscope under water to suppress light reflection also aids in identifying lesions [8,31]. The different findings detected in adults versus adolescents (adolescents primarily show early-stage lesions, whereas adults mainly show fibrosis and scarring) demonstrate that endometriosis is a progressive disease. When assessing and diagnosing endometriosis in adolescents, it is essential to direct careful attention to early-stage lesions, as these can be easily missed.
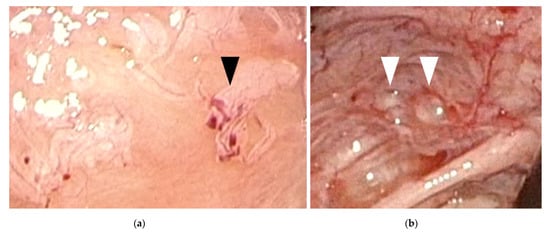
Figure 1.
Early-stage endometriosis lesions found in adolescents. (a) Red and clear peritoneal lesions on the peritoneum (black arrowhead); (b) Vesicular lesions (white arrowhead) found on the peritoneum near the left uterosacral ligament.
4. Pathology
Endometriosis is generally considered in women after menarche. However, it may also be found in adolescents before menarche. Distinct classifications in this age group, including premenarcheal endometriosis and endometriosis associated with congenital uterine anomaly and outflow obstruction, should be noted.
4.1. Premenarcheal Endometriosis
Although documentation is limited, there are reports of biopsy-proven endometriosis diagnosed by laparoscopy in premenarcheal women, who originally sought consultation for pelvic pain. Marsh et al. reported 5 premenarcheal adolescents ranging from 8.5 to 13 years old. They presented with breast development of Tanner stages I–III, chronic pelvic pain lasting for more than 6 months, and no evidence of obstructive uterine anomaly. Red/clear peritoneal endometriotic lesions were observed in all 5 adolescents, and endometrial stromal cells were confirmed by pathology [32]. Ebert et al. reported a 9-year-old girl who underwent laparoscopic surgery for cyclic pelvic pain which started at the age of 8. Although endometriosis was pathologically confirmed, it was only after a year that she had her first menstruation [33]. Gogacz et al. reported an 11-year-old premenarcheal girl without obstructive uterine anomalies who presented with severe abdominal pain and vomiting. Surgery revealed an 8 × 5 cm ovarian cyst with filmy adhesions to the peritoneum and intestines, which was later pathologically confirmed as an ovarian endometrioma [34].
Among the several theories of endometriosis etiology, premenarcheal endometriosis may be explained by those proposing that lesions arise from tissues outside the uterus. These tissues include normal peritoneum undergoing metaplasia, which is suggested to be induced by endocrine disrupting chemicals. Embryonic Müllerian rests, or cells residual from embryogenic Müllerian duct migration, and stem/progenitor cells deriving from the bone marrow may also be the candidates for the extrauterine origins of endometriosis. Mesenchymal stem cells and endometrial stem cells are thought to be potential endometrial progenitor cells [35,36]. These cells may be activated by premenarcheal onset of estrogen stimulation in early puberty [32]. The vascular endothelial growth factor is reported to be expressed more strongly in the proliferative phase than in the secretory phase. The estrogenic effect of maternal steroids or prepubertal estrogen may therefore stimulate angiogenesis in implants leading to its development into endometriosis. Mesenchymal stem cells and endometrial stem cells are thought to be potential endometrial progenitor cells [35,36,37].
Conversely, premenarcheal endometriosis may also be explained by the widely accepted theory of uterine reflux. The phenomenon of neonatal uterine bleeding (NUB) has gained increasing attention as a source of endometrial stem cells. Careful histological observations have shown that NUB results from progesterone withdrawal upon birth [9,23,38]. In a study of 350 newborn girls, although NUB was grossly evident in only 3.3% of neonates, biochemical analysis proved that it actually occurred in 25.4% of neonates. NUB was always seen in the first week, most frequently on the 5th day of life [39]. The neonatal uterine cervix has twice the length of the corpus and is filled with thick mucus, thereby facilitating menstrual blood reflux into the peritoneum rather than cervical outflow [40]. Considering Sampson’s theory of retrograde menstruation and implantation as the most widely accepted theory for the pathogenesis of endometriosis [41], NUB reflux may be the origin of pelvic uterine endometrial stem cells. Neonatal endometrial cells are documented to be able to rapidly attach themselves to the peritoneum. These cells would stay dormant until thelarche, when factors known to stimulate endometrial growth would activate these cells, progressing to endometriosis [9].
4.2. Endometriosis Associated with Congenital Uterine Malformation and Outflow Obstruction
Congenital uterine malformations with outflow obstruction include obstructive Müllerian duct malformations such as unicornuate uterus with non-communicating functional horn, or double uterus with an obstructed vagina. Increased menstrual blood reflux, caused by outflow obstruction, may increase the susceptibility to endometriosis in women with obstructive malformations. In a study of 85 adolescent women younger than 19 years of age, none of the 44 women with genital malformations had a family history of endometriosis, while 8 (19.5%) of the 41 women without genital malformations had a positive family history [42]. In a study of 64 women with Müllerian duct anomalies accompanying functional endometrium and patent fallopian tube, 10 out of 13 (77%) women with outflow obstruction had endometriosis, while only 16 out of 43 women (37%) without outflow obstruction had endometriosis [43]. These results confirm outflow obstruction to be a risk factor for endometriosis, supporting the theory of retrograde menstruation and implantation (Figure 2). Obstructive malformations may also cause earlier onset of endometriosis among young women. Yang et al. examined 63 women younger than 21 years of age with a pathologically confirmed diagnosis of endometriosis. Although the mean age at diagnosis was 18.4 ± 1.8, women with obstructive malformations were diagnosed much younger than those without (16.21 versus 19.0 years). Among the 15 young women with obstructive malformations, the ovaries were involved in 14 women (93%), while the pouch of Douglas and the uterosacral ligaments were affected in only two women (13%). On the other hand, among the 48 women without obstructive malformations, the pouch of Douglas and the uterosacral ligaments were affected in 26 women (54%; p = 0.005) [44]. In adolescents with congenital outflow obstruction, endometriosis is treated by modifying the obstruction under laparoscopic surgery, rather than with medical therapy. Song et al. reported that the mean age at the time of surgery in adolescents with obstructive malformation is reported to be significantly less than those without obstructive malformation (15.1 ± 2.4 versus 17.6 ± 1.7 years; p < 0.001). The delay from symptom onset to surgery was also shorter (1.5 ± 1.3 versus 2.3 ± 2.1 years; p = 0.033) [42]. Surgery drastically alleviates pain, emphasizing the importance of evaluating obstructive malformations in younger adolescent women with pelvic pain. Furthermore, it has been reported that recurrence does not occur after surgical correction of outflow obstruction [10,45].
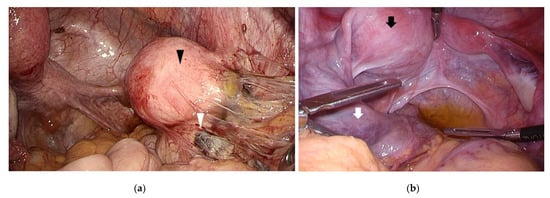
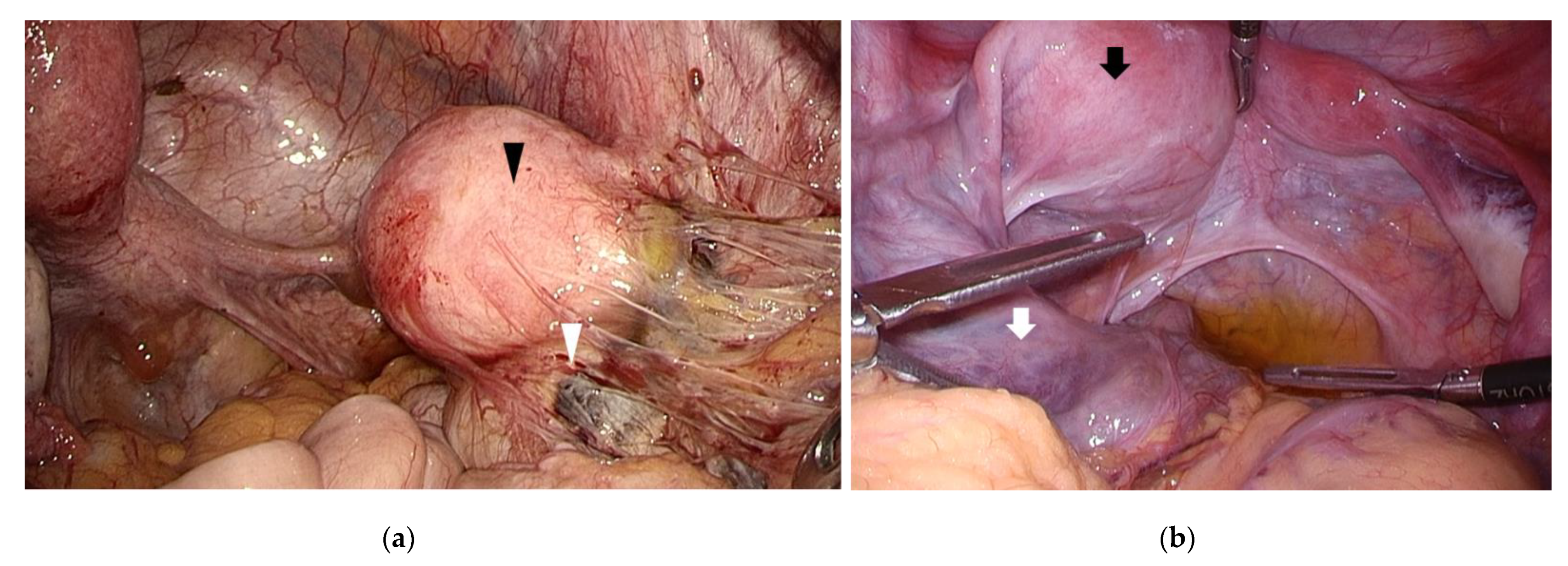
Figure 2.
Congenital uterine anomaly and outflow obstruction. (a) A left unicornuate uterus with non-communicating functional horn (black arrowhead) was observed. An endometriotic cyst (white arrowhead) was found in the right ovary with inflammatory adhesion surrounding the right adnexa; (b) A right unicornuate uterus presenting with hematometra accompanying a non-communicating functional horn (black arrow). Left hematosalpinx (white arrow) and left endometriotic cyst was observed.
5. Treatment
COC and NSAIDs are the mainstream of conservative therapies for adolescents. Continuous- or extended-cycle COC has been reported to be as effective as the conventional cyclic use of these pills [46,47]. Gaining knowledge of dysmenorrhea and maintaining pain records to understand one’s menstrual cycles are important strategies for dealing with pain. Maintaining an orderly life and psychological therapy have also been reported to be effective [1,47]. The likelihood of endometriosis rises when COC and NSAIDs are ineffective. Therefore, laparoscopic diagnosis before introducing GnRH analogs/antagonists or progestins may be recommended [11]. Although the use of GnRH analogs/antagonists has been reported [48], many studies have warned against its use in adolescents, due to ongoing osteogenesis in this population. It has been recommended that GnRH analogs/antagonists should be postponed until the age of 17 years when osteogenesis is completed [11,49,50]. There are limited data on the long-term use of progestins in adolescents. A recent study described 219 nulliparous adolescents under the age of 22 years, who underwent levonorgestrel-releasing intrauterine system (LNG IUS) insertion primarily for non-contraceptive medical indications. One year after insertion, LNG IUS continuation rate was 86%, and the amenorrhea rate was 51%. Approximately 80% of adolescents reported less menstrual bleeding and improved alleviation of abdominal/pelvic pain. The study concluded that LNG IUS is an effective, well-tolerated and safe option for managing menstruation in young nulliparous women, including those who have never been sexually active [51]. However, this study did not report the long-term effects on future fertility and bone density. Although experiences with depot medroxyprogesterone acetate in adults have shown that the decreased bone density generally recovers to pretreatment levels in a year after discontinuation [52], limited results have suggested that long-term use of progestins in adolescents should only be recommended after consideration, as with GnRH analogs/antagonists.
Most of the currently available medications for endometriosis alleviate symptoms by maintaining a hypoestrogenic state. Several new medications are being studied, which target the underlying inflammatory, angiogenic, and hormonal aspects of the disease. In a Cochrane review, mifepristone, a selective progesterone receptor modulator (SPRM), was reported effective in reducing dysmenorrhea in women with endometriosis [53]. A randomized controlled trial also demonstrated that mifepristone may improve surgery outcomes with a higher pregnancy rate and less recurrence [54]. SPRMs are expected to selectively inhibit endometrial proliferation, without depriving estrogen, and relieve pain by suppressing prostaglandin and cytokine synthesis. However, the specific mechanisms are different among each SPRMs, and their effects also depend on the character of the target tissue. Currently, there is insufficient evidence to draw any conclusion about the safety and therapeutic value of using SPRMs in the long-term treatment of endometriosis [55].
Anti-vascular endothelial growth factor agents have been shown to reduce endometriosis size and score in animal models [56]. Drugs targeting inflammation, such as tumor necrosis factor alpha inhibitors, Janus kinase inhibitors and mTOR inhibitors, are also reported with promising results [57]. These new options may also be used by itself or in combination with presently existing medications.
Reports on pain improvement or cure rates after surgery are limited in adolescents with no published comparative trials. A limited retrospective series of 31 teenagers studied post-operative outcomes. All 31 were prescribed NSAIDs or COC with no response prior to laparoscopy. The median age at the time of surgery was 16.5 years (range 13–20 age). Endometriosis was diagnosed in 11 patients, including 5 with mild or moderate and 6 with severe endometriosis. Post-operative medical management consisting of LNG IUS and COCs was prescribed to prevent recurrence. The study reported that approximately 80% were either pain-free or showed improvement in pain after surgery, with a median follow-up of 65 weeks [12]. A study of 20 adolescents with pathologically confirmed endometriosis examined the effect of surgery at a mean follow-up time of 2.6 years (range 1.77–3.43). Surgical excision of endometriosis significantly improved dysmenorrhea and pelvic pain symptoms [13]. Another report studied 17 teenagers with pathologically confirmed endometriosis. Follow-up was up to 66 months with an average of 23.1 months. Although only one-third of patients took post-operative hormonal suppression for any length of time, a statistically significant improvement in pain, including bowel-related symptoms, was noted during this time period. The authors concluded that complete laparoscopic excision of endometriotic lesions in teenagers may eradicate disease, independent of post-operative hormonal suppression [14]. Although most studies have reported improvement in pain after surgery, publication bias may exist.
Post-operative recurrence in adolescents is a major concern. In a retrospective study of 105 women under 20 years of age who underwent conservative laparoscopic endometriotic cyst enucleation, the median time to recurrence was 53.0 months (range 8–111 months). The cumulative endometrioma recurrence rates were 6.4%, 10%, 19.9%, and 30.9%, after 2, 3, 5, and 8 years, respectively. This suggests that the short-term recurrence rate in adolescents after first-line conservative surgery was relatively low. In this study, although most adolescents received post-operative medications, the correlation between medical therapy and recurrence was not examined [15]. In another study of 57 women under the age of 21 years, the cumulative recurrence rate after 5 years of post-operative follow-up was reported to be as high as 56%. In this study, a high recurrence rate was observed, despite the fact that all women were advised to take oral contraceptives post-operatively. However, the majority of women reported to a discontinuation in medication after a short period of time [16]. It has been well demonstrated that post-operative hormonal medication markedly prevents recurrence of endometriosis. Of note, it has also been shown that this protective effect disappears upon discontinuing medication and that only long-term medication would protect women from recurrence [58]. Although the role of post-operative long-term medical therapy in suppressing endometriosis recurrence is clearly demonstrated in adults, its role is yet unclear in adolescents [11,14,59].
Currently, there is no consensus regarding the best timing for surgical intervention. Some have stated that early intervention is preferable since early endometriotic lesions may cause follicular depletion through focal inflammation and less operative damage would occur when the lesion is smaller, thus preventing future iatrogenic ovarian insufficiency [17,18,19]. Others have argued that, considering the high post-operative recurrence rate, early intervention may warrant repeated surgeries. Excised endometrioma tissues have been shown to accompany normal ovarian tissue fractions. Repeated surgeries may lead to ovarian dysfunction as supported by decreased postoperative levels of the anti-Müllerian hormone, which reflects the ovarian reserve [20,47].
6. Conclusions
Endometriosis is difficult to diagnose in adolescents who are less aware of the condition and are more hesitant to undergo gynecologic examinations. Characteristics of adolescent endometriosis, different from that of adult, should be kept in mind in their diagnosis and management. Atypical symptoms and subtle early-stage lesions that are less evident with imaging studies also make the diagnosis more difficult. Premenarcheal endometriosis and endometriosis associated with congenital uterine malformation and outflow obstruction are distinct classifications, which require particular attention in this group of patients. Although COC and NSAIDs are the mainstream of treatment in adolescents, symptoms are frequently resistant to these medications. The use of GnRH agonists/antagonists are recommended to be postponed until osteogenesis is completed. LNG IUS is anticipated to be effective and safe for adolescents; however, long-term effects on future fertility and bone density need to be examined further. New medications are being studied which target the underlying pathophysiology of the disease. The pros and cons of surgery and the precise timing of surgical intervention are also under careful consideration. As endometriosis is a chronic condition, adolescent patients would require an earlier and long-term management that may need to be modulated at various reproductive and social stages of life. Achieving early diagnosis and optimal management may be supported by taking the unique characteristics of endometriosis into account in this age group.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Nagoya University Hospital (2015-03536739).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The author declares no conflict of interest.
References
- Stuparich, M.A.; Donnellan, N.M.; Sanfilippo, J.S. Endometriosis in the Adolescent Patient. Semin. Reprod. Med. 2017, 35, 102–109. [Google Scholar] [PubMed]
- Gupta, J.; Cardoso, L.F.; Harris, C.S.; Dance, A.D.; Seckin, T.; Baker, N.; Ferguson, Y.O. How do adolescent girls and boys perceive symptoms suggestive of endometriosis among their peers? Findings from focus group discussions in New York City. BMJ Open 2018, 8, e020657. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Rijkers, A.; Hoppenbrouwers, K.; Meuleman, C.; D’Hooghe, T. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: A systematic review. Hum. Reprod. Update 2013, 19, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martire, F.G.; Lazzeri, L.; Conway, F.; Siciliano, T.; Pietropolli, A.; Piccione, E.; Solima, E.; Centini, G.; Zupi, E.; Exacoustos, C. Adolescence and endometriosis: Symptoms, ultrasound signs and early diagnosis. Fertil. Steril. 2020, 114, 1049–1057. [Google Scholar] [CrossRef]
- Okaro, E.; Condous, G.; Khalid, A.; Timmerman, D.; Ameye, L.; Huffel, S.V.; Bourne, T. The use of ultrasound-based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain--can we reduce the need for laparoscopy? BJOG Int. J. Obstet. Gynaecol. 2006, 113, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Gerges, B.; Lu, C.; Reid, S.; Chou, D.; Chang, T.; Condous, G. Sonographic evaluation of immobility of normal and endometriotic ovary in detection of deep endometriosis. Ultrasound Obstet. Gynecol. 2017, 49, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Redwine, D.B. Age-related evolution in color appearance of endometriosis. Fertil. Steril. 1987, 48, 1062–1063. [Google Scholar] [CrossRef]
- Laufer, M.R. Current approaches to optimizing the treatment of endometriosis in adolescents. Gynecol. Obstet. Investig. 2008, 66 (Suppl. S1), 19–27. [Google Scholar] [CrossRef]
- Benagiano, G.; Guo, S.-W.; Puttemans, P.; Gordts, S.; Brosens, I. Progress in the diagnosis and management of adolescent endometriosis: An opinion. Reprod. Biomed. Online 2018, 36, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, J.S.; Wakim, N.G.; Schikler, K.N.; Yussman, M.A. Endometriosis in association with uterine anomaly. Am. J. Obstet. Gynecol. 1986, 154, 39–43. [Google Scholar] [CrossRef]
- Saridogan, E. Adolescent endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 46–49. [Google Scholar] [CrossRef]
- Stavroulis, A.; Saridogan, E.; Creighton, S.; Cutner, A. Laparoscopic treatment of endometriosis in teenagers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 248–250. [Google Scholar] [CrossRef]
- Roman, J.D. Adolescent endometriosis in the Waikato region of New Zealand--a comparative cohort study with a mean follow-up time of 2.6 years. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 179–183. [Google Scholar] [CrossRef]
- Yeung, P., Jr.; Sinervo, K.; Winer, W.; Albee, R.B., Jr. Complete laparoscopic excision of endometriosis in teenagers: Is postoperative hormonal suppression necessary? Fertil. Steril. 2011, 95, 1909–1912.e1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, M.-L.; Seong, S.J.; Bae, J.W.; Cho, Y.J. Recurrence of Ovarian Endometrioma in Adolescents after Conservative, Laparoscopic Cyst Enucleation. J. Pediatr. Adolesc. Gynecol. 2017, 30, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Tandoi, I.; Somigliana, E.; Riparini, J.; Ronzoni, S.; Vigano, P.; Candiani, M. High rate of endometriosis recurrence in young women. J. Pediatr. Adolesc. Gynecol. 2011, 24, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.P.; Fedele, L.; Arcaini, L.; Bianchi, S.; Rognoni, M.T.; Candiani, G.B. Laparoscopy in the diagnosis of chronic pelvic pain in adolescent women. J. Reprod. Med. 1989, 34, 827–830. [Google Scholar] [PubMed]
- Kitajima, M.; Defrère, S.; Dolmans, M.-M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Dolmans, M.-M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef]
- Hirokawa, W.; Iwase, A.; Goto, M.; Takikawa, S.; Nagatomo, Y.; Nakahara, T.; Bayasula, B.; Nakamura, T.; Manabe, S.; Kikkawa, F. The post-operative decline in serum anti-Mullerian hormone correlates with the bilaterality and severity of endometriosis. Hum. Reprod. 2011, 26, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Kontoravdis, A.; Hassan, E.; Hassiakos, D.; Botsis, D.; Kontoravdis, N.; Creatsas, G. Laparoscopic evaluation and management of chronic pelvic pain during adolescence. Clin. Exp. Obstet. Gynecol. 1999, 26, 76–77. [Google Scholar]
- Laufer, M.; Goitein, L.; Bush, M.; Cramer, D.; Emans, S. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J. Pediatr. Adolesc. Gynecol. 1997, 10, 199–202. [Google Scholar] [CrossRef]
- Gałczyński, K.; Jóźwik, M.; Lewkowicz, D.; Semczuk-Sikora, A.; Semczuk, A. Ovarian endometrioma—A possible finding in adolescent girls and young women: A mini-review. J. Ovarian Res. 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matalliotakis, M.; Goulielmos, G.N.; Matalliotaki, C.; Trivli, A.; Matalliotakis, I.; Arici, A. Endometriosis in Adolescent and Young Girls: Report on a Series of 55 Cases. J. Pediatr. Adolesc. Gynecol. 2017, 30, 568–570. [Google Scholar] [CrossRef]
- Reid, S.; Lu, C.; Casikar, I.; Reid, G.; Abbott, J.; Cario, G.; Chou, D.; Kowalski, D.; Cooper, M.; Condous, G. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real-time dynamic transvaginal ultrasound technique: The sliding sign. Ultrasound Obstet. Gynecol. 2013, 41, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Ajossa, S.; Gerada, M.; Virgilio, B.; Angioni, S.; Melis, G.B. Diagnostic value of transvaginal ‘tenderness-guided’ ultrasonography for the prediction of location of deep endometriosis. Hum. Reprod. 2008, 23, 2452–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audebert, A.; Lecointre, L.; Afors, K.; Koch, A.; Wattiez, A.; Akladios, C. Adolescent Endometriosis: Report of a Series of 55 Cases With a Focus on Clinical Presentation and Long-Term Issues. J. Minim. Invasive Gynecol. 2015, 22, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Redwine, D.B. The distribution of endometriosis in the pelvis by age groups and fertility. Fertil. Steril. 1987, 47, 173–175. [Google Scholar] [CrossRef]
- Davis, G.D.; Thillet, E.; Lindemann, J. Clinical characteristics of adolescent endometriosis. J. Adolesc. Health 1993, 14, 362–368. [Google Scholar] [CrossRef]
- Jansen, R.P.; Russell, P. Nonpigmented endometriosis: Clinical, laparoscopic, and pathologic definition. Am. J. Obstet. Gynecol. 1986, 155, 1154–1159. [Google Scholar] [CrossRef]
- Laufer, M.R. Identification of clear vesicular lesions of atypical endometriosis: A new technique. Fertil. Steril. 1997, 68, 739–740. [Google Scholar] [CrossRef]
- Marsh, E.E.; Laufer, M.R. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil. Steril. 2005, 83, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Fuhr, N.; David, M.; Schneppel, L.; Papadopoulos, T. Histological confirmation of endometriosis in a 9-year-old girl suffering from unexplained cyclic pelvic pain since her eighth year of life. Gynecol. Obstet. Invest. 2009, 67, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Gogacz, M.; Sarzyński, M.; Napierała, R.; Sierocińska-Sawa, J.; Semczuk, A. Ovarian endometrioma in an 11-year-old girl before menarche: A case study with literature review. J. Pediatr. Adolesc. Gynecol. 2012, 25, e5–e7. [Google Scholar] [CrossRef]
- Lin, J.; Xiang, D.; Zhang, J.-L.; Allickson, J.; Xiang, C. Plasticity of human menstrual blood stem cells derived from the endometrium. J. Zhejiang Univ. Sci. B 2011, 12, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Figueira, P.G.M.; Abrão, M.S.; Krikun, G.; Taylor, H.S. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2011, 1221, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosens, I.; Puttemans, P.; Benagiano, G. Endometriosis: A life cycle approach? Am. J. Obstet. Gynecol. 2013, 209, 307–316. [Google Scholar] [CrossRef]
- Huber, A. The frequency of physiologic vaginal bleeding of newborn infants. Zentralbl. Gynakol. 1976, 98, 1017–1020. [Google Scholar]
- Fluhmann, C.F. The developmental anatomy of the cervix uteri. Obstet. Gynecol. 1960, 15, 62–69. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar] [PubMed]
- Song, X.-C.; Yu, X.; Luo, M.; Yu, Q.; Zhu, L. Clinical Characteristics and Postoperative Symptoms of 85 Adolescents with Endometriosis. J. Pediatr. Adolesc. Gynecol. 2020, 33, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Olive, D.L.; Henderson, D.Y. Endometriosis and mullerian anomalies. Obstet. Gynecol. 1987, 69, 412–415. [Google Scholar]
- Yang, Y.; Wang, Y.; Yang, J.; Wang, S.; Lang, J. Adolescent endometriosis in China: A retrospective analysis of 63 cases. J. Pediatr. Adolesc. Gynecol. 2012, 25, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Gordts, S.; Benagiano, G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Hum. Reprod. 2013, 28, 2026–2031. [Google Scholar] [CrossRef] [Green Version]
- Nanda, K.; Lendvay, A.; Kwok, C.; Tolley, E.; Dubé, K.; Brache, V. Continuous compared with cyclic use of oral contraceptive pills in the Dominican Republic: A randomized controlled trial. Obstet. Gynecol. 2014, 123, 1012–1022. [Google Scholar] [CrossRef]
- Steenberg, C.K.; Tanbo, T.G.; Qvigstad, E. Endometriosis in adolescence: Predictive markers and management. Acta Obstet. Gynecol. Scand. 2013, 92, 491–495. [Google Scholar] [CrossRef]
- DiVasta, A.D.; Laufer, M.R. The use of gonadotropin releasing hormone analogues in adolescent and young patients with endometriosis. Curr. Opin. Obstet. Gynecol. 2013, 25, 287–292. [Google Scholar] [CrossRef]
- Laufer, M.R. Helping “adult gynecologists” diagnose and treat adolescent endometriosis: Reflections on my 20 years of personal experience. J. Pediatr. Adolesc. Gynecol. 2011, 24, S13–S17. [Google Scholar] [CrossRef]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Schwartz, B.I.; Alexander, M.; Breech, L.L. Levonorgestrel Intrauterine Device Use for Medical Indications in Nulliparous Adolescents and Young Adults. J. Adolesc. Health 2021, 68, 357–363. [Google Scholar] [CrossRef]
- ACOG Practice bulletin, no. 114: Management of endometriosis. Obstet. Gynecol. 2010, 116, 223–236. [Google Scholar]
- Fu, J.; Song, H.; Zhou, M.; Zhu, H.; Wang, Y.; Chen, H.; Huang, W. Progesterone receptor modulators for endometriosis. Cochrane Database Syst. Rev. 2017, 25, CD009881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X. Effect of mifepristone in the different treatments of endometriosis. Clin. Exp. Obstet. Gynecol. 2016, 43, 350–353. [Google Scholar]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind the therapeutic success and failure. Hum. Reprod. Update 2020, 26, 565–585. [Google Scholar] [CrossRef]
- Liu, S.; Xin, X.; Hua, T.; Shi, R.; Chi, S.; Jin, Z.; Wang, H. Efficacy of Anti-VEGF/VEGFR agents on animal models of endometriosis: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0166658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, K.; Llarena, N.C.; Rehmer, J.M.; Richards, E.; Falcone, T. The role of pharmacotherapy in the treatment of endometriosis across the lifespan. Expert Opin. Pharmacother. 2020, 21, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Dowlut-McElroy, T.; Strickland, J.L. Endometriosis in adolescents. Curr. Opin. Obstet. Gynecol. 2017, 29, 306–309. [Google Scholar] [CrossRef]
- Vercellini, P.; Crosignani, P.; Somigliana, E.; Viganò, P.; Frattaruolo, M.P.; Fedele, L. “Waiting for Godot”: A commonsense approach to the medical treatment of endometriosis. Hum. Reprod. 2011, 26, 3–13. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).