Thyroid, Adrenal, PRL Impairments and Ovarian Function
Abstract
1. Introduction: Endocrine System and Reproductive Ability
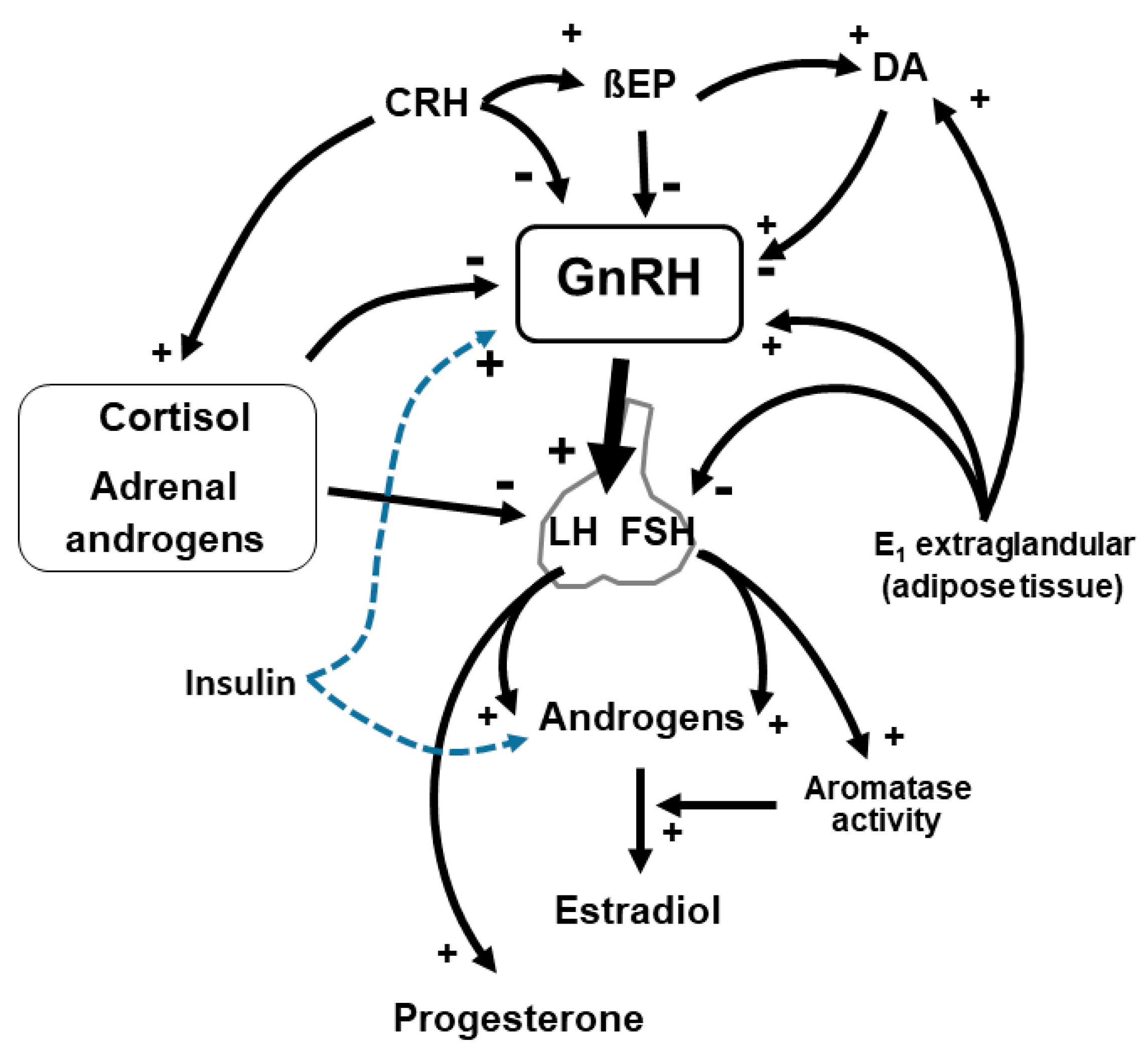
2. Impaired Gonadotropin Release, Altered Menstrual Cyclicity and Body Weight
3. Hyperandrogenism
4. Prolactin Disorders
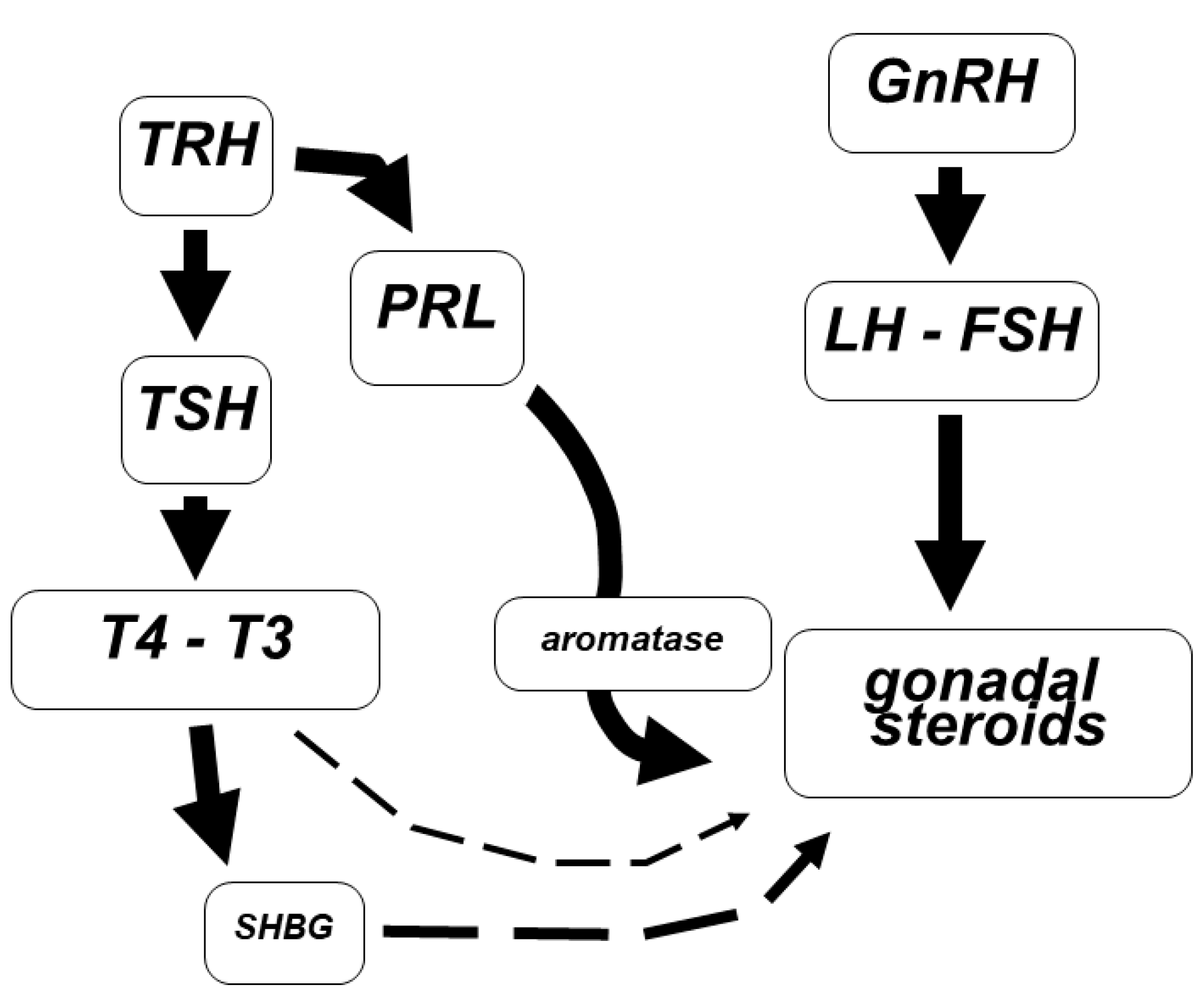
5. Thyroid Dysfunctions
6. Hypothyroidism
7. Autoimmune Thyroid Disease
8. Hyperthyroidism
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gargiulo, A.R. Yen & Jaffe’s Reproductive Endocrinology, 8th ed.; Elsevier Publ.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Podfigurna, A.; Maciejewska-Jeske, M.; Meczekalski, B.; Genazzani, A.D. Kisspeptin and LH pulsatility in patients with functional hypothalamic amenorrhea. Endocrine 2020, 70, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Meczekalski, B.; Katulski, K.; Czyzyk, A.; Podfigurna-Stopa, A.; Maciejewska-Jeske, M. Functional hypothalamic amenorrhea and its influence on women’s health. J. Endocrinol. Investig. 2014, 37, 1049–1056. [Google Scholar] [CrossRef]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petraglia, F.; Bonati, M.; Genazzani, A.R. Developmental of endogenous opioids system and control of gonadotropin secretion. In Frontiers in Endocrinology 191–197; Bergadà, C., Moguilevsky, J.A., Eds.; Ares-Serono Symposia Publication: Rome, Italy, 1995. [Google Scholar]
- Genazzani, A.D. Neuroendocrine aspects of amenorrhea related to stress. Pediatr. Endocrinol. Rev. 2005, 2, 661–668. [Google Scholar] [PubMed]
- Hall, J.E. The Female Reproductive System, Infertility, and Contraception. In Harrison’s Endocrinology; McGraw-Hill Education: New York, NY, USA, 2011; pp. 178–193. [Google Scholar]
- Genazzani, A.D.; Santagni, S.; Chierchia, E.; Rattighieri, E.; Campedelli, A.; Prati, A.; Federica, R.; Tommaso, S. Estimation of instantaneous secretory rates and intrinsic characteristics of luteinizing hormone secretion in women with Kallmann syndrome before and after estriol administration. Reprod. Biol. 2011, 11, 284–293. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Meczekalski, B.; Podfigurna-Stopa, A.; Santagni, S.; Rattighieri, E.; Ricchieri, F.; Chierchia, E.; Simoncini, T. Estriol administration modulates luteinizing hormone secretion in women with functional hypothalamic amenorrhea. Fertil. Steril. 2012, 97, 483–488. [Google Scholar] [CrossRef]
- Genazzani, A.; Chierchia, E.; Santagni, S.; Rattighieri, E.; Farinetti, A.; Lanzoni, C. Hypothalamic amenorrhea: From diagnosis to therapeutical approach. Ann. d’Endocrinologie 2010, 71, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Geisler, C.; Heymsfield, S.B.; Bosy-Westphal, A. Recent advances in understanding body weight homeostasis in humans. F1000Research 2018, 7, 1025. [Google Scholar] [CrossRef]
- Fontana, R.; Della Torre, S. The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. Nutrients 2016, 8, 87. Available online: https://pubmed.ncbi.nlm.nih.gov/26875986/ (accessed on 26 July 2021). [CrossRef]
- Katulski, K.; Podfigurna, A.; Czyzyk, A.; Meczekalski, B.; Genazzani, A. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine 2018, 61, 149–157. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Petraglia, F.; Gamba, O.; Sgarbi, L.; Greco, M.M.; Genazzani, A.D. Neuroendocrinology of the menstrual cycle. In Gynecologic Endoscopic Surgery 48–54 (1994); The New York Academy of Sciences: New York, NY, USA, 1997. [Google Scholar]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Prati, A.; Ricchieri, F. PCOS and Insulin Resistance (IR): From Lifestyle to Insulin Sensitizers; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–23. [Google Scholar]
- Genazzani, A.D.; Despini, G.; Bonacini, R.; Prati, A. Functional Hypothalamic Amenorrhea as Stress Induced Defensive System; Springer: Berlin/Heidelberg, Germany, 2017; pp. 111–118. [Google Scholar]
- Genazzani, A.D.; Prati, A.; Simoncini, T.; Napolitano, A. Modulatory role of D-chiro-inositol and alpha lipoic acid combination on hormonal and metabolic parameters of overweight/obese PCOS patients. Eur. Gynecol. Obs. 2019, 1, 29–33. [Google Scholar]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef] [PubMed]
- Astapova, O.; Minor, B.M.N.; Hammes, S.R. Physiological and Pathological Androgen Actions in the Ovary. Endocrinology 2019, 160, 1166–1174. [Google Scholar] [CrossRef]
- Sen, A.; Hammes, S.R. Granulosa Cell-Specific Androgen Receptors Are Critical Regulators of Ovarian Development and Function. Mol. Endocrinol. 2010, 24, 1393–1403. [Google Scholar] [CrossRef]
- Walters, K.A.; Gilchrist, R.; Ledger, W.; Teede, H.J.; Handelsman, D.J.; Campbell, R.E. New Perspectives on the Pathogenesis of PCOS: Neuroendocrine Origins. Trends Endocrinol. Metab. 2018, 29, 841–852. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol. Metab. 2017, 28, 399–415. [Google Scholar] [CrossRef]
- Magiakou, M.A.; Mastorakos, G.; Webster, E.; Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and the female reproductive system. Ann. N. Y. Acad. Sci. 1997, 816, 42–56. [Google Scholar] [CrossRef]
- Barbarino, A.; De Marinis, L.; Tofani, A.; della Casa, S.; D’Amico, C.; Mancini, A.; Corsello, S.M.; Sciuto, R.; Barini, A. Corticotropin-Releasing Hormone Inhibition of Gonadotropin Release and the Effect of Opioid Blockade. J. Clin. Endocrinol. Metab. 1989, 68, 523–528. [Google Scholar] [CrossRef]
- Ortega, M.T.; McGrath, J.A.; Carlson, L.; Poccia, V.F.; Larson, G.; Douglas, C.; Sun, B.Z.; Zhao, S.; Beery, B.; Vesper, H.W.; et al. Longitudinal Investigation of Pubertal Milestones and Hormones as a Function of Body Fat in Girls. J. Clin. Endocrinol. Metab. 2021, 106, 1668–1683. [Google Scholar] [CrossRef]
- Genazzani, A.D. Inositol as putative integrative treatment for PCOS. Reprod. Biomed. Online 2016, 33, 770–780. [Google Scholar] [CrossRef]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e25. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Prati, A.; Chierchia, E.; Santagni, S. Gli stati iperandrogenici. Boll. Ginecol. Endocrinol. 2014, 8, 35–44. [Google Scholar]
- Genazzani, A.D. Expert’s opinion: Integrative treatment with inositols and lipoic acid for insulin resistance of PCOS. Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 146–157. Available online: https://gremjournal.com/journal/03-2020/experts-opinion-integrative-treatment-with-inositols-and-lipoic-acid-for-insulin-resistance-of-pcos/ (accessed on 26 July 2021).
- Genazzani, A. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil. Steril. 2004, 81, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.V.; Gavrilova-Jordan, L.; Walker, W.; Azziz, R. Androgen excess: Investigations and management. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 98–118. [Google Scholar] [CrossRef]
- Farah-Eways, L.; Reyna, R.; Knochenhauer, E.S.; Bartolucci, A.A.; Azziz, R. Glucose action and adrenocortical biosynthesis in women with polycystic ovary syndrome. Fertil. Steril. 2004, 81, 120–125. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petraglia, F.; Pianazzi, F.; Volpogni, C.; Genazzani, A.R. The concomitant release of androstenedione with Cortisol and luteinizing hormone pulsatile releases distinguishes adrenal from ovarian hyperandrogenism. Gynecol. Endocrinol. 1993, 7, 33–41. [Google Scholar] [CrossRef]
- Moran, C.; Azziz, R. 21-Hydroxylase-Deficient Nonclassic Adrenal Hyperplasia: The Great Pretender. Semin. Reprod. Med. 2003, 21, 295–300. [Google Scholar] [CrossRef]
- Ding, T.; Hardiman, P.J.; Petersen, I.; Wang, F.-F.; Qu, F.; Baio, G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: A systematic review and meta-analysis. Oncotarget 2017, 8, 96351–96358. [Google Scholar] [CrossRef] [PubMed]
- Speiser, P.W.; Azziz, R.; Baskin, L.S.; Ghizzoni, L.; Hensle, T.W.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Montori, V.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2010, 95, 4133–4160. [Google Scholar] [CrossRef]
- Azziz, R.; Dewailly, D.; Owerbach, D. Clinical review 56: Nonclassic adrenal hyperplasia: Current concepts. J. Clin. Endocrinol. Metab. 1994, 78, 810–815. [Google Scholar] [CrossRef]
- Nappi, R.E.; Di Ciaccio, S.; Genazzani, A.D. Prolactin as a neuroendocrine clue in sexual function of women across the reproductive life cycle: An expert point of view. Gynecol. Endocrinol. 2021, 37, 490–496. [Google Scholar] [CrossRef]
- Timmerman, W.; Deinum, M.E.; Poelman, R.T.; Westernik, B.H.; Schuiling, G.A. Characterisation of the DA-ergic system in the mediobasal hypothalamus: A new approach to simultaneously monitor the release of DA from the TIDA neurons and the PRL secretion from the adenohypophysis in awake rats. Brain Res. 1994, 657, 275–280. [Google Scholar] [CrossRef]
- Cetel, N.; Yen, S.S.C. Concomitant Pulsatile Release of Prolactin and Luteinizing Hormone in Hypogonadal Women*. J. Clin. Endocrinol. Metab. 1983, 56, 1313–1315. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef] [PubMed]
- Torner, L. Actions of Prolactin in the Brain: From Physiological Adaptations to Stress and Neurogenesis to Psychopathology. Front. Endocrinol. 2016, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.E.; Wyatt, A.; Herbison, R.E.; Knowles, P.J.; Ladyman, S.R.; Binart, N.; Banks, W.A.; Grattan, D.R. Prolactin transport into mouse brain is independent of prolactin receptor. FASEB J. 2016, 30, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.A.; Frawley, L.S.; Neill, J.D. Neuroendocrine Control of Prolactin Secretion. Annu. Rev. Physiol. 1983, 45, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H.M. Williams Textbook of Endocrinology, 13th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.; Schlechte, J.; Wass, J.A.H. Diagnosis and Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, A.K.; Halder, A.; Jain, M.; Chaturvedi, P.K.; Sharma, J.B. Prevalence and reproductive manifestations of macroprolactinemia. Endocrine 2019, 63, 332–340. [Google Scholar] [CrossRef]
- De Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef]
- Ribeiro, A.; Leite, C.M.; Kalil, B.; Franci, C.R.; Anselmo-Franci, J.A.; Szawka, R.E. Kisspeptin Regulates Tuberoinfundibular Dopaminergic Neurones and Prolactin Secretion in an Oestradiol-Dependent Manner in Male and Female Rats. J. Neuroendocr. 2015, 27, 88–99. [Google Scholar] [CrossRef]
- Yang, J.A.; Song, C.I.; Hughes, J.K.; Kreisman, M.J.; Parra, R.A.; Haisenleder, D.J.; Kauffman, A.S.; Breen, K.M. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice.-Abstract-Europe PMC. Endocrinology 2017, 158, 3716–3723. [Google Scholar] [CrossRef]
- Levine, S.; Muneyyirci-Delale, O. Stress-Induced Hyperprolactinemia: Pathophysiology and Clinical Approach. Obstet. Gynecol. Int. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Salvatore, D.; Davies, T.; Schlumberger, M.; Hay, I.; Reed Larsen, P. Thyroid Physiology and Diagnostic Evaluation of Patients with Thyroid Disorders. In Williams Textbook of Endocrinology, 12th ed.; Melmed, S., Polonsky, K.S., Kronenberg, H.M., Eds.; Saunders, Elsevier Science: Amsterdam, The Netherlands, 2011; pp. 327–361. [Google Scholar]
- Poppe, K.; Velkeniers, B.; Glinoer, D. Thyroid disease and female reproduction. Clin. Endocrinol. 2007, 66, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Rucci, N.; Scaldaferri, M.L.; Masciulli, M.P.; Rossi, G.; Moretti, C.; D’Armiento, M.; Ulisse, S. Thyroid hormone effects on mouse oocyte maturation and granulosa cell aromatase activity. Endocrinology 1999, 140, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, L.; Zhu, B.; Feng, Y.; Yu, S.; An, N.; Wang, X. Effects of 3, 5, 3’-Triiodothyronine (T3) and Follicle Stimulating Hormone on Apoptosis and Proliferation of Rat Ovarian Granulosa Cells. Chin. J. Physiol. 2013, 56, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, T.I.M.; Medici, M.; Visser, T.J.; Peeters, R.P. Thyroid disease in pregnancy: New insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 2017, 13, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.A.; Dorfman, D.M.; Genest, D.R.; Salvatore, D.; Larsen, P.R. Type 3 Iodothyronine Deiodinase Is Highly Expressed in the Human Uteroplacental Unit and in Fetal Epithelium. J. Clin. Endocrinol. Metab. 2003, 88, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Ain, K.B.; Mori, Y.; Refetoff, S. Reduced Clearance Rate of Thyroxine-Binding Globulin (TBG) with Increased Sialylation: A Mechanism for Estrogen-Induced Elevation of Serum TBG Concentration*. J. Clin. Endocrinol. Metab. 1987, 65, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Steingold, K.A.; Matt, D.W.; DeZiegler, D.; Sealey, J.E.; Fratkin, M.; Reznikov, S. Comparison of Transdermal to Oral Estradiol Administration on Hormonal and Hepatic Parameters in Women with Premature Ovarian Failure. J. Clin. Endocrinol. Metab. 1991, 73, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; DeFazio, J.D.; Erlik, Y.; Lu, J.K.; Wolfsen, A.F.; Carlson, H.E.; Hershman, J.M.; Judd, H.L. Pituitary hormones during the menopausal hot flash. Obstet. Gynecol. 1984, 64, 752–756. [Google Scholar] [PubMed]
- Krassas, G.G.; Poppe, K.; Glinoer, D. Thyroid Function and Human Reproductive Health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: Past, present, and future. Nutr. Rev. 2012, 70, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P.J.; Tunbrldge, W.M.G.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Evans, J.G.; Hasan, D.M.; Rodgers, H.; Tunbridge, W.M.; et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef]
- Krassas, G.E. Thyroid disease and female reproduction. Fertil. Steril. 2000, 74, 1063–1070. [Google Scholar] [CrossRef]
- Gordon, G.G.; Southren, A.L. Thyroid-hormone effects on steroid-hormone metabolism. Bull. N. Y. Acad. Med. 1977, 53, 241–259. [Google Scholar]
- Longcope, C.; Abend, S.; Braverman, L.E.; Emerson, C.H. Androstenedione and Estrone Dynamics in Hypothyroid Women*. J. Clin. Endocrinol. Metab. 1990, 70, 903–907. [Google Scholar] [CrossRef]
- Valenti, G.; Ceda, G.P.; Denti, L.; Tarditi, E.; Speroni, G. Gonadotropin secretion in hyperthyroidism and hypothyroidism. La Ric. Clin. e Lab. 1984, 14, 53–63. [Google Scholar]
- Honbo, K.S.; van Herle, A.J.; Kellett, K.A. Serum prolactin levels in untreated primary hypothyroidism. Am. J. Med. 1978, 64, 782–787. [Google Scholar] [CrossRef]
- Phillips, D.I.W.; Lazarus, J.H.; Butland, B.K. The influence of Pregnancy and Reproductive span on the occurrence of autoimmune thyroiditis. Clin. Endocrinol. 1990, 32, 301–306. [Google Scholar] [CrossRef]
- Sawin, C.T.; Castelli, W.P.; Hershman, J.M.; McNamara, P.; Bacharach, P. The aging thyroid. Thyroid deficiency in the Framingham Study. Arch. Intern. Med. 1985, 145, 1386–1388. [Google Scholar] [CrossRef]
- Carlé, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. Epidemiology of subtypes of hyperthyroidism in Denmark: A population-based study. Eur. J. Endocrinol. 2011, 164, 801–809. [Google Scholar] [CrossRef]
- Vitti, P.; Rago, T.; Chiovato, L.; Pallini, S.; Santini, F.; Fiore, E.; Rocchi, R.; Martino, E.; Pinchera, A. Clinical Features of Patients with Graves’ Disease Undergoing Remission After Antithyroid Drug Treatment. Thyroid 1997, 7, 369–375. [Google Scholar] [CrossRef]
- Tonacchera, M.; Vitti, P.; Agretti, P.; Giulianetti, B.; Mazzi, B.; Cavaliere, R.; Ceccarini, G.; Fiore, E.; Viacava, P.; Naccarato, A.; et al. Activating Thyrotropin Receptor Mutations in Histologically Heterogeneous Hyperfunctioning Nodules of Multinodular Goiter. Thyroid 1998, 8, 559–564. [Google Scholar] [CrossRef]
- Kahaly, G.K. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J. Clin. Endocrinol. Metab. 2020, 105, 3704–3720. [Google Scholar] [CrossRef]
- Akande, E.O.; Hockaday, T.D. Plasma oestrogen and luteinizing hormone concentrations in thyrotoxic menstrual disturbance. Proc. R. Soc. Med. 1972, 65, 789–790. [Google Scholar] [CrossRef][Green Version]
- Ridgway, E.C.; Longcope, C.; Maloof, F. Metabolic Clearance and Blood Production Rates of Estradiol in Hyperthyroidism. J. Clin. Endocrinol. Metab. 1975, 41, 491–497. [Google Scholar] [CrossRef] [PubMed]
- The Male and Female Reproductive System in Thyrotoxicosis-Werner & Ingbar’s The Thyroid: A Fundamental & Clinical Text, 9th Edition. Available online: https://doctorlib.info/medical/thyroid/50.html (accessed on 26 July 2021).
- Southren, A.L.; Olivo, J.; Gordon, G.G.; Vittek, J.; Brener, J.; Rafii, F. The Conversion of Androgens to Estrogens in Hyperthyroidism. J. Clin. Endocrinol. Metab. 1974, 38, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Akande, E.O.; Hockaday, T.D.R.; Pereira, B.; Rosa, L.F.B.C.; Safi, D.A.; Bechara, E.J.H.; Curi, R. Plasma luteinizing hormone Levels in women with thyrotoxicosis. J. Endocrinol. 1972, 53, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Akande, E.O. The effect of oestrogen on plasma levels of luteinizing hormone in euthyroid and thyrotoxic postmenopausal women. BJOG Int. J. Obstet. Gynaecol. 1974, 81, 795–803. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar]
- Ganie, M.A.; Marwaha, R.K.; Aggarwal, R.; Singh, S. High prevalence of polycystic ovary syndrome characteristics in girls with euthyroid chronic lymphocytic thyroiditis: A case-control study. Eur. J. Endocrinol. 2010, 162, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomatis, V.; Battipaglia, C.; Genazzani, A.D. Thyroid, Adrenal, PRL Impairments and Ovarian Function. Endocrines 2021, 2, 212-225. https://doi.org/10.3390/endocrines2030021
Tomatis V, Battipaglia C, Genazzani AD. Thyroid, Adrenal, PRL Impairments and Ovarian Function. Endocrines. 2021; 2(3):212-225. https://doi.org/10.3390/endocrines2030021
Chicago/Turabian StyleTomatis, Veronica, Christian Battipaglia, and Alessandro D. Genazzani. 2021. "Thyroid, Adrenal, PRL Impairments and Ovarian Function" Endocrines 2, no. 3: 212-225. https://doi.org/10.3390/endocrines2030021
APA StyleTomatis, V., Battipaglia, C., & Genazzani, A. D. (2021). Thyroid, Adrenal, PRL Impairments and Ovarian Function. Endocrines, 2(3), 212-225. https://doi.org/10.3390/endocrines2030021






