Abstract
MEN1 mutation predisposes patients to multiple endocrine neoplasia type 1 (MEN1), a genetic syndrome associated with the predominant co-occurrence of endocrine tumors. Intriguingly, recent evidence has suggested that MEN1 could also be involved in the development of breast and prostate cancers, two major hormone-related cancers. The first clues as to its possible role arose from the identification of the physical and functional interactions between the menin protein, encoded by MEN1, and estrogen receptor α and androgen receptor. In parallel, our team observed that aged heterozygous Men1 mutant mice developed cancerous lesions in mammary glands of female and in the prostate of male mutant mice at low frequencies, in addition to endocrine tumors. Finally, observations made both in MEN1 patients and in sporadic breast and prostate cancers further confirmed the role played by menin in these two cancers. In this review, we present the currently available data concerning the complex and multifaceted involvement of MEN1 in these two types of hormone-dependent cancers.
1. Introduction
The most frequently encountered hormone-dependent cancers are breast and prostate cancers. The prevalence of breast cancer (BC) has increased such that its incidence is ranked second after lung cancer among cancers occurring in women [1], with 18.1 million new cases and 9.6 million cancer deaths in 2018. Similarly, prostate cancer (PCa), with its 174,650 new cases and 31,620 deaths estimated in 2019 in the USA alone [2], continues to represent a major cause of cancer-related mortality and morbidity in men. Hence, their global health burden is enormous, especially in developed countries, where their incidence is increasing [3]. Intriguingly, several lines of evidence have recently suggested that the tumor suppressor gene MEN1, the mutation of which predisposes patients to multiple endocrine neoplasia type 1 (MEN1, OMIM131100), may be involved in the development of these two cancers. In this review, we present the currently available data concerning the seemingly complex and multifaceted implications of MEN1 in these two types of hormone-dependent cancers. We believe that a better understanding of the role played by MEN1 should provide useful insights, not only into the mechanisms underlying the development of these two cancers, but also into their treatment, and may provide new markers for their diagnosis and prognosis.
2. Background about Breast and Prostate Cancers
2.1. Histopathology and Classification
2.1.1. Breast Cancer
BC is histologically divided into two subtypes based on its invasive features—in situ carcinoma or invasive (infiltrating) carcinoma. BCs can also be divided into ductal or lobular types, depending on the tissue of origin, whether arising from the inner wall of the mammary ducts or the mammary glands, respectively [4]. More recently, a classification based on molecular markers such as estrogen receptor alpha (ERα), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) was purposed to facilitate the diagnosis and treatment of the four main subtypes. These include: (i) Luminal A, which represents approximately 40% of diagnosed BCs, is ERα-positive, PR-positive, or both; HER2-negative; Ki-67-low; and is associated with a slow proliferation and a significantly good prognosis, being sensitive to hormonotherapy. (ii) Luminal B is ERα-positive, PR-positive, or both; either HER2-positive or -negative and Ki-67-high; and has a worse prognosis than the luminal A subtype. (iii) The HER2-enriched subtype is ERα- and PR- negative, HER2-positive, and more aggressive than luminal subtypes. (iv) Triple-negative breast cancers (TNBCs) are ERα-negative, PR-negative, and HER2-negative [5], and are the most aggressive subtype, with the worst prognosis. Recent studies have further attempted to divide this classification into six subtypes by including basal-like and androgen receptor (LAR) subtypes, the latter displaying a high level of androgen receptor (AR) expression and an enrichment in AR signaling [6]. Treatments for BC including surgery, radiotherapy, chemotherapy, hormonotherapy, and rapidly developing targeted therapies, depend on the BC subtypes. ERα-positive BC subtypes are the most sensitive to hormonotherapy using either selective estrogen receptor modulators (SERMs) or selective estrogen receptor downregulators (SERD), whereas the treatment for HER2-enriched BCs has been greatly improved owing to therapies targeting the HER2 receptor. Unfortunately, there are very limited therapeutic options for TNBCs, although inhibitors of poly (ADP-ribose) polymerase (PARP) have shown promising results [7].
2.1.2. Prostate Cancer
PCa classifications mainly revolve around the Gleason grading system, based entirely on the histological pattern of carcinoma cells in Hematoxylin and Eosin (H&E)-stained prostate tissue sections [8,9] and the local disease state [10]. Aberrant signaling in the androgen pathway is critical in the development and progression of PCa. Androgen deprivation therapies (ADT) are the frontline treatment for PCa [11,12]. Although highly effective, ADT are characterized by the predictable emergence of resistance, termed castration-resistant PCas (CRPCs) [13,14], with a high mortality rate [15]. Genomic characterizations of CRPCs have led to the subdivision of CRPCs into two subtypes: (1) AR-dependent CRPCs, containing alterations in the AR gene, such as amplification, point mutations, and generation of splice variants; and (2) AR-independent CRPCs, in which resistant cells or metastatic CRPC (mCRPC) lack AR expression or signaling. The latter subtype has recently been reported to be associated with cellular plasticity and neuroendocrine (NE) molecular features. Importantly, there are mCRPCs that neither express the AR nor markers of NE differentiation (“AR null–NE null”) [16,17]—their incidence has risen over the past 2 decades from 5% in 1998–2011 to 23% in 2012–2016 [18]. Neuroendocrine prostate cancer (NEPC) displays a more complex spectrum of phenotypes, ranging from anaplastic carcinomas to pure small-cell carcinomas (SCCs) [18,19]. Several studies [20,21,22] have shown that 10–20% of lethal PCa display SCC features with a very poor prognosis [23,24].
2.2. Estrogen Receptor-Alpha and Androgen Receptor
2.2.1. Estrogen Receptor-Alpha (ERα)
Structure
ERα belongs to the steroid-stimulated nuclear receptors, which are transcriptional factors involved in regulating the transcription of hundreds of target genes [25]. The gene encoding ERα is called ESR1. This gene is highly conserved, localized on chromosome 6q25.1, and composed of 8 exons on a 140 kb genomic locus (Figure 1, upper panel) [26]. ERα consists of 595 amino acids, with two transactivation domains AF1 and AF2 located in the N-terminal domain (NTD) and domain E, respectively. The NTD is involved in both inter-molecular and intra-molecular interactions, as well as in the regulation of gene transcription, while the DNA-binding domain (DBD) allows ERα to dimerize and to bind to specific estrogen response element (ERE) sequences on DNA. The hinge domain (D region) containing the nuclear localization sequence (NLS) plays a role in receptor dimerization and in binding to chaperone heat shock proteins (Hsp). The ligand-binding domain (LBD, E/F region, C-terminal) comprises the E2-binding domain and works synergistically with the NTD in the regulation of gene transcription (Figure 1, upper panel) [27]. At least 2 isoforms of Erα have been identified: Erα-46, lacking the AF1 domain [28]; and ERα-36, devoid of both transcriptional activation domains (AF1 and AF2) and localized in both the plasma membrane and cytoplasm, where it mediates non-genomic ERα signaling [29,30]. ESR1 mutations, such as ESR1 amplifications or point mutations, were found in endocrine-therapy-resistant breast tumors, and occur predominantly in the LBD, leading to constitutive hormone-independent activation of ERα [31,32].
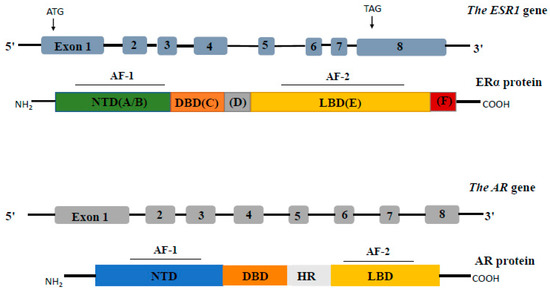
Figure 1.
Schematic representation of the structure of the ESR1 gene and the AR gene and their proteins. Different protein domains are indicated, including the N-terminal domain (NTD), the DNA binding domain (DBD), the hinge domain (D for ERα protein and HR for AR protein), and the ligand binding domain (LBD).
ESR1 Gene Regulation
Several studies have demonstrated that the ESR1 promoter is positively or negatively regulated by epigenetic factors. In 1994, Ottaviano et al. showed that the lack of ERα expression in ERα-negative BC cell lines was due to the hypermethylation of ESR1 CpG islands [33]. It was subsequently shown that the ESR1 promoter was occupied by several complexes with inhibitory components such as DNA methyltransferases (DNMTs) and histone modifiers such as HDAC1 and msin3A [34,35]. Many studies have also reported that the ESR1 promoter was occupied by several transcription factors, including members of the AP1 [36] and Forkhead box (FOX) family (FOXO3A [37], FOXM1 [38]), as well as metastasis-associated protein 1 (MTA1) and Twist [39]. The most extensively described ERα-associated transcription factors are GATA3 [40] and FOXA1 [41], which activate the transcription of the ESR1 gene and are necessary for its proper functioning [40,42,43]. Recently, a study also revealed a regulation of the ESR1 distal promoter by a loop-like complex involving GATA3, FOXA1, and menin [44]. In this study, they showed that menin binds to the ESR1 enhancer region at sites that are also bound by FOXA1 and GATA3, and recruits the mixed lineage leukemia (MLL) compass-like complex containing MLL1/2, menin, ASH2L, RBBP5, and WDR5 [45] to these sites, thus forming a complex and regulating its expression.
Gene Targets and Gene Functions
Estrogens, through the ERα signaling pathway, play important and various developmental, physiological, and pathological roles. ERα is essential for the normal development of the female reproductive tract, including the uterus and the ovaries, as well as the proliferation and differentiation of mammary glands [46]. Furthermore, ERα plays a role in male fertility and in other non-reproductive organs, such as the neuroendocrine and cardiovascular systems and bone metabolism [46,47]. Estrogens can bind to ERα in the cytoplasm and causes their release from bound chaperones, dimerization, and their nuclear translocation, where they bind to ERE and regulate transcription of downstream ERα genes, triggering the “genomic signaling pathway”. ERα can also indirectly bind to promoters via protein–protein interactions, activating a variety of transcription factors, such as the activator protein (AP)-1 or the nuclear factor-κB (NF-κB) [48]. Finally, estrogens can bind to ERα in the plasma membrane, thus inducing the “non-genomic pathway”.
Among the thousands of ERα target genes, one of the earliest identified was pS2/TFF1 [49,50], followed by many genes that were discovered by monitoring the global expression changes upon estradiol induction [51,52,53,54,55]. ERα target genes display a wide variety of functions, such that they can be divided into (i) pro-proliferative genes, such as Cyclin D1 [56], cMyc [57,58], and IGF-1 [59]; (ii) anti-apoptotic factors, such as TIT-5 and EIT-6 [55]; (iii) enzymes, such as the lysosomal proteinase cathepsin D [60]; (iv) and nuclear receptors, such as progesterone receptor [61], in addition to many other genes of as yet unknown function. Interestingly, these global expression experiments indicated that approximately half of ERα target genes are downregulated upon estrogen induction, reinforcing the view that estrogen promotes cell survival by downregulating pro-apoptotic genes.
2.2.2. Androgen Receptor (AR)
Structure
The AR gene is located on chromosome X (Xq11–12) and consists of 8 exons coding a protein of about 110 kDa (Figure 1, lower panel). The full-length AR has four domains, namely from the N-terminal, the NTD, the DBD, the hinge domain, and the LBD [62,63]. The NTD includes the transcriptional regulatory domain AF1, while the LBD includes AF2. Over 20 splice variants of the AR have been reported in the last 2 decades [64]. Most of them are lacking the C-terminal region containing the LBD [65,66] and are, therefore, functionally active independently of the presence of androgens. Among them, AR-V1 and AR-V7 are the most abundant variants [65]. Somatic AR mutations may occur selectively in response to androgen deprivation [67]. A review of 27 clinical studies revealed that AR mutations in androgen-dependent tumors ranged from 2 to 25%, while the incidence in CRPC tumors was slightly higher at 10–40% [67,68]. Furthermore, the AR LBD was described as a mutational hotspot, placing the incidence of its point mutations in CRPC at ~15–20% [69,70].
AR Gene Regulation
A better understanding of the regulation of AR transcription is crucial for studying prostate cell tumorigenesis. SP1, a zinc finger transcription factor, binds to GC-rich motifs of the AR promoter and activates the transcription of AR, whereas the associated antagonistic transcription factor pur-α can bind to the same region and inhibit AR transcription [71]. More recently, Deng et al. demonstrated that PRMT5 promotes prostate cancer cell growth by epigenetically activating the transcription of AR in prostate cancer cells. PRMT5 binds to the proximal promoter region of the AR gene and mainly contributes to the enriched symmetric dimethylation of H4R3 in the same region. Mechanistically, PRMT5 is recruited to the AR promoter upon its interaction with Sp1, forming a complex with Brg1, an ATP-dependent chromatin remodeler [72]. In addition, Grad and colleagues found that AR is regulated by AR itself in osteoblast-like U2OS cells. Indeed, two androgen response elements (AREs) were identified in exons 4 and 5 of the AR gene that were responsible for the androgen-mediated upregulation of AR mRNA [73].
Gene Targets and Functions
AR, playing a key role in both normal prostate development and prostate cancer, is a hormonal transcription factor. Upon binding to androgens, testosterone, or dihydrotestosterone (DHT), the AR localizes to the nucleus [74,75]. There, the receptor dimers bind to AREs in the promoter regions of target genes, such as prostate-specific antigen (PSA) and transmembrane protease serine 2 (TMPRSS2), to regulate transcription [76]. Similarly to other transcription factors, AR-enhanced transcription depends on the recruitment of RNA polymerase II to its target gene promoter. Some dynamic changes in the state of covalent histone modifications, relying on methyltransferase activities, are related to androgen-stimulated transcription. Fu et al. demonstrated direct interactions between p300, CBP, P/CAF, and AR. Moreover, several signaling pathways are known to enhance AR activity [77], including the EGF, IGF, IL6, Wnt, Ras-Raf-MAP kinase, PI3K/AKT, and MAPK/ERK pathways [75,78,79,80,81,82,83]. Mounir et al. reported that PMRT5 display inhibitory effects on the transactivation of differentiated genes by AR via AR methylation [84].
For prostate cancer cells, studies have shown that AR is a critical regulator of the G1-S transition in AR-dependent cell cycle progression. Indeed, Xu et al. demonstrated that androgen induces Cyclin D expression via mTOR-dependent enhancement of translation [85]. The p21cip has been validated as a direct AR target [86], consistent with the findings revealing that p21cip expression is enhanced in tumors and is correlated with a higher proliferative index and Gleason grade [87,88]. Furthermore, Knudsen et al. showed that androgen depletion induces p27Kip1, which likely contributes to the observed reduction in CDK2 activity [89].
Rokhlin et al. found that androgen and AR signaling could directly regulate p53 to suppress apoptosis. Mechanistically, androgen suppresses TNF-α/Fas-induced apoptosis through the inhibition of p53 expression and caspase-2 activation [90]. Interestingly, Frezza et al. reported that a significant decrease in AR expression leads to an increase in caspase-3 activity in LNCaP and PC-3AR cells, suggesting that AR might suppress caspase-3 expression [91]. Liao et al. also showed that knockdown of AR via siRNA leads to apoptotic death in PCa cells [92]. Blockade of AR degradation and ectopic expression of Bcl-2 or selected caspase inhibitors can suppress this pro-apoptotic activity [93].
Zhao et al. demonstrated that AR can act as a transcriptional repressor to directly inhibit gene expression. This repression is mediated by the binding of AR to AREs, and is facilitated by EZH2-mediated repressive chromatin remodeling [94]. More recently and interestingly, Song et al. revealed that AR upregulated EZH2 expression by binding to the EZH2 promoter and stimulating its transcriptional activity in hepatocellular carcinoma (HCC) cells. EZH2 overexpression increased H3K27me3 levels, thereby silencing the expression of Wnt signal inhibitors, resulting in the activation of Wnt/β-Catenin signaling and subsequent induction of cell proliferation and tumorigenesis [95].
3. The Involvement of the MEN1 Gene in Breast and Prostate Cancers
Multiple endocrine neoplasia type 1 (MEN1) is a hereditary syndrome characterized by the multiple occurrence of endocrine tumors of the parathyroid, pancreas, and anterior pituitary. The large tissue spectrum of the disease, affecting a dozen different endocrine cell lineages [96], indicates that the predisposition gene, MEN1, possesses a relevant role in all of the endocrine tissues affected. The MEN1 gene, the mutation of which predisposes patients to MEN1 syndrome, was first identified in 1997 [97,98], and functional studies have since further improved our understanding of the gene. In particular, both genetic and biochemical experiments suggest that the MEN1 gene has a large spectrum of expression and that the menin protein encoded by the gene plays multifaceted biological functions in a broad range of different tissues and cells, likely through physical and functional interactions with its numerous protein partners. Menin primarily has a nuclear localization, although it can also be located in the cytoplasm and membranes [99]. Menin may act as an adaptor protein involved in the regulation of gene expression via its physical interaction with several transcription factors, such as JunD, Smad1/3/5, β-Catenin, MafA/B, Foxa2, and P53, as well as epigenetic factors, including KMT2A/2B, Sin3A, and EZH2 [44,100,101,102,103,104,105,106,107]. The interactions between menin and several nuclear receptors were recently unveiled (see below). Importantly, various analyses demonstrated that menin is involved in different cellular activities controlled by many signaling transduction pathways, in particular cell proliferation, cell cycle, and cell death. Finally, the experiments using various in vivo models have also revealed that the biological functions of menin extend far beyond endocrine cells to hematopoiesis, adipogenesis, myogenesis, fibrogenesis, or even osteogenesis [108,109,110].
3.1. Molecular Studies
The first clues as to the possible involvement of menin in BC came from the observation that the menin protein binds physically to ERα. In 2006, Dreijerink et al. revealed that menin, owing to an evolutionarily conserved amino acid sequence LXXLL, could physically interact with several nuclear receptors, such as the vitamin D receptor, RXR, and ERα, and played the role of a cofactor. In the same study, they showed that menin binds to the AF2 domain of ERα and coactivates the transcription of TFF1, an estrogen-responsive ERα target gene, through the recruitment of the compass-like complex trimethylating H3K4me3 on the TFF1 promoter [111]. In 2009, Imachi et al. confirmed the previous results by showing that menin coactivates ERα in an estrogen-dependent manner in the ERα-positive MCF7 BC cell line [112]. A recent study conducted by Dreijerink et al. demonstrated that menin regulates the expression of the ESR1 gene (as described above) through an upstream enhancer via a looping mechanism that connects the TSS bound menin&MLL1/2 to the enhancer-bound transcription factors GATA3 and FOXA1 [44].
Almost a decade after discovering the interaction between menin and ERα, menin was identified as an important cofactor for AR signaling due to its physical interaction with AR-NTD and the recruitment of the MLL histone methyltransferase complex to AR target genes [113]. Inhibition of menin–MLL interaction with a small-molecule inhibitor (MI) impaired AR signaling and inhibited the growth of castration-resistant tumors in xenograft experiments in mice [113]. Hence, these results suggest that menin can facilitate oncogene activation through AR signaling in PCa (Figure 2).
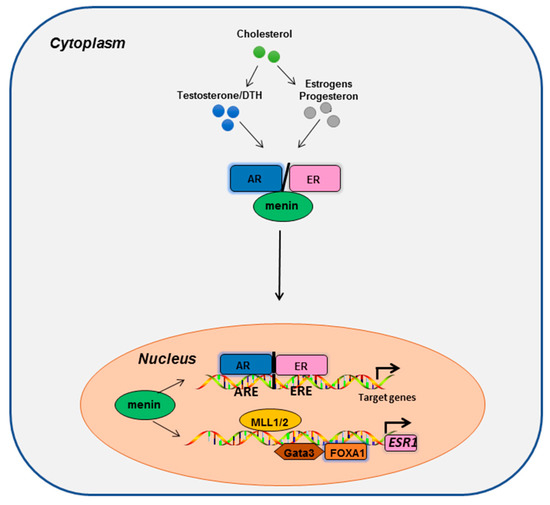
Figure 2.
The menin protein interacts physically and functionally with ERα and AR, and is involved in the regulation of ESR1 transcription and the transactivation of the target genes of both ERα and AR.
3.2. Mouse Models
3.2.1. Mammary Gland Lesions in Mouse Men1 Models
Our team is at the forefront of studies on the role of MEN1 using Men1 mutant mouse models. We have observed that aged heterozygous Men1 mutant mice, in addition to endocrine tumors, developed mammary gland carcinomas in female and prostate cancers in male mutant mice at low frequencies [114]. To further confirm and understand the role of menin in the development of mammary lesions, we generated a conditional mammary-specific Men1 knock-out mouse model by crossing the mice carrying floxed Men1 alleles (Men1F/F) with WapCre transgenic mice expressing Cre recombinase under the control of the whey acidic protein (Wap) promoter, which is known to be expressed in luminal mammary epithelial cells. Our results demonstrated that female Men1F/F-WapCre mice developed substantially higher amounts of early mammary intraepithelial neoplasia (MIN), which are precursor lesions, in comparison with control Men1+/+-WapCre mice. Interestingly, we found that ERα expression and the number of ERα-positive cells were clearly reduced in MIN lesions of mutant mice compared with normal mammary glands. In addition, cell membrane expression of β-Catenin and E-Cadherin was almost absent in the mammary lesions of Men1F/F-WapCre mice compared with control mice; neither β-Catenin nor E-cadherin were detected in the TS1 cell line derived from a mouse Men1 BC [115].
3.2.2. Prostate Lesions in Mouse Men1 Models
By following a cohort of 47 male heterozygous Men1 mutant mice (Men1+/−) and 23 male wild-type (Men1+/+), age-matched littermate mice from 18 to 26 months of age, our group found that six Men1+/− mice (6/47, 12.8%) developed prostate cancer, including two adenocarcinomas and four in situ carcinomas, while none of the control mice developed cancerous lesions. No prostate carcinoma was found in age-matched Men1+/+ littermates (0/23). In addition, these carcinomas exhibited loss of the non-target Men1 allele (LOH), therefore supporting a tumor suppressor role for the Men1 gene in prostate glands. Moreover, the AR and p27 expression decreased in tumor lesions, likely facilitating prostate cell tumorigenesis due to Men1 inactivation [116].
Taken together, all of the data obtained from mouse models suggest a tumor-suppressive role for menin during the initiation and development of murine breast and prostate cancers.
3.3. Human Studies
3.3.1. MEN1 in Human Breast Cancer
Over the last two decades, several case reports have described breast cancer cases related to MEN1. In 2004, a 44-year-old Japanese woman was diagnosed with MEN1 syndrome, having hyperparathyroidism, primary aldosteronism, and also scirrhous breast carcinoma. The DNA taken from her parathyroid adenoma and breast cancer tissues showed germline MEN1 mutation at codon 451 in exon 10, which resulted in alanine-to-tyrosine substitution (A541T), as well as LOH [117]. Another study by Jeong et al. reported a case of a patient with both MEN1-associated tumors and breast cancer. They found a germline MEN1 mutation manifested as a 5-bp duplication in exon 3, named c.196_200dupAGCCC), which resulted in a frameshift mutation. In addition, the tested exon 10 showed a polymorphism at codon 423 with substitution of a cytidine to a thymidine (C423T), causing a change of amino acid [118]. More recently, a 41-year old patient with no familial history of breast cancer but with a mother with primary hyperparathyroidism (PHP) was found carrying a variant p.C421R/p.426R in the MEN1 gene. The patient’s histopathological study revealed hormone receptor negativity, as well as HER-2 and p53 negativity. A family study showed positive findings for MEN1 in a sister, two maternal nephews, and one of the patient’s daughters, with no record of breast cancer development in any of these people [119]
Evidence of the likely involvement of menin in BC arose from the observation that female MEN1 patients were at a higher risk of developing BC [120]. In this study, Dreijerink et al. referred to the Dutch longitudinal MEN1 database to assess the incidence of BC in MEN1 patients, and found that out of 190 female patients, the relative risk of invasive BC was 2.83 (p < 0.001) and the mean (±SD) age at diagnosis of essentially luminal-type BC was 48 ± 8.8 years, compared with an age range of 60 to 65 years in the general population. This feature is often observed in the patients harboring a genetic predisposition. The authors validated their results using 3 other independent MEN1 patient cohorts from the United States (p = 0.11), Tasmania (p = 0.22), and France (p = 0.03), which provided similar values for relative risk as those obtained in the Dutch cohort, with an average age at diagnosis of 51 years. Furthermore, 8 out of 10 BC samples obtained from Dutch MEN1 patients displayed more than 50% reduction of menin expression in the nucleus, and subsequent analysis showed loss of heterozygosity at the MEN1 locus in 3 of 9 tumors. Overall, these observations strongly suggest that MEN1 mutations could be involved in human breast tumorigenesis as a tumor suppressor.
Concomitantly to our work carried out in mice, we also observed that a substantial proportion of human sporadic BCs displayed reduced menin expression, as observed through the analyses of two series of human BCs [115]. More recently, a study in which the whole-genome sequences of 560 BCs were analyzed highlighted sporadic MEN1 mutations, albeit at low frequency, as being among driver mutations (such as BRCA1, TP53, PIK3CA, MYC, CCND1, PTEN) in BC [121]. In addition, several other case reports identified MEN1 mutations among sporadic BC patients, independent or not of germline mutations in BRCA1 and BRCA2 genes that are usually associated with hereditary BC [118,122,123,124].
However, in a clinical study conducted by Imachi et al. with 65 ERα-positive BC samples treated with tamoxifen for 2–5 years as adjuvant therapies, they observed that menin-positive tumors (20 patients) had a worse clinical outcome and were more resistant to tamoxifen than menin-negative tumors (46 patients) [112]. They, therefore, proposed that menin could be a predictive factor of resistance to tamoxifen. Furthermore, they found that raloxifene could inhibit the binding of menin to the AF2 domain of ERα and proposed raloxifene as the therapeutic options for menin-positive and ERα-positive BC [125]. Their works suggest an oncogenic role for menin, which raised the controversy as to its precise role in BC.
3.3.2. MEN1 in Human Prostate Cancer
Perakakis et al. reported two cases of PCa seen in a MEN1 family with atypical tumor spectrum [126]. The DNA sequencing analysis revealed a novel mutation—Ser38Cys (TCC > TGC) in exon 2, located in a region of menin that is responsible for interaction with the transcription factor JunD. The latter has recently been associated with prostate cancer.
Only limited sporadic MEN1 mutations have so far been reported in human sporadic PCa [127]. Manson-Bahr et al. found that missense mutations of the MEN1 gene were detected in 2 of 8 formalin-fixed prostate needle biopsy materials [125]. Interestingly, Grasso et al. analyzed 58 human CRPC samples by aCGH and found that 17.2% of all samples (10 of 58) harbored mutations in the MLL complex, including the MEN1 gene [69]. MLL functions as part of a multi-protein complex containing menin [128]. Many members of the complex have different levels of aberrations in CRPC [69]. Noticeably, Chen et al. analyzed 150 cases for advanced and metastatic human PCa. They observed that the percentage of PTEN and MEN1 co-loss was almost the same as the co-loss of PTEN and PML (Promyelocytic Leukemia), which is around 11% in all cases [129]. Conversely, Paris et al. reported that the MEN1 locus was amplified in some patients and was predictive of post-operative recurrence [130]. The similar observation was made Kerstin et al. [131]. Moreover, MEN1 knockdown resulted in a decrease in cell proliferation in DU145 cells [132,133], but curiously not in the PC3 cell line [132].
In total, the current data obtained from human studies suggest that the MEN1 gene could play a complex even opposite role in the development of human breast and prostate cancers.
4. Further Clues for the Role of Menin in Breast and Prostate Cancers
As we mentioned above, many different factors and signaling pathways are involved in mammary and prostate cell tumorigenesis. By investigating the possible molecular links between the former and menin, we speculated that we might gain further insight into the possible role played by menin in these cancers (Figure 3).
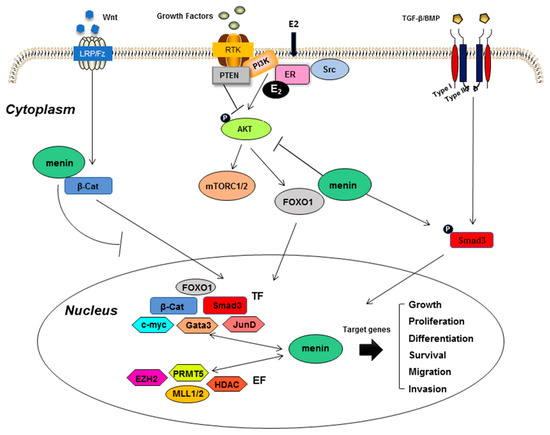
Figure 3.
Mechanistic clues underlying the involvement of menin in mammary and prostate cell tumorigenesis. Menin interacts with numerous menin-interacting factors, consequently participating in the regulation of many target genes and interfering with different signaling pathways strongly implicated in breast and prostate cancers. EF: epigenetic factors; TF: transcriptional factors.
4.1. Epigenetic Factors
Interestingly, several epigenetic factors reported to be involved in mammary cell tumorigenesis are known to be partners of menin. Histone methylase MLL1 (KMT2A) and MLL4 (KMT2B), which are the most characterized partners of menin, were shown to act synergistically with ERs (ERα and ERβ) to mediate the estrogen-induced transcriptional activation of the HOXB9 gene, which is critical for mammary gland development and BC [134]. Menin was also shown to upregulate several members of the same family (mainly HOXA9 gene) in leukemia by associating with the compass-like complex and lens-epithelium-derived growth factor (LEDGF) [45,135]. The HDAC family, which contains known partners of menin [54,55], is implicated in the regulation of ERα expression, mainly by silencing the ESR1 gene. It was proposed that HDAC may be responsible for loss of ERα expression in ER-negative BC [34,35]. EZH2 and PRMT5 are two shared partners of menin [100,136,137] and the ERα pathway. Indeed, EZH2 inhibits the transcription of estrogen-responsive genes through its association with the transcriptional corepressor repressor of estrogen receptor activity (REA) [138]. Although there is no direct evidence of the interaction between PRMT5 and ERα, PRMT5 plays an important role in BC by methylating programmed cell death 4 (PDCD4), a tumor suppressive protein with anti-proliferative functions on arginine residue 110 [139].
4.2. Transcription Factors
JunD, a member of the AP-1 family that interacts physically with menin [140,141], has a higher level of expression in BCs [142]. JunD and menin co-expression was found in the mouse submandibular gland, an AR-responsive tissue, with their expression pattern and localization changing with cell differentiation status [143]. Moreover, JunD physically binds to ERα and facilitates its binding to target genes [36]. Intriguingly, it has been shown that prostaglandin E2 (PGE2) induces JunD and JunB expression, resulting in the activation of the aromatase promoters I.3/II, while JunD and c-Jun mediate the suppression of the aromatase promoter I.4, leading to high levels of local estrogen, and thus to BC progression [144]. JunD is crucial for cell proliferation in PCa cells, as it controls cell cycle regulatory genes [145,146]. Their analyses further suggest that the essential role played by JunD in prostate cancer cell proliferation is mediated by MYC signaling [147]. Furthermore, Mehraein-Ghomi et al. highlighted JunD as an AR co-activator, as it triggers the oxidative stress pathway in prostate cancer cells by regulating the SSAT promoter, which produces large amounts of metabolic reactive oxygen species (ROS) [148].
Another important factor is cMyc, a well-known estrogen-regulated oncogene [149,150], which is overexpressed in approximately 20–30% of BCs [151] and has also been shown to interact with ERα to modulate estrogen-mediated signaling [152]. The cMyc overexpression in PCa has been a well-recognized phenomenon since 1986, when Fleming et al. showed a significantly higher level of its expression in adenocarcinoma of the prostate than in benign prostate hyperplasia by Northern blotting [153]. Furthermore, Sato et al. reported that cMyc amplification is strongly associated with higher histopathological grades and Gleason scores, as well as with earlier disease progression and cancer-associated death [154]. It is now known that cMyc is a partner of menin and that they collaborate to either activate or repress the expression of certain genes. The most recent report shows that menin can directly interact with the transactivation domain (TAD) of cMyc, and that they in turn bind to E boxes to enhance the transcription of cMyc target genes [155]. Interestingly, menin can interact with the cMyc promoter to regulate its transcription in HEK293 cells [156].
Finally, menin was recently shown to interact with GATA3 and FOXA1 [44] in BC to regulate the ESR1 promoter (see details above), both of which are markers of luminal BC, especially for the luminal A subtype [42,43,146], and which are highly associated with ERα and are required for the proper function of most of its target genes [40,157,158]. Menin interacts with GATA3 to activate Th2 cell maturation in primary human peripheral blood T cells [159] and to physically interact with a member of the FOXA family, namely FOXA2 [103]. It is worth mentioning that FOXA1 plays a crucial role in the AR signaling, and possibly in CRPC occurrence [160].
4.3. Signal Transduction Pathways
Menin is known to interfere with different signaling pathways that play important roles in breast and prostate cancers.
4.3.1. The PI3K/PTEN/AKT/mTOR Pathways
Activation of the PI3K/PTEN/AKT/mTOR pathways occurs in 70% of BCs overall [161]. PIK3CA (a subclass of the PI3K family of genes) is the most commonly mutated gene in ER-positive BCs [162]. This mutation is present in approximately 35% of HR-positive BCs, 20–25% of HER2-overexpressing BCs, and with a lower frequency (8.3%) in TNBCs [163].
PTEN is one of the most commonly deleted and mutated genes in human breast and prostate cancers. Loss of PTEN in BC is negatively correlated with ERα and PR status, and is associated with the basal-like phenotype [164,165], with more aggressive behaviors (tumor size, lymph node metastasis, etc.), and with worse outcome (disease-free survival DFS and overall survival OS) [166]. Accumulating evidence has highlighted an association between loss of PTEN and the development of CRPC, likely due to AR phosphorylation [167,168]. Moreover, loss of PTEN and AR expression has been clinically correlated with increased mortality in CRPC patients [169]. More recently, Wong and colleagues generated mouse models with insulin-specific biallelic inactivation of Men1 and Pten in β-cells, and showed that concomitant loss of Pten and Men1 accelerated islet cell tumorigenesis. Co-mutations of MEN1 and PTEN were observed in a small percentage of human PanNETs [170,171], suggesting that menin and Pten may function synergistically to suppress tumorigenesis.
Several studies have focused on the relationship between the PAM (PI3K/Akt/mTOR) and resistance to endocrine therapy in pre-clinical BC models [172], in which the authors showed that Akt can activate the ERα pathway independently of estrogen availability and that the combination of mTOR inhibitors and endocrine therapy can overcome this resistance [173,174]. In addition, the PAM pathway has also been implicated in trastuzumab resistance in HER2-overexpressing BCs [175]. Interestingly, menin interacts with AKT1, downregulates its kinase activity and suppresses both AKT1 induced proliferation and anti-apoptosis in endocrine and non-endocrine cells, mainly by reducing the translocation of AKT1 from the cytoplasm to the plasma membrane during growth factor stimulation [176]. Another study showed that menin can interact with FOXO1, a downstream effector of Akt, in the hepatocytic cancer cell line HepG2 and in MEFs [177]. In the same year, a study also showed that MEN1 and genes from the mTOR pathway are frequently altered in pancreatic neuroendocrine tumors [170]. A recent study revealed that menin regulates milk protein synthesis through mTOR signaling in normal mammary epithelial cells [178]. According to the authors, menin overexpression caused significant suppression of factors involved in the mTOR pathway, as well as milk protein κ-casein (CSNK). All of the abovementioned data suggest that menin may regulate the PI3K/Akt/mTOR pathway in mammary cells.
4.3.2. Cell Cycle, Growth, and Death Control
Kaji et al. demonstrated that menin could suppress cell proliferation via the transforming growth factor-β (TGF-β) pathway in the rat pituitary cell line by interacting with Smad3 [179]. Agarwal et al. reported that menin is essential for JunD-mediated inhibition of cell proliferation [140]. Ratineau et al. showed that menin represses cell proliferation in rat intestinal epithelial cells [180] by inhibiting the expression of Cyclin D1, Cyclin D3, and CDK4. Based on a transcriptomic study of differentially expression genes, our team demonstrated that Men1 ablation in mouse islet cells greatly affected the expression of factors involved in cell cycle and cell growth control, such as Cyclin A2, B2, and D2 for the former; and IGF2, IGFBP3, and 6 for the latter [181]. Menin can also repress cell proliferation by interacting and inhibiting ASK (S-phase kinase) [182]. In addition, menin was reported to upregulate the expression of Cyclin-dependent kinase inhibitors p18ink4c and p27kip1 with the help of the MLL compass-like complex, which adds H3K4 trimethylation marks on their promoters, thus activating gene expression [183,184,185]. Interestingly, p18 has recently been shown to be a downstream target of GATA3 in luminal BC and to suppress luminal progenitor cell proliferation and tumorigenesis [186]. P27 is ranked as one of the 18 most significantly mutated genes in luminal A BC, and loss of p27 was associated with poor outcome in BC patients [187].
Schnepp et al. revealed that the infection of cells using menin-expressing adenoviruses could trigger apoptosis in MEFs [188,189] by activating an apoptotic pathway that depends on Bax [186]. They also highlighted that Men1 disruption in vivo increased resistance to TNFα-induced apoptosis, further supporting a vital role for menin in regulating apoptosis.
4.3.3. Wnt Signaling
It is well known that the Wnt pathway plays a crucial role in the development of breast and prostate cancers, in particular at late stages [190]. We observed that in Men1-deficient mice insulinomas, β-Catenin expression switched from a membrane expression to a cytoplasmic or even nuclear expression [187]. Along with our collaborators, we also showed that menin physically interacts with β-Catenin, and menin overexpression reduced the nuclear accumulation of β-Catenin and suppressed its transcriptional activity in Men1-null MEFs [104]. Jiang et al. further demonstrated that β-Catenin ablation leads to the suppression of tumorigenesis and significantly improved hypoglycemia and the survival rate of Men1-deficient mice [105]. Applying the small molecule inhibitor, PKF115–584, in Men1-deficient mice to antagonize β-Catenin signaling suppressed tumor cell proliferation in vitro and in vivo [105]. Kim et al. reported that menin promotes ubiquitin-mediated degradation of β-Catenin and menin overexpression downregulates the transcriptional activity of β-Catenin and target gene expression, as well as the proliferation of human renal carcinoma cells with an activated β-Catenin pathway [191].
5. Finishing Words
5.1. The Dual Role of Menin
The abovementioned data provide clues on the complex and sometimes paradoxical role of the MEN1 gene in mammary and prostate cell tumorigenesis (Figure 2 and Figure 3). Dreijerink et al. proposed a hypothesis on the dual role of menin in BC, which may shed light on these discrepancies and the surrounding confusion [44]. They proposed that menin could act as a tumor suppressor in normal luminal mammary epithelial cells and as an oncogene in sporadic ER-positive BCs, the key point being its essential role in the regulation of the ESR1 gene mediated by the MLL–menin complex via H3K4me3 sites. Therefore, when MEN1 is mutated or inactivated in normal mammary and prostate cells, it could result in dysregulated ERα and AR pathways, leading to aberrant cell proliferation and differentiation, and to tumor development with the participation of other oncogenic alterations. Conversely, in ER-positive BC and AR-positive prostate cancer cells, menin could act as a co-activator of these two nuclear receptors, playing a crucial role in promoting cell proliferation by the latter.
5.2. Remaining Questions
The currently available data and the abovementioned molecular clues suggest that menin may play a multifaceted but non-negligible role in the tumorigenesis of both mammary and prostate cells. However, concerning the detailed mechanisms underlying its involvement, many questions remain. Among them, one may wonder about the molecular pathophysiological consequences of MEN1 inactivation in these two tissues during the initiation of tumorigenesis. In addition, since menin interacts and regulates the ERα and AR pathways, does menin play different roles in HR-positive than in HR-negative cancers? Last but not least, as menin acts as a scaffold protein, what are the other factors, in particular interacting partners, involved in the process?
To further understand the involvement of menin in these two cancers, there is an urgent need to generate adequate cell, tissue, and animal models in order to better investigate the distinct roles played by menin during the initiation of carcinogenesis on the one hand, and during cancer progression on the other hand. Concurrently, strengthening MEN1 mutation detection and menin expression analysis for breast and prostate cancer samples collected from young and aged patients or in different subtypes would be informative. The availability of more relevant models and crucial data from clinical samples, together with the rapidly improved tools in molecular study, should be of great help in obtaining rightful answers for the abovementioned questions.
6. Summary
Even though the role of menin in the development of neuroendocrine cancers is well known, its role in human breast and prostate cancers is slowly emerging. Based on the literature presented above, we speculate that future research could unveil further crosstalk between menin and the ERα and AR pathways. Finally, a better understanding of the mechanisms underlying its role in the mammary and prostate cell tumorigenesis could also make menin a potential therapeutic target for the treatment of these cancers, as well as a new marker for their diagnosis and prognosis.
Author Contributions
R.A.Z. and Y.L. prepared manuscript and figures. M.L.R. and V.V.-G. participated in study design and provided critical revision of the manuscript. C.X.Z. conceived and supervised the manuscript preparation and obtained funding. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fondation de l’Association pour la Recherche contre le Cancer (SFI20101201530), the Ligue Inter-Régionale contre le Cancer (R19040CC), and the Région Auvergne, Rhône-Alpes (SICORRA22425). R.A.Z. was the recipient of a PhD-fellowship from Association “G04MEDIA S.A.R.L”, Lebanon. Y.L. was the recipient of a PhD-fellowship from China Scholarship Council.
Acknowledgments
We are grateful to Brigitte Manship for her assistance in editing and proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADT | Androgen deprivation therapies |
| AP-1 | Activator protein 1 |
| AR | Androgen receptor |
| AREs | Androgen response elements |
| ASK | Activator of S-phase kinase |
| BAX | BCL2 Associated X |
| BC | Breast cancer |
| BRCA1&2 | Breast cancers 1 and 2 |
| CDK | Cyclin-dependent kinase |
| CRPC | Castration-resistant prostate cancer |
| DBD | DNA-binding domain |
| DHT | Dihydrotestosterone |
| DNMTs | DNA methyltransferases |
| EIT-6 | Estrogen Induced Tag-6 |
| ER | Estrogen receptor |
| ERE | Estrogen response element |
| EZH2 | Enhancer of zeste homolog 2 |
| FOX | Forkhead box |
| H3K4me3 | Tri-methylation at the 4th lysine residue of the histone H3 protein |
| HCC | Hepatocellular carcinoma |
| HDAC | Histone deacetylase |
| HER2 | Human epidermal growth factor receptor 2 |
| HOX | Homeobox |
| Hsp | Heat-shock proteins |
| IGF-1 | insulin-like growth factor-1 |
| IGFBP-3 | Insulin-like growth factor-binding protein 3 |
| LAR | Luminal-androgen receptor |
| LBD | Ligand-binding domain |
| LEDGF | Lens epithelium-derived growth factor |
| LOH | Loss of heterozygosity |
| mCRPC | Metastatic CRPC |
| MEF | Mouse embryonic fibroblast |
| MEN1 | Multiple Endocrine Neoplasia type 1 |
| MI | Molecule inhibitor of menin-MLL interaction |
| MIN | Mammary intraepithelial neoplasia |
| MLL1&2 | mixed lineage leukemia 1&2 (KMT2A and 2B) |
| MTA1 | metastasis-associated protein 1 |
| mTOR | Mammalian target of rapamycin |
| NEPC | Neuroendocrine prostate cancer |
| NF-κB | nuclear factor-κB |
| NLS | Nuclear localization sequence |
| NTD | N-terminal Domain |
| PCa | Prostate cancer |
| PR | Progesterone receptor |
| PRMT5 | Protein arginine N-methyltransferase 5 |
| PSA | Prostate-specific antigen |
| PTEN | Phosphatase and TENsin homolog |
| ROS | Reactive oxygen species |
| SCC | Small cell carcinomas |
| SERDs | Selective estrogen receptor downregulators |
| SERMs | Selective estrogen receptor modulators |
| TGF-β | Transforming growth factor beta |
| Th2 | T- helper type 2 |
| TIT-5 | Tamoxifen Induced Tag-5 |
| TMPRSS2 | Transmembrane protease serine 2 |
| TNBC | Triple negative breast cancer |
| TNFα | Tumor necrosis factor alpha |
| TSS | Transcription start site |
| Wap | Whey acidic protein |
References
- Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; The International Agency for Research on Cancer (IARC): Geneva, Switzerland, 2018; Volume 263, p. 3.
- Siegel, R.L.; Miller, K.D. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Boyle, P.; Ferlay, J. Cancer Incidence and Mortality in Europe, 2004. Ann. Oncol. 2005, 16, 481–488. [Google Scholar] [CrossRef]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, Molecular and Functional Subtypes of Breast Cancers. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Eliyatkın, N.; Yalçın, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Gleason, D.F. Classification of Prostatic Carcinomas. Cancer Chem. Rep. 1966, 50, 125–128. [Google Scholar]
- Gleason, D.F.; Mellinger, G.T. Prediction of Prognosis for Prostatic Adenocarcinoma by Combined Histological Grading and Clinical Staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Humphrey, P.A. Gleason Grading and Prognostic Factors in Carcinoma of the Prostate. Mod. Pathol. Off. J. U.S. Can. Acad. Pathol. Inc. 2004, 17, 292–306. [Google Scholar] [CrossRef]
- Nelson, P.S.; Clegg, N.; Arnold, H.; Ferguson, C.; Bonham, M.; White, J.; Hood, L.; Lin, B. The Program of Androgen-Responsive Genes in Neoplastic Prostate Epithelium. Proc. Natl. Acad. Sci. USA 2002, 99, 11890–11895. [Google Scholar] [CrossRef]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of Intratumoral Androgens in Metastatic Prostate Cancer: A Mechanism for Castration-Resistant Tumor Growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 2017, 32, 474–489.e6. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Cook, R.; Lee, K.A.; Nelson, J.B. Disease and Host Characteristics as Predictors of Time to First Bone Metastasis and Death in Men with Progressive Castration-Resistant Nonmetastatic Prostate Cancer. Cancer 2011, 117, 2077–2085. [Google Scholar] [CrossRef]
- Roudier, M.P.; True, L.D.; Higano, C.S.; Vesselle, H.; Ellis, W.; Lange, P.; Vessella, R.L. Phenotypic Heterogeneity of End-Stage Prostate Carcinoma Metastatic to Bone. Hum. Pathol. 2003, 34, 646–653. [Google Scholar] [CrossRef]
- Wang, W.; Epstein, J.I. Small Cell Carcinoma of the Prostate. A Morphologic and Immunohistochemical Study of 95 Cases. Am. J. Surg. Pathol. 2008, 32, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.; Tzelepi, V.; Araujo, J.C.; Guo, C.C.; Liang, S.; Troncoso, P.; Logothetis, C.J.; Navone, N.M.; Maity, S.N. Neuroendocrine Prostate Cancer Xenografts with Large-Cell and Small-Cell Features Derived from a Single Patient’s Tumor: Morphological, Immunohistochemical, and Gene Expression Profiles. Prostate 2011, 71, 846–856. [Google Scholar] [CrossRef]
- Tzelepi, V.; Zhang, J.; Lu, J.F.; Kleb, B.; Wu, G.; Wan, X.; Hoang, A.; Efstathiou, E.; Sircar, K.; Navone, N.M.; et al. Modeling a Lethal Prostate Cancer Variant with Small-Cell Carcinoma Features. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 666–677. [Google Scholar] [CrossRef]
- Turbat-Herrera, E.A.; Herrera, G.A.; Gore, I.; Lott, R.L.; Grizzle, W.E.; Bonnin, J.M. Neuroendocrine Differentiation in Prostatic Carcinomas. A Retrospective Autopsy Study. Arch. Pathol. Lab. Med. 1988, 112, 1100–1105. [Google Scholar]
- Tanaka, M.; Suzuki, Y.; Takaoka, K.; Suzuki, N.; Murakami, S.; Matsuzaki, O.; Shimazaki, J. Progression of Prostate Cancer to Neuroendocrine Cell Tumor. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2001, 8, 431–436. [Google Scholar] [CrossRef]
- Shah, R.B.; Mehra, R.; Chinnaiyan, A.M.; Shen, R.; Ghosh, D.; Zhou, M.; Macvicar, G.R.; Varambally, S.; Harwood, J.; Bismar, T.A.; et al. Androgen-Independent Prostate Cancer Is a Heterogeneous Group of Diseases: Lessons from a Rapid Autopsy Program. Cancer Res. 2004, 64, 9209–9216. [Google Scholar] [CrossRef] [PubMed]
- Têtu, B.; Ro, J.Y.; Ayala, A.G.; Johnson, D.E.; Logothetis, C.J.; Ordonez, N.G. Small Cell Carcinoma of the Prostate. Part I. A Clinicopathologic Study of 20 Cases. Cancer 1987, 59, 1803–1809. [Google Scholar] [CrossRef]
- Oesterling, J.E.; Hauzeur, C.G.; Farrow, G.M. Small Cell Anaplastic Carcinoma of the Prostate: A Clinical, Pathological and Immunohistological Study of 27 Patients. J. Urol. 1992, 147 Pt 2, 804–807. [Google Scholar] [CrossRef]
- Kos, M.; Reid, G.; Denger, S.; Gannon, F. Minireview: Genomic Organization of the Human ERalpha Gene Promoter Region. Mol. Endocrinol. 2001, 15, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Sakai, M.; Muramatsu, M. Molecular Cloning and Characterization of Rat Estrogen Receptor CDNA. Nucleic Acids Res. 1987, 15, 2499–2513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Zakharov, M.N.; Khan, S.H.; Miki, R.; Jang, H.; Toraldo, G.; Singh, R.; Bhasin, S.; Jasuja, R. The Dynamic Structure of the Estrogen Receptor. J. Amino Acids 2011, 2011, 812540. [Google Scholar] [CrossRef]
- Flouriot, G.; Brand, H.; Denger, S.; Metivier, R.; Kos, M.; Reid, G.; Sonntag-Buck, V.; Gannon, F. Identification of a New Isoform of the Human Estrogen Receptor-Alpha (HER-Alpha) That Is Encoded by Distinct Transcripts and That Is Able to Repress HER-Alpha Activation Function 1. EMBO J. 2000, 19, 4688–4700. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Shen, P.; Loggie, B.W.; Chang, Y.; Deuel, T.F. A Variant of Estrogen Receptor-{alpha}, HER-{alpha}36: Transduction of Estrogen- and Antiestrogen-Dependent Membrane-Initiated Mitogenic Signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 9063–9068. [Google Scholar] [CrossRef]
- Omarjee, S.; Jacquemetton, J.; Poulard, C.; Rochel, N.; Dejaegere, A.; Chebaro, Y.; Treilleux, I.; Marangoni, E.; Corbo, L.; Romancer, M.L. The Molecular Mechanisms Underlying the ERα-36-Mediated Signaling in Breast Cancer. Oncogene 2017, 36, 2503–2514. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Borg, A.; Wolf, D.M.; Oesterreich, S.; Fuqua, S.A. An Estrogen Receptor Mutant with Strong Hormone-Independent Activity from a Metastatic Breast Cancer. Cancer Res. 1997, 57, 1244–1249. [Google Scholar]
- Basudan, A.; Priedigkeit, N.; Hartmaier, R.J.; Sokol, E.S.; Bahreini, A.; Watters, R.J.; Boisen, M.M.; Bhargava, R.; Weiss, K.R.; Karsten, M.M.; et al. Frequent ESR1 and CDK Pathway Copy-Number Alterations in Metastatic Breast Cancer. Mol. Cancer Res. 2019, 17, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, Y.L.; Issa, J.P.; Parl, F.F.; Smith, H.S.; Baylin, S.B.; Davidson, N.E. Methylation of the Estrogen Receptor Gene CpG Island Marks Loss of Estrogen Receptor Expression in Human Breast Cancer Cells. Cancer Res. 1994, 54, 2552–2555. [Google Scholar] [PubMed]
- Kawai, H.; Li, H.; Avraham, S.; Jiang, S.; Avraham, H.K. Overexpression of Histone Deacetylase HDAC1 Modulates Breast Cancer Progression by Negative Regulation of Estrogen Receptor Alpha. Int. J. Cancer 2003, 107, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, M.; Cinti, C.; Russo, G.; Russo, A.; Giordano, A. PRb2/P130-E2F4/5-HDAC1-SUV39H1-P300 and PRb2/P130-E2F4/5-HDAC1-SUV39H1-DNMT1 Multimolecular Complexes Mediate the Transcription of Estrogen Receptor-Alpha in Breast Cancer. Oncogene 2003, 22, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Paech, K.; Webb, P.; Kuiper, G.G.; Nilsson, S.; Gustafsson, J.; Kushner, P.J.; Scanlan, T.S. Differential Ligand Activation of Estrogen Receptors ERalpha and ERbeta at AP1 Sites. Science 1997, 277, 1508–1510. [Google Scholar] [CrossRef]
- Belguise, K.; Guo, S.; Sonenshein, G.E. Activation of FOXO3a by the Green Tea Polyphenol Epigallocatechin-3-Gallate Induces Estrogen Receptor Alpha Expression Reversing Invasive Phenotype of Breast Cancer Cells. Cancer Res. 2007, 67, 5763–5770. [Google Scholar] [CrossRef]
- Madureira, P.A.; Varshochi, R.; Constantinidou, D.; Francis, R.E.; Coombes, R.C.; Yao, K.-M.; Lam, E.W.-F. The Forkhead Box M1 Protein Regulates the Transcription of the Estrogen Receptor Alpha in Breast Cancer Cells. J. Biol. Chem. 2006, 281, 25167–25176. [Google Scholar] [CrossRef]
- Campbell, T.M.; Castro, M.A.A.; de Oliveira, K.G.; Ponder, B.A.J.; Meyer, K.B. ERα Binding by Transcription Factors NFIB and YBX1 Enables FGFR2 Signaling to Modulate Estrogen Responsiveness in Breast Cancer. Cancer Res. 2018, 78, 410–421. [Google Scholar] [CrossRef]
- Lacroix, M.; Leclercq, G. About GATA3, HNF3A, and XBP1, Three Genes Co-Expressed with the Oestrogen Receptor-Alpha Gene (ESR1) in Breast Cancer. Mol. Cell. Endocrinol. 2004, 219, 1–7. [Google Scholar] [CrossRef]
- Bernardo, G.M.; Lozada, K.L.; Miedler, J.D.; Harburg, G.; Hewitt, S.C.; Mosley, J.D.; Godwin, A.K.; Korach, K.S.; Visvader, J.E.; Kaestner, K.H.; et al. FOXA1 Is an Essential Determinant of ERalpha Expression and Mammary Ductal Morphogenesis. Development 2010, 137, 2045–2054. [Google Scholar] [CrossRef]
- van de Vijver, M.J.; He, Y.D.; van’t Veer, L.J.; Dai, H.; Hart, A.A.M.; Voskuil, D.W.; Schreiber, G.J.; Peterse, J.L.; Roberts, C.; Marton, M.J.; et al. A Gene-Expression Signature as a Predictor of Survival in Breast Cancer. N. Engl. J. Med. 2002, 347, 1999–2009. [Google Scholar] [CrossRef]
- Oh, D.S.; Troester, M.A.; Usary, J.; Hu, Z.; He, X.; Fan, C.; Wu, J.; Carey, L.A.; Perou, C.M. Estrogen-Regulated Genes Predict Survival in Hormone Receptor-Positive Breast Cancers. J. Clin. Oncol. 2006, 24, 1656–1664. [Google Scholar] [CrossRef]
- Dreijerink, K.M.A.; Groner, A.C.; Vos, E.S.M.; Font-Tello, A.; Gu, L.; Chi, D.; Reyes, J.; Cook, J.; Lim, E.; Lin, C.Y.; et al. Enhancer-Mediated Oncogenic Function of the Menin Tumor Suppressor in Breast Cancer. Cell Rep. 2017, 18, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Wang, Z.; Wysocka, J.; Sanyal, M.; Aufiero, D.J.; Kitabayashi, I.; Herr, W.; Cleary, M.L. Leukemia Proto-Oncoprotein MLL Forms a SET1-like Histone Methyltransferase Complex with Menin to Regulate Hox Gene Expression. Mol. Cell. Biol. 2004, 24, 5639–5649. [Google Scholar] [CrossRef] [PubMed]
- Korach, K.S.; Emmen, J.M.A.; Walker, V.R.; Hewitt, S.C.; Yates, M.; Hall, J.M.; Swope, D.L.; Harrell, J.C.; Couse, J.F. Update on Animal Models Developed for Analyses of Estrogen Receptor Biological Activity. J. Steroid Biochem. Mol. Biol. 2003, 86, 387–391. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Ström, A.; Vega, V.B.; Kong, S.L.; Yeo, A.L.; Thomsen, J.S.; Chan, W.C.; Doray, B.; Bangarusamy, D.K.; Ramasamy, A.; et al. Discovery of Estrogen Receptor Alpha Target Genes and Response Elements in Breast Tumor Cells. Genome Biol. 2004, 5, R66. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen Signaling Multiple Pathways to Impact Gene Transcription. Curr. Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef]
- Brown, A.M.; Jeltsch, J.M.; Roberts, M.; Chambon, P. Activation of PS2 Gene Transcription Is a Primary Response to Estrogen in the Human Breast Cancer Cell Line MCF-7. Proc. Natl. Acad. Sci. USA 1984, 81, 6344–6348. [Google Scholar] [CrossRef]
- Jakowlew, S.B.; Breathnach, R.; Jeltsch, J.M.; Masiakowski, P.; Chambon, P. Sequence of the PS2 MRNA Induced by Estrogen in the Human Breast Cancer Cell Line MCF-7. Nucleic Acids Res. 1984, 12, 2861–2878. [Google Scholar] [CrossRef]
- Charpentier, A.H.; Bednarek, A.K.; Daniel, R.L.; Hawkins, K.A.; Laflin, K.J.; Gaddis, S.; MacLeod, M.C.; Aldaz, C.M. Effects of Estrogen on Global Gene Expression: Identification of Novel Targets of Estrogen Action. Cancer Res. 2000, 60, 5977–5983. [Google Scholar]
- Cunliffe, H.E.; Ringnér, M.; Bilke, S.; Walker, R.L.; Cheung, J.M.; Chen, Y.; Meltzer, P.S. The Gene Expression Response of Breast Cancer to Growth Regulators: Patterns and Correlation with Tumor Expression Profiles. Cancer Res. 2003, 63, 7158–7166. [Google Scholar] [PubMed]
- Frasor, J.; Danes, J.M.; Komm, B.; Chang, K.C.N.; Lyttle, C.R.; Katzenellenbogen, B.S. Profiling of Estrogen Up- and down-Regulated Gene Expression in Human Breast Cancer Cells: Insights into Gene Networks and Pathways Underlying Estrogenic Control of Proliferation and Cell Phenotype. Endocrinology 2003, 144, 4562–4574. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Yoshida, N.; Omoto, Y.; Oguchi, S.; Yamori, T.; Kiyama, R.; Hayashi, S. Development of CDNA Microarray for Expression Profiling of Estrogen-Responsive Genes. J. Mol. Endocrinol. 2002, 29, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.; Krop, I.; Porter, D.; Polyak, K. Novel Estrogen and Tamoxifen Induced Genes Identified by SAGE (Serial Analysis of Gene Expression). Oncogene 2002, 21, 836–843. [Google Scholar] [CrossRef]
- Altucci, L.; Addeo, R.; Cicatiello, L.; Dauvois, S.; Parker, M.G.; Truss, M.; Beato, M.; Sica, V.; Bresciani, F.; Weisz, A. 17beta-Estradiol Induces Cyclin D1 Gene Transcription, P36D1-P34cdk4 Complex Activation and P105Rb Phosphorylation during Mitogenic Stimulation of G(1)-Arrested Human Breast Cancer Cells. Oncogene 1996, 12, 2315–2324. [Google Scholar]
- Dubik, D.; Dembinski, T.C.; Shiu, R.P. Stimulation of C-Myc Oncogene Expression Associated with Estrogen-Induced Proliferation of Human Breast Cancer Cells. Cancer Res. 1987, 47 Pt 1, 6517–6521. [Google Scholar]
- Dubik, D.; Shiu, R.P. Transcriptional Regulation of C-Myc Oncogene Expression by Estrogen in Hormone-Responsive Human Breast Cancer Cells. J. Biol. Chem. 1988, 263, 12705–12708. [Google Scholar]
- Umayahara, Y.; Kawamori, R.; Watada, H.; Imano, E.; Iwama, N.; Morishima, T.; Yamasaki, Y.; Kajimoto, Y.; Kamada, T. Estrogen Regulation of the Insulin-like Growth Factor I Gene Transcription Involves an AP-1 Enhancer. J. Biol. Chem. 1994, 269, 16433–16442. [Google Scholar]
- Elangovan, S.; Moulton, B.C. Progesterone and Estrogen Control of Rates of Synthesis of Uterine Cathepsin D. J. Biol. Chem. 1980, 255, 7474–7479. [Google Scholar]
- Yu, W.C.; Leung, B.S.; Gao, Y.L. Effects of 17 Beta-Estradiol on Progesterone Receptors and the Uptake of Thymidine in Human Breast Cancer Cell Line CAMA-1. Cancer Res. 1981, 41 Pt 1, 5004–5009. [Google Scholar]
- Jenster, G.; van der Korput, H.A.; van Vroonhoven, C.; van der Kwast, T.H.; Trapman, J.; Brinkmann, A.O. Domains of the Human Androgen Receptor Involved in Steroid Binding, Transcriptional Activation, and Subcellular Localization. Mol. Endocrinol. (Baltim. Md.) 1991, 5, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Bryce, A.H.; Antonarakis, E.S. Androgen Receptor Splice Variant 7 in Castration-Resistant Prostate Cancer: Clinical Considerations. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2016, 23, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Dunn, T.A.; Wei, S.; Isharwal, S.; Veltri, R.W.; Humphreys, E.; Han, M.; Partin, A.W.; Vessella, R.L.; Isaacs, W.B.; et al. Ligand-Independent Androgen Receptor Variants Derived from Splicing of Cryptic Exons Signify Hormone-Refractory Prostate Cancer. Cancer Res. 2009, 69, 16–22. [Google Scholar] [CrossRef]
- Van der Steen, T.; Tindall, D.J.; Huang, H. Posttranslational Modification of the Androgen Receptor in Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 14833–14859. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Iczkowski, K.A.; Kilari, D.; See, W.; Nevalainen, M.T. Androgen Receptor-Dependent and -Independent Mechanisms Driving Prostate Cancer Progression: Opportunities for Therapeutic Targeting from Multiple Angles. Oncotarget 2017, 8, 3724–3745. [Google Scholar] [CrossRef]
- Koochekpour, S. Androgen Receptor Signaling and Mutations in Prostate Cancer. Asian J. Androl. 2010, 12, 639–657. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The Mutational Landscape of Lethal Castration-Resistant Prostate Cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Hay, C.W.; Hunter, I.; MacKenzie, A.; McEwan, I.J. An Sp1 Modulated Regulatory Region Unique to Higher Primates Regulates Human Androgen Receptor Promoter Activity in Prostate Cancer Cells. PLoS ONE 2015, 10, e0139990. [Google Scholar] [CrossRef]
- Deng, X.; Shao, G.; Zhang, H.T.; Li, C.; Zhang, D.; Cheng, L.; Elzey, B.D.; Pili, R.; Ratliff, T.L.; Huang, J.; et al. Protein Arginine Methyltransferase 5 Functions as an Epigenetic Activator of the Androgen Receptor to Promote Prostate Cancer Cell Growth. Oncogene 2017, 36, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Grad, J.M.; Lyons, L.S.; Robins, D.M.; Burnstein, K.L. The Androgen Receptor (AR) Amino-Terminus Imposes Androgen-Specific Regulation of AR Gene Expression via an Exonic Enhancer. Endocrinology 2001, 142, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Yen, A.E.; Weigel, N.L. Androgen Receptors in Hormone-Dependent and Castration-Resistant Prostate Cancer. Pharmacol. Ther. 2013, 140, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen Receptor: Structure, Role in Prostate Cancer and Drug Discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Fu, M.; Wang, C.; Reutens, A.T.; Wang, J.; Angeletti, R.H.; Siconolfi-Baez, L.; Ogryzko, V.; Avantaggiati, M.-L.; Pestell, R.G. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J. Biol. Chem. 2000, 275, 20853–20860. [Google Scholar] [CrossRef]
- Bakin, R.E.; Gioeli, D.; Bissonette, E.A.; Weber, M.J. Attenuation of Ras Signaling Restores Androgen Sensitivity to Hormone-Refractory C4-2 Prostate Cancer Cells. Cancer Res. 2003, 63, 1975–1980. [Google Scholar]
- Bakin, R.E.; Gioeli, D.; Sikes, R.A.; Bissonette, E.A.; Weber, M.J. Constitutive Activation of the Ras/Mitogen-Activated Protein Kinase Signaling Pathway Promotes Androgen Hypersensitivity in LNCaP Prostate Cancer Cells. Cancer Res. 2003, 63, 1981–1989. [Google Scholar]
- Gregory, C.W.; Fei, X.; Ponguta, L.A.; He, B.; Bill, H.M.; French, F.S.; Wilson, E.M. Epidermal Growth Factor Increases Coactivation of the Androgen Receptor in Recurrent Prostate Cancer. J. Biol. Chem. 2004, 279, 7119–7130. [Google Scholar] [CrossRef]
- Wu, J.D.; Haugk, K.; Woodke, L.; Nelson, P.; Coleman, I.; Plymate, S.R. Interaction of IGF Signaling and the Androgen Receptor in Prostate Cancer Progression. J. Cell. Biochem. 2006, 99, 392–401. [Google Scholar] [CrossRef]
- Schweizer, L.; Rizzo, C.A.; Spires, T.E.; Platero, J.S.; Wu, Q.; Lin, T.A.; Gottardis, M.M.; Attar, R.M. The Androgen Receptor Can Signal through Wnt/Beta-Catenin in Prostate Cancer Cells as an Adaptation Mechanism to Castration Levels of Androgens. BMC Cell Boil. 2008, 9, 4. [Google Scholar] [CrossRef]
- Leung, J.K.; Sadar, M.D. Non-Genomic Actions of the Androgen Receptor in Prostate Cancer. Front. Endocrinol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Mounir, Z.; Korn, J.M.; Westerling, T.; Lin, F.; Kirby, C.A.; Schirle, M.; McAllister, G.; Hoffman, G.; Ramadan, N.; Hartung, A.; et al. ERG Signaling in Prostate Cancer Is Driven through PRMT5-Dependent Methylation of the Androgen Receptor. eLife 2016, 5, e13964. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, S.Y.; Ross, K.N.; Balk, S.P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006, 66, 7783–7792. [Google Scholar]
- Lu, S.; Liu, M.; Epner, D.E.; Tsai, S.Y.; Tsai, M.J. Androgen Regulation of the Cyclin-Dependent Kinase Inhibitor P21 Gene through an Androgen Response Element in the Proximal Promoter. Mol. Endocrinol. (Baltim. Md.) 1999, 13, 376–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aaltomaa, S.; Lipponen, P.; Eskelinen, M.; Ala-Opas, M.; Kosma, V.M. Prognostic Value and Expression of P21(Waf1/Cip1) Protein in Prostate Cancer. Prostate 1999, 39, 8–15. [Google Scholar] [CrossRef]
- Baretton, G.B.; Klenk, U.; Diebold, J.; Schmeller, N.; Löhrs, U. Proliferation- and Apoptosis-Associated Factors in Advanced Prostatic Carcinomas before and after Androgen Deprivation Therapy: Prognostic Significance of P21/WAF1/CIP1 Expression. Br. J. Cancer 1999, 80, 546–555. [Google Scholar] [CrossRef]
- Knudsen, K.E.; Arden, K.C.; Cavenee, W.K. Multiple G1 Regulatory Elements Control the Androgen-Dependent Proliferation of Prostatic Carcinoma Cells. J. Boil. Chem. 1998, 273, 20213–20222. [Google Scholar] [CrossRef]
- Rokhlin, O.W.; Taghiyev, A.F.; Guseva, N.V.; Glover, R.A.; Chumakov, P.M.; Kravchenko, J.E.; Cohen, M.B. Androgen Regulates Apoptosis Induced by TNFR Family Ligands via Multiple Signaling Pathways in LNCaP. Oncogene 2005, 24, 6773–6784. [Google Scholar] [CrossRef]
- Frezza, M.; Yang, H.; Dou, Q.P. Modulation of the Tumor Cell Death Pathway by Androgen Receptor in Response to Cytotoxic Stimuli. J. Cell. Physiol. 2011, 226, 2731–2739. [Google Scholar] [CrossRef]
- Liao, X.; Tang, S.; Thrasher, J.B.; Griebling, T.L.; Li, B. Small-Interfering RNA-Induced Androgen Receptor Silencing Leads to Apoptotic Cell Death in Prostate Cancer. Mol. Cancer Ther. 2005, 4, 505–515. [Google Scholar] [CrossRef]
- Godfrey, B.; Lin, Y.; Larson, J.; Haferkamp, B.; Xiang, J. Proteasomal Degradation Unleashes the Pro-Death Activity of Androgen Receptor. Cell Res. 2010, 20, 1138–1147. [Google Scholar] [CrossRef]
- Zhao, J.C.; Yu, J.; Runkle, C.; Wu, L.; Hu, M.; Wu, D.; Liu, J.S.; Wang, Q.; Qin, Z.S.; Yu, J. Cooperation between Polycomb and Androgen Receptor during Oncogenic Transformation. Genome Res. 2012, 22, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yu, Z.; Sun, X.; Feng, J.; Yu, Q.; Khan, H.; Zhu, X.; Huang, L.; Li, M.; Mok, M.T.S.; et al. Androgen Receptor Drives Hepatocellular Carcinogenesis by Activating Enhancer of Zeste Homolog 2-Mediated Wnt/β-Catenin Signaling. EBioMedicine 2018, 35, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Thakker, R.V. Multiple Endocrine Neoplasia Type 1 (MEN1) and Type 4 (MEN4). Mol. Cell. Endocrinol. 2014, 386, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, I.; Van de Ven, W.J.; Kas, K.; Zhang, C.X.; Giraud, S.; Wautot, V.; Buisson, N.; De Witte, K.; Salandre, J.; Lenoir, G.; et al. Identification of the Multiple Endocrine Neoplasia Type 1 (MEN1) Gene. The European Consortium on MEN1. Hum. Mol. Genet. 1997, 6, 1177–1183. [Google Scholar] [CrossRef]
- Chandrasekharappa, S.C. Positional Cloning of the Gene for Multiple Endocrine Neoplasia-Type 1. Science 1997, 276, 404–407. [Google Scholar] [CrossRef]
- Wautot, V.; Khodaei, S.; Frappart, L.; Buisson, N.; Baro, E.; Lenoir, G.M.; Calender, A.; Zhang, C.X.; Weber, G. Expression Analysis of Endogenous Menin, the Product of the Multiple Endocrine Neoplasia Type 1 Gene, in Cell Lines and Human Tissues. Int. J. Cancer 2000, 85, 877–881. [Google Scholar] [CrossRef]
- Matkar, S.; Thiel, A.; Hua, X. Menin: A Scaffold Protein That Controls Gene Expression and Cell Signaling. Trends Biochem. Sci. 2013, 38, 394–402. [Google Scholar] [CrossRef]
- Lu, J.; Hamze, Z.; Bonnavion, R.; Herath, N.; Pouponnot, C.; Assade, F.; Fontanière, S.; Bertolino, P.; Cordier-Bussat, M.; Zhang, C.X. Reexpression of Oncoprotein MafB in Proliferative β-Cells and Men1 Insulinomas in Mouse. Oncogene 2012, 31, 3647–3654. [Google Scholar] [CrossRef]
- Hamze, Z.; Vercherat, C.; Bernigaud-Lacheretz, A.; Bazzi, W.; Bonnavion, R.; Lu, J.; Calender, A.; Pouponnot, C.; Bertolino, P.; Roche, C.; et al. Altered MENIN Expression Disrupts the MAFA Differentiation Pathway in Insulinoma. Endocr. Relat. Cancer 2013, 20, 833–848. [Google Scholar] [CrossRef]
- Bonnavion, R.; Teinturier, R.; Gherardi, S.; Leteurtre, E.; Yu, R.; Cordier-Bussat, M.; Du, R.; Pattou, F.; Vantyghem, M.-C.; Bertolino, P.; et al. Foxa2, a Novel Protein Partner of the Tumour Suppressor Menin, Is Deregulated in Mouse and Human MEN1 Glucagonomas. J. Pathol. 2017, 242, 90–101. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, R.; Jiang, X.; Lu, J.; Jiang, J.; Zhang, C.; Li, X.; Ning, G. Nuclear-Cytoplasmic Shuttling of Menin Regulates Nuclear Translocation of {beta}-Catenin. Mol. Cell. Biol. 2009, 29, 5477–5487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cao, Y.; Li, F.; Su, Y.; Li, Y.; Peng, Y.; Cheng, Y.; Zhang, C.; Wang, W.; Ning, G. Targeting β-Catenin Signaling for Therapeutic Intervention in MEN1-Deficient Pancreatic Neuroendocrine Tumours. Nat. Commun. 2014, 5, 5809. [Google Scholar] [CrossRef] [PubMed]
- Bazzi, W.; Renon, M.; Vercherat, C.; Hamze, Z.; Lacheretz-Bernigaud, A.; Wang, H.; Blanc, M.; Roche, C.; Calender, A.; Chayvialle, J.-A.; et al. MEN1 Missense Mutations Impair Sensitization to Apoptosis Induced by Wild-Type Menin in Endocrine Pancreatic Tumor Cells. Gastroenterology 2008, 135, 1698–1709.e2. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, S.; Ripoche, D.; Mikaelian, I.; Chanal, M.; Teinturier, R.; Goehrig, D.; Cordier-Bussat, M.; Zhang, C.X.; Hennino, A.; Bertolino, P. Menin Regulates Inhbb Expression through an Akt/Ezh2-Mediated H3K27 Histone Modification. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2017, 1860, 427–437. [Google Scholar] [CrossRef]
- Dreijerink, K.M.A.; Varier, R.A.; van Beekum, O.; Jeninga, E.H.; Höppener, J.W.M.; Lips, C.J.M.; Kummer, J.A.; Kalkhoven, E.; Timmers, H.T.M. The Multiple Endocrine Neoplasia Type 1 (MEN1) Tumor Suppressor Regulates Peroxisome Proliferator-Activated Receptor Gamma-Dependent Adipocyte Differentiation. Mol. Cell. Biol. 2009, 29, 5060–5069. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Miyake, T.; Engleka, K.A.; Epstein, J.A.; McDermott, J.C. Menin Expression Modulates Mesenchymal Cell Commitment to the Myogenic and Osteogenic Lineages. Dev. Biol. 2009, 332, 116–130. [Google Scholar] [CrossRef]
- Maillard, I.; Chen, Y.-X.; Friedman, A.; Yang, Y.; Tubbs, A.T.; Shestova, O.; Pear, W.S.; Hua, X. Menin Regulates the Function of Hematopoietic Stem Cells and Lymphoid Progenitors. Blood 2009, 113, 1661–1669. [Google Scholar] [CrossRef]
- Dreijerink, K.M.A.; Mulder, K.W.; Winkler, G.S.; Höppener, J.W.M.; Lips, C.J.M.; Timmers, H.T.M. Menin Links Estrogen Receptor Activation to Histone H3K4 Trimethylation. Cancer Res. 2006, 66, 4929–4935. [Google Scholar] [CrossRef]
- Imachi, H.; Murao, K.; Dobashi, H.; Bhuyan, M.M.; Cao, X.; Kontani, K.; Niki, S.; Murazawa, C.; Nakajima, H.; Kohno, N.; et al. Menin, a Product of the MENI Gene, Binds to Estrogen Receptor to Enhance Its Activity in Breast Cancer Cells: Possibility of a Novel Predictive Factor for Tamoxifen Resistance. Breast Cancer Res. Treat. 2010, 122, 395–407. [Google Scholar] [CrossRef]
- Malik, R.; Khan, A.P.; Asangani, I.A.; Cieślik, M.; Prensner, J.R.; Wang, X.; Iyer, M.K.; Jiang, X.; Borkin, D.; Escara-Wilke, J.; et al. Targeting the MLL Complex in Castration-Resistant Prostate Cancer. Nat. Med. 2015, 21, 344–352. [Google Scholar] [CrossRef]
- Bertolino, P.; Tong, W.-M.; Galendo, D.; Wang, Z.-Q.; Zhang, C.-X. Heterozygous Men1 Mutant Mice Develop a Range of Endocrine Tumors Mimicking Multiple Endocrine Neoplasia Type 1. Mol. Endocrinol. 2003, 17, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Seigne, C.; Auret, M.; Treilleux, I.; Bonnavion, R.; Assade, F.; Carreira, C.; Goddard-Léon, S.; Lavergne, E.; Chabaud, S.; Garcia, A.; et al. High Incidence of Mammary Intraepithelial Neoplasia Development in Men1-Disrupted Murine Mammary Glands: Men—1 and Pre-Cancerous Mammary Glands Lesions. J. Pathol. 2013, 229, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Seigne, C.; Fontanière, S.; Carreira, C.; Lu, J.; Tong, W.M.; Fontanière, B.; Wang, Z.Q.; Zhang, C.X.; Frappart, L. Characterisation of Prostate Cancer Lesions in Heterozygous Men1 Mutant Mice. BMC Cancer 2010, 10, 395. [Google Scholar] [CrossRef]
- Honda, M.; Tsukada, T.; Horiuchi, T.; Tanaka, R.; Yamaguchi, K.; Obara, T.; Miyakawa, H.; Yamaji, T.; Ishibashi, M. Primary Hyperparathyroidism Associatiated with Aldosterone-Producing Adrenocortical Adenoma and Breast Cancer: Relation to MEN1 Gene. Intern. Med. 2004, 43, 310–314. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Oh, H.K.; Bong, J.G. Multiple Endocrine Neoplasia Type 1 Associated with Breast Cancer: A Case Report and Review of the Literature. Oncol. Lett. 2014, 8, 230–234. [Google Scholar] [CrossRef]
- Herranz-Antolín, S.; Gil-García, S.; Álvarez-de Frutos, V. Multiple Endocrine Neoplasia Type 1 and Breast Cancer. An Association to Consider. Endocrinol. Diabetes Nutr. 2018, 65, 468–469. [Google Scholar] [CrossRef]
- Dreijerink, K.M.A.; Goudet, P.; Burgess, J.R.; Valk, G.D. International Breast Cancer in MEN1 Study Group. Breast-Cancer Predisposition in Multiple Endocrine Neoplasia Type 1. N. Engl. J. Med. 2014, 371, 583–584. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of Somatic Mutations in 560 Breast Cancer Whole-Genome Sequences. Nature 2016, 534, 47–54. [Google Scholar] [CrossRef]
- Inic, Z.M.; Inic, M.; Dzodic, R.; Pupic, G.; Damjanovic, S. Breast Cancer in a Patient with Multiple Endocrine Neoplasia Type 1 (MEN 1): A Case Report and Review of the Literature. JCO 2012, 30 (Suppl. 15), e21136. [Google Scholar] [CrossRef]
- Papi, L.; Palli, D.; Masi, L.; Putignano, A.L.; Congregati, C.; Zanna, I.; Marini, F.; Giusti, F.; Luzi, E.; Tonelli, F.; et al. Germline Mutations in MEN1 and BRCA1 Genes in a Woman with Familial Multiple Endocrine Neoplasia Type 1 and Inherited Breast-Ovarian Cancer Syndromes: A Case Report. Cancer Genet. Cytogenet. 2009, 195, 75–79. [Google Scholar] [CrossRef]
- Ghataorhe, P.; Kurian, A.W.; Pickart, A.; Trapane, P.; Norton, J.A.; Kingham, K.; Ford, J.M. A Carrier of Both MEN1 and BRCA2 Mutations: Case Report and Review of the Literature. Cancer Genet. Cytogenet. 2007, 179, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Imachi, H.; Yu, X.; Nishiuchi, T.; Miyai, Y.; Masugata, H.; Murao, K. Raloxifene Inhibits Menin-Dependent Estrogen Receptor Activation in Breast Cancer Cells. J. Endocrinol. Investig. 2011, 34, 813–815. [Google Scholar] [CrossRef]
- Perakakis, N.; Flohr, F.; Kayser, G.; Thomusch, O.; Parsons, L.; Billmann, F.; von Dobschuetz, E.; Rondot, S.; Seufert, J.; Laubner, K. Multiple Endocrine Neoplasia Type 1 Associated with a New Germline Men1 Mutation in a Family with Atypical Tumor Phenotype. Hormones 2016, 15, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Manson-Bahr, D.; Ball, R.; Gundem, G.; Sethia, K.; Mills, R.; Rochester, M.; Goody, V.; Anderson, E.; O’Meara, S.; Flather, M.; et al. Mutation Detection in Formalin-Fixed Prostate Cancer Biopsies Taken at the Time of Diagnosis Using next-Generation DNA Sequencing. J. Clin. Pathol. 2015, 68, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Varier, R.A.; Timmers, H.T. Histone Lysine Methylation and Demethylation Pathways in Cancer. Biochim. Biophys. Acta 2011, 1815, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.S.; Lee, Y.R.; Fung, J.; Katon, J.M.; et al. An Aberrant SREBP-Dependent Lipogenic Program Promotes Metastatic Prostate Cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef]
- Paris, P.L.; Andaya, A.; Fridlyand, J.; Jain, A.N.; Weinberg, V.; Kowbel, D.; Brebner, J.H.; Simko, J.; Watson, J.E.V.; Volik, S.; et al. Whole Genome Scanning Identifies Genotypes Associated with Recurrence and Metastasis in Prostate Tumors. Hum. Mol. Genet. 2004, 13, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Heselmeyer-Haddad, K.M.; Berroa Garcia, L.Y.; Bradley, A.; Hernandez, L.; Hu, Y.; Habermann, J.K.; Dumke, C.; Thorns, C.; Perner, S.; Pestova, E.; et al. Single-Cell Genetic Analysis Reveals Insights into Clonal Development of Prostate Cancers and Indicates Loss of PTEN as a Marker of Poor Prognosis. Am. J. Pathol. 2014, 184, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Paris, P.L.; Sridharan, S.; Hittelman, A.B.; Kobayashi, Y.; Perner, S.; Huang, G.; Simko, J.; Carroll, P.; Rubin, M.A.; Collins, C. An Oncogenic Role for the Multiple Endocrine Neoplasia Type 1 Gene in Prostate Cancer. Prostate Cancer Prostatic Dis. 2009, 12, 184–191. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, Y.; Song, M.; Chen, G.; Wang, H.; Zhao, Y.; Wang, Z.; Li, F. PRDM16 Is Associated with Evasion of Apoptosis by Prostatic Cancer Cells According to RNA Interference Screening. Mol. Med. Rep. 2016, 14, 3357–3361. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.I.; Shrestha, B.; Hussain, I.; Kasiri, S.; Mandal, S.S. Histone Methylases MLL1 and MLL3 Coordinate with Estrogen Receptors in Estrogen-Mediated HOXB9 Expression. Biochemistry 2011, 50, 3517–3527. [Google Scholar] [CrossRef]
- Yokoyama, A.; Cleary, M.L. Menin Critically Links MLL Proteins with LEDGF on Cancer-Associated Target Genes. Cancer Cell 2008, 14, 36–46. [Google Scholar] [CrossRef]
- Gao, S.-B.; Feng, Z.-J.; Xu, B.; Wu, Y.; Yin, P.; Yang, Y.; Hua, X.; Jin, G.-H. Suppression of Lung Adenocarcinoma through Menin and Polycomb Gene-Mediated Repression of Growth Factor Pleiotrophin. Oncogene 2009, 28, 4095–4104. [Google Scholar] [CrossRef]
- Gurung, B.; Feng, Z.; Hua, X. Menin Directly Represses Gli1 Expression Independent of Canonical Hedgehog Signaling. Mol. Cancer Res. 2013, 11, 1215–1222. [Google Scholar] [CrossRef]
- Hwang, C.; Giri, V.N.; Wilkinson, J.C.; Wright, C.W.; Wilkinson, A.S.; Cooney, K.A.; Duckett, C.S. EZH2 Regulates the Transcription of Estrogen-Responsive Genes through Association with REA, an Estrogen Receptor Corepressor. Breast Cancer Res. Treat. 2008, 107, 235–242. [Google Scholar] [CrossRef]
- Powers, M.A.; Fay, M.M.; Factor, R.E.; Welm, A.L.; Ullman, K.S. Protein Arginine Methyltransferase 5 Accelerates Tumor Growth by Arginine Methylation of the Tumor Suppressor Programmed Cell Death 4. Cancer Res. 2011, 71, 5579–5587. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Guru, S.C.; Heppner, C.; Erdos, M.R.; Collins, R.M.; Park, S.Y.; Saggar, S.; Chandrasekharappa, S.C.; Collins, F.S.; Spiegel, A.M.; et al. Menin Interacts with the AP1 Transcription Factor JunD and Represses JunD-Activated Transcription. Cell 1999, 96, 143–152. [Google Scholar] [CrossRef]
- Huang, J.; Gurung, B.; Wan, B.; Matkar, S.; Veniaminova, N.A.; Wan, K.; Merchant, J.L.; Hua, X.; Lei, M. The Same Pocket in Menin Binds Both MLL and JUND but Has Opposite Effects on Transcription. Nature 2012, 482, 542–546. [Google Scholar] [CrossRef]
- Kharman-Biz, A.; Gao, H.; Ghiasvand, R.; Zhao, C.; Zendehdel, K.; Dahlman-Wright, K. Expression of Activator Protein-1 (AP-1) Family Members in Breast Cancer. BMC Cancer 2013, 13, 441. [Google Scholar] [CrossRef]
- Hipkaeo, W.; Sakulsak, N.; Wakayama, T.; Yamamoto, M.; Nakaya, M.-A.; Keattikunpairoj, S.; Kurobo, M.; Iseki, S. Coexpression of Menin and JunD during the Duct Cell Differentiation in Mouse Submandibular Gland. Tohoku J. Exp. Med. 2008, 214, 231–245. [Google Scholar] [CrossRef][Green Version]
- Chen, D.; Reierstad, S.; Fang, F.; Bulun, S.E. JunD and JunB Integrate Prostaglandin E2 Activation of Breast Cancer-Associated Proximal Aromatase Promoters. Mol. Endocrinol. 2011, 25, 767–775. [Google Scholar] [CrossRef]
- Millena, A.C.; Vo, B.T.; Khan, S.A. JunD Is Required for Proliferation of Prostate Cancer Cells and Plays a Role in Transforming Growth Factor-β (TGF-β)-Induced Inhibition of Cell Proliferation. J. Boil. Chem. 2016, 291, 17964–17976. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Elliott, B.; Millena, A.C.; Matyunina, L.; Zhang, M.; Zou, J.; Wang, G.; Zhang, Q.; Bowen, N.; Eaton, V.; Webb, G.; et al. Essential Role of JunD in Cell Proliferation Is Mediated via MYC Signaling in Prostate Cancer Cells. Cancer Lett. 2019, 448, 155–167. [Google Scholar] [CrossRef]
- Mehraein-Ghomi, F.; Basu, H.S.; Church, D.R.; Hoffmann, F.M.; Wilding, G. Androgen Receptor Requires JunD as a Coactivator to Switch on an Oxidative Stress Generation Pathway in Prostate Cancer Cells. Cancer Res. 2010, 70, 4560–4568. [Google Scholar] [CrossRef]
- Shang, Y.; Hu, X.; DiRenzo, J.; Lazar, M.A.; Brown, M. Cofactor Dynamics and Sufficiency in Estrogen Receptor-Regulated Transcription. Cell 2000, 103, 843–852. [Google Scholar] [CrossRef]
- Shang, Y.; Brown, M. Molecular Determinants for the Tissue Specificity of SERMs. Science 2002, 295, 2465–2468. [Google Scholar] [CrossRef]
- Bièche, I.; Laurendeau, I.; Tozlu, S.; Olivi, M.; Vidaud, D.; Lidereau, R.; Vidaud, M. Quantitation of MYC Gene Expression in Sporadic Breast Tumors with a Real-Time Reverse Transcription-PCR Assay. Cancer Res. 1999, 59, 2759–2765. [Google Scholar]
- Cheng, A.S.L.; Jin, V.X.; Fan, M.; Smith, L.T.; Liyanarachchi, S.; Yan, P.S.; Leu, Y.-W.; Chan, M.W.Y.; Plass, C.; Nephew, K.P.; et al. Combinatorial Analysis of Transcription Factor Partners Reveals Recruitment of C-MYC to Estrogen Receptor-Alpha Responsive Promoters. Mol. Cell 2006, 21, 393–404. [Google Scholar] [CrossRef]
- Fleming, W.H.; Hamel, A.; MacDonald, R.; Ramsey, E.; Pettigrew, N.M.; Johnston, B.; Dodd, J.G.; Matusik, R.J. Expression of the C-Myc Protooncogene in Human Prostatic Carcinoma and Benign Prostatic Hyperplasia. Cancer Res. 1986, 46, 1535–1538. [Google Scholar]
- Sato, H.; Minei, S.; Hachiya, T.; Yoshida, T.; Takimoto, Y. Fluorescence in Situ Hybridization Analysis of C-Myc Amplification in Stage TNM Prostate Cancer in Japanese Patients. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2006, 13, 761–766. [Google Scholar] [CrossRef]
- Wu, G.; Yuan, M.; Shen, S.; Ma, X.; Fang, J.; Zhu, L.; Sun, L.; Liu, Z.; He, X.; Huang, D.; et al. Menin Enhances C-Myc-Mediated Transcription to Promote Cancer Progression. Nat. Commun. 2017, 8, 15278. [Google Scholar] [CrossRef]
- Zaman, S.; Sukhodolets, K.; Wang, P.; Qin, J.; Levens, D.; Agarwal, S.K.; Marx, S.J. FBP1 Is an Interacting Partner of Menin. Int. J. Endocrinol. 2014, 2014, 535401. [Google Scholar] [CrossRef]
- Carroll, J.S.; Brown, M. Estrogen Receptor Target Gene: An Evolving Concept. Mol. Endocrinol. 2006, 20, 1707–1714. [Google Scholar] [CrossRef]
- Carroll, J.S.; Liu, X.S.; Brodsky, A.S.; Li, W.; Meyer, C.A.; Szary, A.J.; Eeckhoute, J.; Shao, W.; Hestermann, E.V.; Geistlinger, T.R.; et al. Chromosome-Wide Mapping of Estrogen Receptor Binding Reveals Long-Range Regulation Requiring the Forkhead Protein FoxA1. Cell 2005, 122, 33–43. [Google Scholar] [CrossRef]
- Nakata, Y.; Brignier, A.C.; Jin, S.; Shen, Y.; Rudnick, S.I.; Sugita, M.; Gewirtz, A.M. C-Myb, Menin, GATA-3, and MLL Form a Dynamic Transcription Complex That Plays a Pivotal Role in Human T Helper Type 2 Cell Development. Blood 2010, 116, 1280–1290. [Google Scholar] [CrossRef]
- Obinata, D.; Takayama, K.; Takahashi, S.; Inoue, S. Crosstalk of the Androgen Receptor with Transcriptional Collaborators: Potential Therapeutic Targets for Castration-Resistant Prostate Cancer. Cancers 2017, 9, 22. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Cortes-Funes, H.; Gomez, H.L.; Ciruelos, E.M. The Phosphatidyl Inositol 3-Kinase/AKT Signaling Pathway in Breast Cancer. Cancer Metastasis Rev. 2010, 29, 751–759. [Google Scholar] [CrossRef]
- Ellis, M.J.; Perou, C.M. The Genomic Landscape of Breast Cancer as a Therapeutic Roadmap. Cancer Discov. 2013, 3, 27–34. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef]
- Jones, N.; Bonnet, F.; Sfar, S.; Lafitte, M.; Lafon, D.; Sierankowski, G.; Brouste, V.; Banneau, G.; Tunon de Lara, C.; Debled, M.; et al. Comprehensive Analysis of PTEN Status in Breast Carcinomas. Int. J. Cancer 2013, 133, 323–334. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Wang, Q.; Wang, X.; Li, F.; Zhang, C.; Wu, L.; Long, H.; Liu, Y.; Li, X.; et al. Glucose-Mediated Repression of Menin Promotes Pancreatic β-Cell Proliferation. Endocrinology 2012, 153, 602–611. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Wang, M.; Yang, J.; Lv, M.; Li, P.; Chen, Z.; Yang, J. Loss of PTEN Expression in Breast Cancer: Association with Clinicopathological Characteristics and Prognosis. Oncotarget 2017, 8, 32043–32054. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Lei, Q.; Rozengurt, N.; Pritchard, C.; Jiao, J.; Thomas, G.V.; Li, G.; Roy-Burman, P.; Nelson, P.S.; et al. Prostate-Specific Deletion of the Murine Pten Tumor Suppressor Gene Leads to Metastatic Prostate Cancer. Cancer Cell 2003, 4, 209–221. [Google Scholar] [CrossRef]
- Shen, M.M.; Abate-Shen, C. Pten Inactivation and the Emergence of Androgen-Independent Prostate Cancer. Cancer Res. 2007, 67, 6535–6538. [Google Scholar] [CrossRef]
- Sircar, K.; Yoshimoto, M.; Monzon, F.A.; Koumakpayi, I.H.; Katz, R.L.; Khanna, A.; Alvarez, K.; Chen, G.; Darnel, A.D.; Aprikian, A.G.; et al. PTEN Genomic Deletion Is Associated with P-Akt and AR Signalling in Poorer Outcome, Hormone Refractory Prostate Cancer. J. Pathol. 2009, 218, 505–513. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and MTOR Pathway Genes Are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.-M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-Genome Landscape of Pancreatic Neuroendocrine Tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef]
- Boulay, A.; Rudloff, J.; Ye, J.; Zumstein-Mecker, S.; O’Reilly, T.; Evans, D.B.; Chen, S.; Lane, H.A. Dual Inhibition of MTOR and Estrogen Receptor Signaling in Vitro Induces Cell Death in Models of Breast Cancer. Clin. Cancer Res. 2005, 11, 5319–5328. [Google Scholar] [CrossRef]
- Crowder, R.J.; Phommaly, C.; Tao, Y.; Hoog, J.; Luo, J.; Perou, C.M.; Parker, J.S.; Miller, M.A.; Huntsman, D.G.; Lin, L.; et al. PIK3CA and PIK3CB Inhibition Produce Synthetic Lethality When Combined with Estrogen Deprivation in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2009, 69, 3955–3962. [Google Scholar] [CrossRef]
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of Phosphatidylinositol-3 Kinase Promotes Escape from Hormone Dependence in Estrogen Receptor-Positive Human Breast Cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar] [CrossRef]
- Nagata, Y.; Lan, K.-H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN Activation Contributes to Tumor Inhibition by Trastuzumab, and Loss of PTEN Predicts Trastuzumab Resistance in Patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef]
- Wang, Y.; Ozawa, A.; Zaman, S.; Prasad, N.B.; Chandrasekharappa, S.C.; Agarwal, S.K.; Marx, S.J. The Tumor Suppressor Protein Menin Inhibits AKT Activation by Regulating Its Cellular Localization. Cancer Res. 2011, 71, 371–382. [Google Scholar] [CrossRef]
- Wuescher, L.; Angevine, K.; Hinds, T.; Ramakrishnan, S.; Najjar, S.M.; Mensah-Osman, E.J. Insulin Regulates Menin Expression, Cytoplasmic Localization, and Interaction with FOXO1. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E474–E483. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Wang, Z.; Lin, X.; Yan, Z.; Cao, Q.; Zhao, M.; Shi, K. MEN1/Menin Regulates Milk Protein Synthesis through MTOR Signaling in Mammary Epithelial Cells. Sci. Rep. 2017, 7, 5479. [Google Scholar] [CrossRef]
- Kaji, H.; Canaff, L.; Lebrun, J.J.; Goltzman, D.; Hendy, G.N. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 3837–3842. [Google Scholar]
- Ratineau, C.; Bernard, C.; Poncet, G.; Blanc, M.; Josso, C.; Fontanière, S.; Calender, A.; Chayvialle, J.A.; Zhang, C.X.; Roche, C. Reduction of Menin Expression Enhances Cell Proliferation and Is Tumorigenic in Intestinal Epithelial Cells. J. Biol. Chem. 2004, 279, 24477–24484. [Google Scholar] [CrossRef]
- Fontanière, S.; Tost, J.; Wierinckx, A.; Lachuer, J.; Lu, J.; Hussein, N.; Busato, F.; Gut, I.; Wang, Z.Q.; Zhang, C.X. Gene Expression Profiling in Insulinomas of Men1 Beta-Cell Mutant Mice Reveals Early Genetic and Epigenetic Events Involved in Pancreatic Beta-Cell Tumorigenesis. Endocr. Relat. Cancer 2006, 13, 1223–1236. [Google Scholar] [CrossRef]
- Schnepp, R.W.; Hou, Z.; Wang, H.; Petersen, C.; Silva, A.; Masai, H.; Hua, X. Functional Interaction between Tumor Suppressor Menin and Activator of S-Phase Kinase. Cancer Res. 2004, 64, 6791–6796. [Google Scholar] [CrossRef]
- Pei, X.-H.; Bai, F.; Smith, M.D.; Usary, J.; Fan, C.; Pai, S.-Y.; Ho, I.-C.; Perou, C.M.; Xiong, Y. CDK Inhibitor P18(INK4c) Is a Downstream Target of GATA3 and Restrains Mammary Luminal Progenitor Cell Proliferation and Tumorigenesis. Cancer Cell 2009, 15, 389–401. [Google Scholar] [CrossRef]
- Stephens, P.J.; Tarpey, P.S.; Davies, H.; Van Loo, P.; Greenman, C.; Wedge, D.C.; Nik-Zainal, S.; Martin, S.; Varela, I.; Bignell, G.R.; et al. The Landscape of Cancer Genes and Mutational Processes in Breast Cancer. Nature 2012, 486, 400–404. [Google Scholar] [CrossRef]
- Karnik, S.K.; Hughes, C.M.; Gu, X.; Rozenblatt-Rosen, O.; McLean, G.W.; Xiong, Y.; Meyerson, M.; Kim, S.K. Menin Regulates Pancreatic Islet Growth by Promoting Histone Methylation and Expression of Genes Encoding P27Kip1 and P18INK4c. Proc. Natl. Acad. Sci. USA 2005, 102, 14659–14664. [Google Scholar] [CrossRef]
- Milne, T.A.; Hughes, C.M.; Lloyd, R.; Yang, Z.; Rozenblatt-Rosen, O.; Dou, Y.; Schnepp, R.W.; Krankel, C.; LiVolsi, V.A.; Gibbs, D.; et al. Menin and MLL Cooperatively Regulate Expression of Cyclin-Dependent Kinase Inhibitors. Proc. Natl. Acad. Sci. USA 2005, 102, 749–754. [Google Scholar] [CrossRef]
- Schnepp, R.W.; Chen, Y.-X.; Wang, H.; Cash, T.; Silva, A.; Diehl, J.A.; Brown, E.; Hua, X. Mutation of Tumor Suppressor Gene Men1 Acutely Enhances Proliferation of Pancreatic Islet Cells. Cancer Res. 2006, 66, 5707–5715. [Google Scholar] [CrossRef]
- Schnepp, R.W.; Mao, H.; Sykes, S.M.; Zong, W.X.; Silva, A.; La, P.; Hua, X. Menin Induces Apoptosis in Murine Embryonic Fibroblasts. J. Biol. Chem. 2004, 279, 10685–10691. [Google Scholar] [CrossRef]
- Lindsten, T.; Ross, A.J.; King, A.; Zong, W.X.; Rathmell, J.C.; Shiels, H.A.; Ulrich, E.; Waymire, K.G.; Mahar, P.; Frauwirth, K.; et al. The Combined Functions of Proapoptotic Bcl-2 Family Members Bak and Bax Are Essential for Normal Development of Multiple Tissues. Mol. Cell 2000, 6, 1389–1399. [Google Scholar] [CrossRef]
- Yeh, Y.; Guo, Q.; Connelly, Z.; Cheng, S.; Yang, S.; Prieto-Dominguez, N.; Yu, X. Wnt/Beta-Catenin Signaling and Prostate Cancer Therapy Resistance. Adv. Exp. Med. Biol. 2019, 1210, 351–378. [Google Scholar] [CrossRef]
- Kim, B.; Song, T.Y.; Jung, K.Y.; Kim, S.G.; Cho, E.J. Direct Interaction of Menin Leads to Ubiquitin-Proteasomal Degradation of β-Catenin. Biochem. Biophys. Res. Commun. 2017, 492, 128–134. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).