Administration of Very Low Doses of Estradiol Modulates the LH Response to a GnRH Bolus and the LH and Cortisol Responses to Naloxone Infusion in Patients with Functional Hypothalamic Amenorrhea (FHA): A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assay
2.3. Instantaneous Secretory Rates (ISR) Computation for the GnRH Stimulation Test
2.4. Statistical Analysis
3. Results
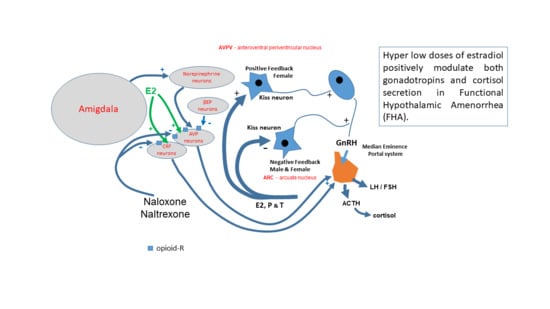
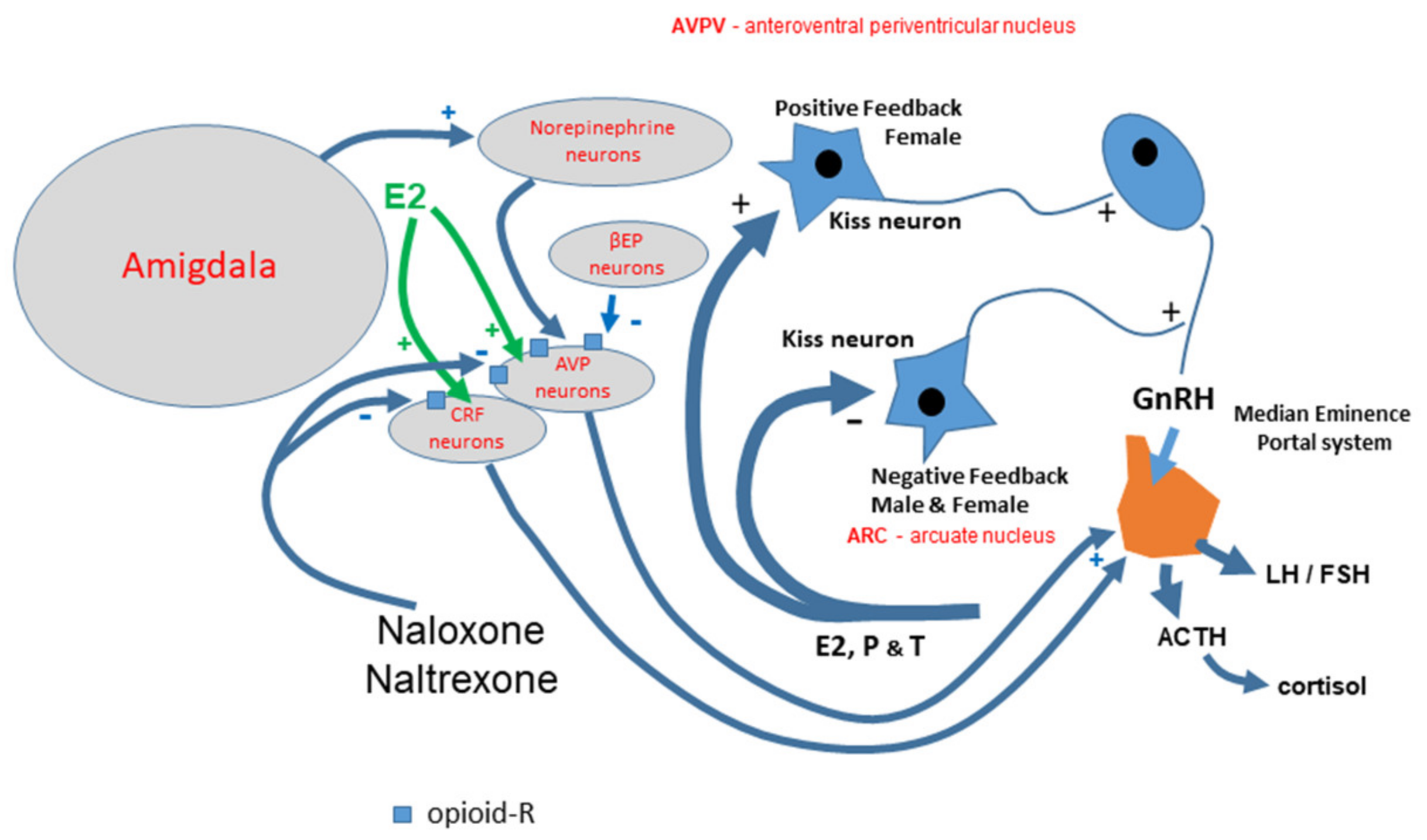
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Genazzani, A.D. Neuroendocrine aspects of amenorrhea related to stress. Pediatr. Endocrinol. Rev. 2005, 2, 661–668. [Google Scholar] [PubMed]
- Meczekalski, B.; Podfigurna-Stopa, A.; Warenik-Szymankiewicz, A.; Genazzani, A.R. Functional hypothalamic amenorrhea: Current view on neuroendocrine aberrations. Gynecol. Endocrinol. 2008, 24, 4–11. [Google Scholar] [CrossRef]
- Berga, S.L.; Mortola, S.F.; Girton, L.; Suh, B.; Laughlin, G.; Pham, P.; Yen, S.S.C. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 1989, 68, 301–308. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petraglia, F.; Volpe, A.; Genazzani, A.R. Hypothalamic amenorrhea: Neuroendocrine mechanisms/stress-induced anomalies. Assist. Reprod. Technol. Androl. 1997, 9, 1–13. [Google Scholar]
- Genazzani, A.D.; Despini, G.; Chierchia, E.; Benedetti, C.; Prati, A. Pharmacological and integrative treatment of stress-induced hypothalamic amenorrhea. In Frontiers in Gynecological Endocrinology; ISGE series; Genazzani, A.R., Tarlatzis, B., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 3, Chapter 9; pp. 69–84. [Google Scholar]
- Yen, S.S.C. Effects of lifestyle and body composition on the ovary. Endocrinol. Metab. Clin. N. Am. 1998, 27, 915–926. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Gastaldi, M.; Volpe, A.; Petraglia, F.; Genazzani, A. Spontaneous episodic release of adenohypophyseal hormones in hypothalamic amenorrhea. Gynecol. Endocrinol. 1995, 9, 325–334. [Google Scholar] [CrossRef]
- Meczekalski, B.; Podfigurna-Stopa, A.; Genazzani, A.R. Why kisspeptin is such important for reproduction? Gynecol. Endocrinol. 2011, 27, 8–13. [Google Scholar] [CrossRef]
- De Nicolao, G.; Liberati, D.; Veldhuis, J.D.; Sartorio, A. LH and FSH secretory responses to GnRH in normal individuals: A nonparametric deconvolution approach. Eur. J. Endocrinol. 1999, 141, 245–256. [Google Scholar]
- Genazzani, A.D.; Petraglia, F.; Benatti, R.; Montanini, V.; Algeri, I.; Volpe, A.; Genazzani, A.R. Luteinizing hormone (LH) secretory burst duration is independent from LH, prolactin, or gonadal steroid plasma levels in amenorrheic women. J. Clin. Endocrinol. Metab. 1991, 72, 1220–1225. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Petraglia, F.; Fabbri, G.; Monzani, A.; Montanini, V.; Genazzani, A.R. Evidence of luteinizing hormone secretion in hypothalamic amenorrhea associated with weight loss. Fertil. Steril. 1990, 54, 222–226. [Google Scholar] [CrossRef]
- Dobson, H.; Ghuman, S.; Prabhakar, S.; Smith, R. A conceptual model of the influence of stress on female reproduction. Reprod. Cambr. 2003, 125, 151–163. [Google Scholar]
- Klein, D.A.; Paradise, S.L.; Reeder, R.M. Amenorrhea: A Systematic Approach to Diagnosis and Management. Am. Fam. Physician 2019, 100, 39–48. [Google Scholar]
- Meczekalski, B.; Katulski, K.; Czyzyk, A.; Podfigurna-Stopa, A.; Maciejewska-Jeske, M. Functional hypothalamic amenorrhea and its influence on women’s health. J. Endocrinol. Investig. 2014, 37, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Gibson, M.E.S.; Fleming, N.; Zuijdwijk, C.; Dumont, T. Where Have the Periods Gone? The Evaluation and Management of Functional Hypothalamic Amenorrhea. J. Clin. Res. Pediatr. Endocrinol. 2020, 12 (Suppl. 1), 18. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Podfigurna-Stopa, A.; Czyzyk, A.; Katulski, K.; Prati, A.; Despini, G.; Angioni, S.; Simoncini, T.; Meczekalski, B. Short-term estriol administration modulates hypothalamo-pituitary function in patients with functional hypothalamic amenorrhea (FHA). Gynecol. Endocrinol. 2016, 32, 253–257. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Meczekalski, B.; Podfigurna-Stopa, A.; Santagni, S.; Rattighieri, E.; Ricchieri, F.; Chierchia, E.; Simoncini, T. Estriol administration modulates luteinizing hormone secretion in women with functional hypothalamic amenorrhea. Fertil. Steril. 2012, 97, 483–488. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1995. [Google Scholar]
- Genazzani, A.D.; Petraglia, F.; Pianazzi, F.; Volpogni, C.; Genazzani, A.R. The concomitant release of androstenedione with cortisol and luteinizing hormone pulsatile releases distinguishes adrenal from ovarian hyperandrogenism. Gynecol. Endocrinol. 1993, 7, 33–41. [Google Scholar] [CrossRef]
- Oerter, K.E.; Guardabasso, V.; Rodbard, D. Detection and characterization of peaks and estimation of instantaneous secretory rate for episodic pulsatile hormone secretion. Comput. Biomed. Res. 1986, 19, 170–191. [Google Scholar] [CrossRef]
- Genazzani, A.D. Application of peak-detection programs to clinical data. In Computers in Endocrinology: Recent Advances; Guardabasso, V., Rodbard, D., Forti, G., Eds.; Raven Press: New York, NY, USA, 1990; pp. 71–82. [Google Scholar]
- Genazzani, A.D.; Rodbard, D. Evaluation of methods for detection of pulsatile hormone secretion: Sensitivity vs. specificity. Acta Endocrinol. (Cph.) 1991, 124, 295–306. [Google Scholar]
- Genazzani, A.D.; Rodbard, D.; Forti, G.; Petraglia, F.; Baraghini, G.F.; Genazzani, A.R. Estimation of instantaneous secretory rate of luteinizing hormone in woman during the menstrual cycle and in man. Clin. Endocrinol. 1990, 32, 573–581. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Fraioli, F.; Rogol, A.G.; Dufau, M.L. Metabolic clearance of biologically active luteinizing hormone in man. J. Clin. Investig. 1986, 77, 1122–1128. [Google Scholar] [CrossRef]
- Gallinelli, A.; Matteo, M.L.; Volpe, A.; Facchinetti, F. Autonomic and neuroendocrine responses to stress in patients with functional hypothalamic secondary amenorrhea. Fertil. Steril. 2000, 73, 812–816. [Google Scholar] [CrossRef]
- Facchinetti, F.; Fava, M.; Fioroni, L.; Genazzani, A.D.; Genazzani, A.R. Stressful life events and affective disorders inhibit pulsatile LH secretion in hypothalamic amenorrhea. Psychoneuroendocrinology 1993, 18, 397–404. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Santagni, S.; Chierchia, E.; Rattighieri, E.; Campedelli, A.; Prati, A.; Ricchieri, F.; Simoncini, T. Estimation of instantaneous rates and intrinsic characteristics of luteinizing hormone secretion in women with Kallmann syndrome before and after estriol administration. Reprod. Biol. 2011, 3, 284–293. [Google Scholar] [CrossRef]
- Terasawa, E.; Kurian, J.R.; Guerriero, K.A.; Kenealy, B.P.; Hutz, E.D.; Keen, K.L. Recent discoveries on the control of gonadotrophin-releasing hormone neurones in nonhuman primates. J. Neuroendocrinol. 2010, 22, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Genazzani, A.R.; Facchinetti, F.; De Leo, V.; Picciolini, E.; Franchi, F.; Parrini, D.; Kicovic, P.M. Effect of epimestrol on gonadotropin and prolactin plasma levels and response to luteinizing hormone-releasing hormone/thyrotropin-releasing hormone in secondary amenorrhea and oligomenorrhea. Fertil. Steril. 1978, 30, 654–660. [Google Scholar] [CrossRef]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin Signaling in the Brain. Endocr. Rev. 2009, 30, 713–743. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Petraglia, F. Opioid Control of Luteinizing Hormone Secretion in Humans. J. Steroid Biochem. 1989, 33, 751–755. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Menozzi, R.; Del Rio, G.; Luisi, S.; Petraglia, F.; Genazzani, A.R. Acute infusion of naloxone, an opioid receptor antagonist, does not modify serum leptin concentrations in amenorrheic and healthy women. Fertil. Steril. 1998, 70, 924–926. [Google Scholar] [CrossRef]
- Nappi, R.E.; Petraglia, F.; Genazzani, A.D.; D’Ambrogio, G.; Zara, C.; Genazzani, A.R. Hypothalamic amenorrhea: Evidence for a central derangement of hypothalamic-pituitary-adrenal cortex axis activity. Fertil. Steril. 1993, 59, 571–576. [Google Scholar] [CrossRef]
- Meczekalski, B.; Tonetti, A.; Monteleone, P.; Bernardi, F.; Luisi, S.; Stomati, M.; Luisi, M.; Petraglia, F.; Genazzani, A.R. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropinreleasing hormone test. Eur. J. Endocrinol. 2000, 142, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Lovallo, W.R.; Acheson, A.; Vincent, A.S.; Sorocco, K.H.; Cohoon, A.J. Early life adversity diminishes the cortisol response to opioid blockade in women: Studies from the Family Health Patterns Project. PLoS ONE 2018, 13, e0205723. [Google Scholar] [CrossRef]
- De Kloet, E.R. Hormones and the stressed brain. Ann. N. Y. Acad. Sci. 2004, 1018, 1–15. [Google Scholar] [CrossRef]


| Patient sn = 17 | LH mUI/mL | FSH mUI/mL | PRL ng/mL | E2 pg/mL | P ng/mL | Cortisol µg/100 mL | T ng/mL | A ng/mL | Insulin µUI/mL |
|---|---|---|---|---|---|---|---|---|---|
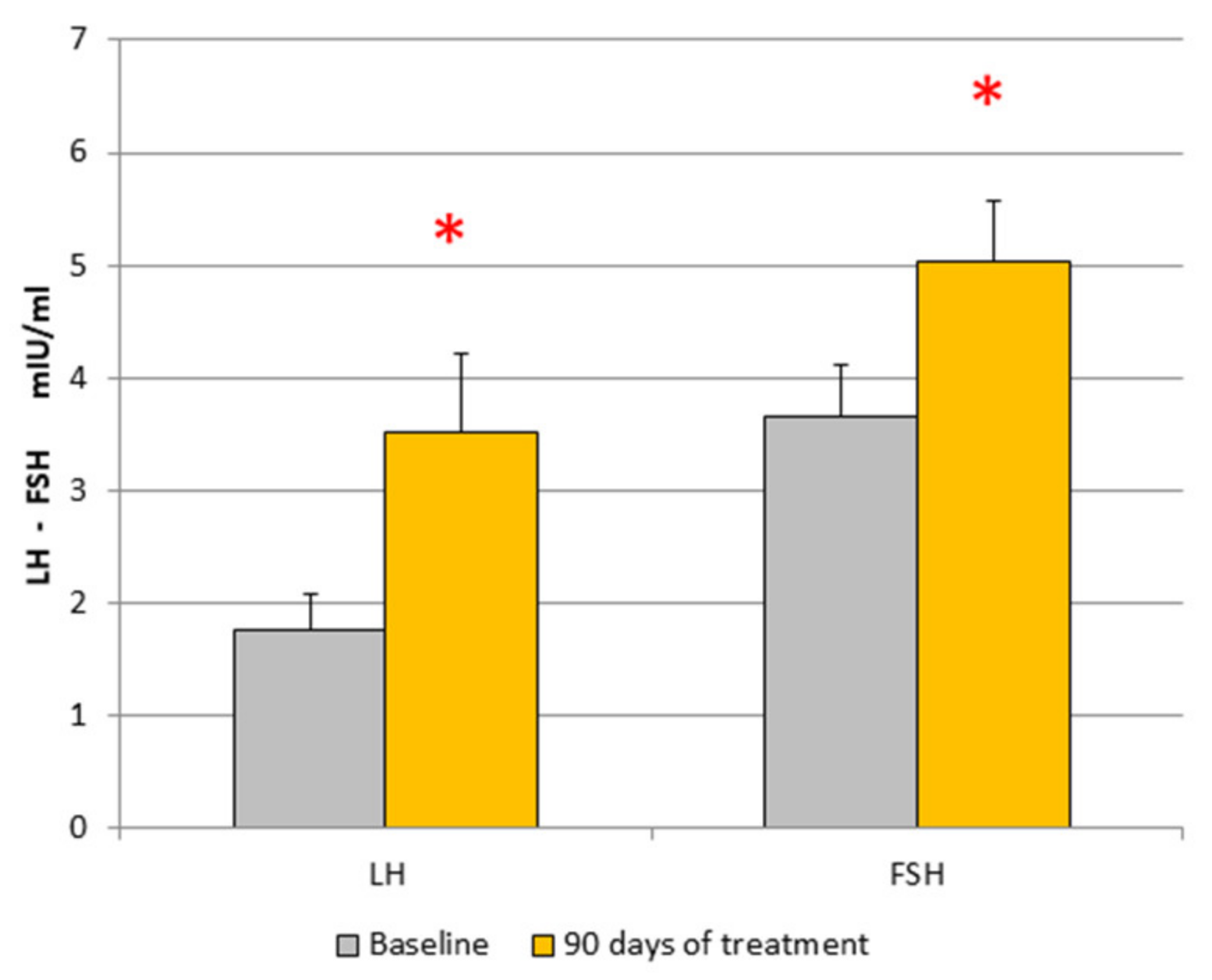
| Baseline | 1.85 ± 0.32 | 3.44 ± 0.43 | 11.99 ± 1.86 | 33.46 ± 8.05 | 0.46 ± 0.06 | 15.9 ± 3.5 | 19 ± 2.1 | 190.8 ± 10.4 | 2.86 ± 0.45 |
| After treatment | 3.52 ± 0.7 * | 5.02 ± 0.54 * | 10.8 ± 1.9 | 28.67 ± 4.9 | 0.85 ± 0.34 | 16.1 ± 2.9 | 28.3 ± 13 | 214 ± 29.76 | 3.78 ± 0.7 |
| LH Pulse After GnRH Test | Amplitude (Plasma Concentration) mIU/mL | Amplitude (ISR) mIU/mL |
|---|---|---|
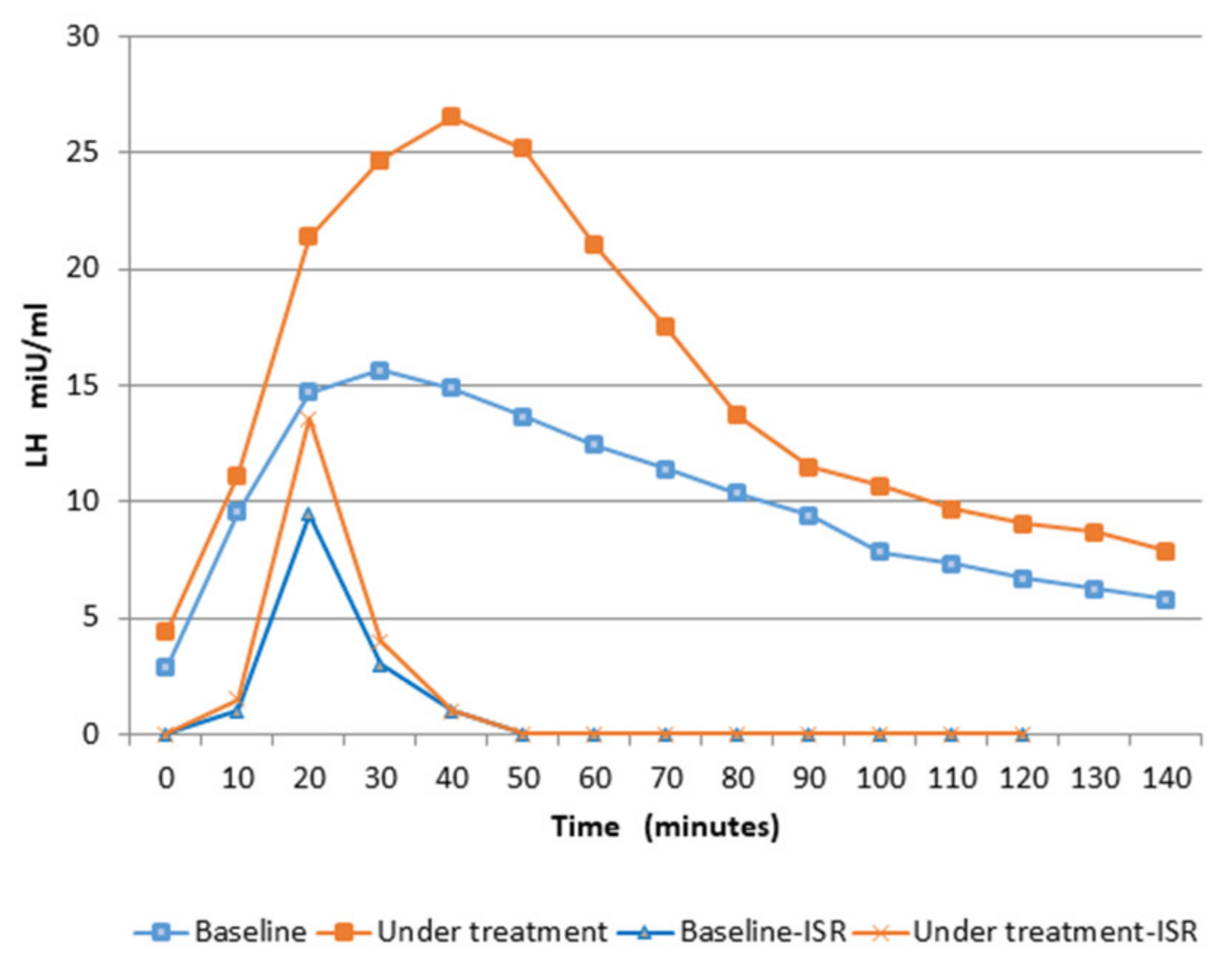
| Baseline | 15.2 ± 1.6 | 9.5 ± 0.9 $ |
| After 12 weeks of treatment | 24.6 ± 5.7 * | 13.5 ± 2.4 * $ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genazzani, A.D.; Despini, G.; Prati, A.; Manzo, A.; Petrillo, T.; Tomatis, V.; Giannini, A.; Simoncini, T. Administration of Very Low Doses of Estradiol Modulates the LH Response to a GnRH Bolus and the LH and Cortisol Responses to Naloxone Infusion in Patients with Functional Hypothalamic Amenorrhea (FHA): A Pilot Study. Endocrines 2020, 1, 35-45. https://doi.org/10.3390/endocrines1010004
Genazzani AD, Despini G, Prati A, Manzo A, Petrillo T, Tomatis V, Giannini A, Simoncini T. Administration of Very Low Doses of Estradiol Modulates the LH Response to a GnRH Bolus and the LH and Cortisol Responses to Naloxone Infusion in Patients with Functional Hypothalamic Amenorrhea (FHA): A Pilot Study. Endocrines. 2020; 1(1):35-45. https://doi.org/10.3390/endocrines1010004
Chicago/Turabian StyleGenazzani, Alessandro D., Giulia Despini, Alessia Prati, Alba Manzo, Tabatha Petrillo, Veronica Tomatis, Andrea Giannini, and Tommaso Simoncini. 2020. "Administration of Very Low Doses of Estradiol Modulates the LH Response to a GnRH Bolus and the LH and Cortisol Responses to Naloxone Infusion in Patients with Functional Hypothalamic Amenorrhea (FHA): A Pilot Study" Endocrines 1, no. 1: 35-45. https://doi.org/10.3390/endocrines1010004