Liver Transplantation in the Era of Metabolic Dysfunction–Associated Fatty Liver Disease: Challenges, Ethical Dilemmas, and Future Directions
Abstract
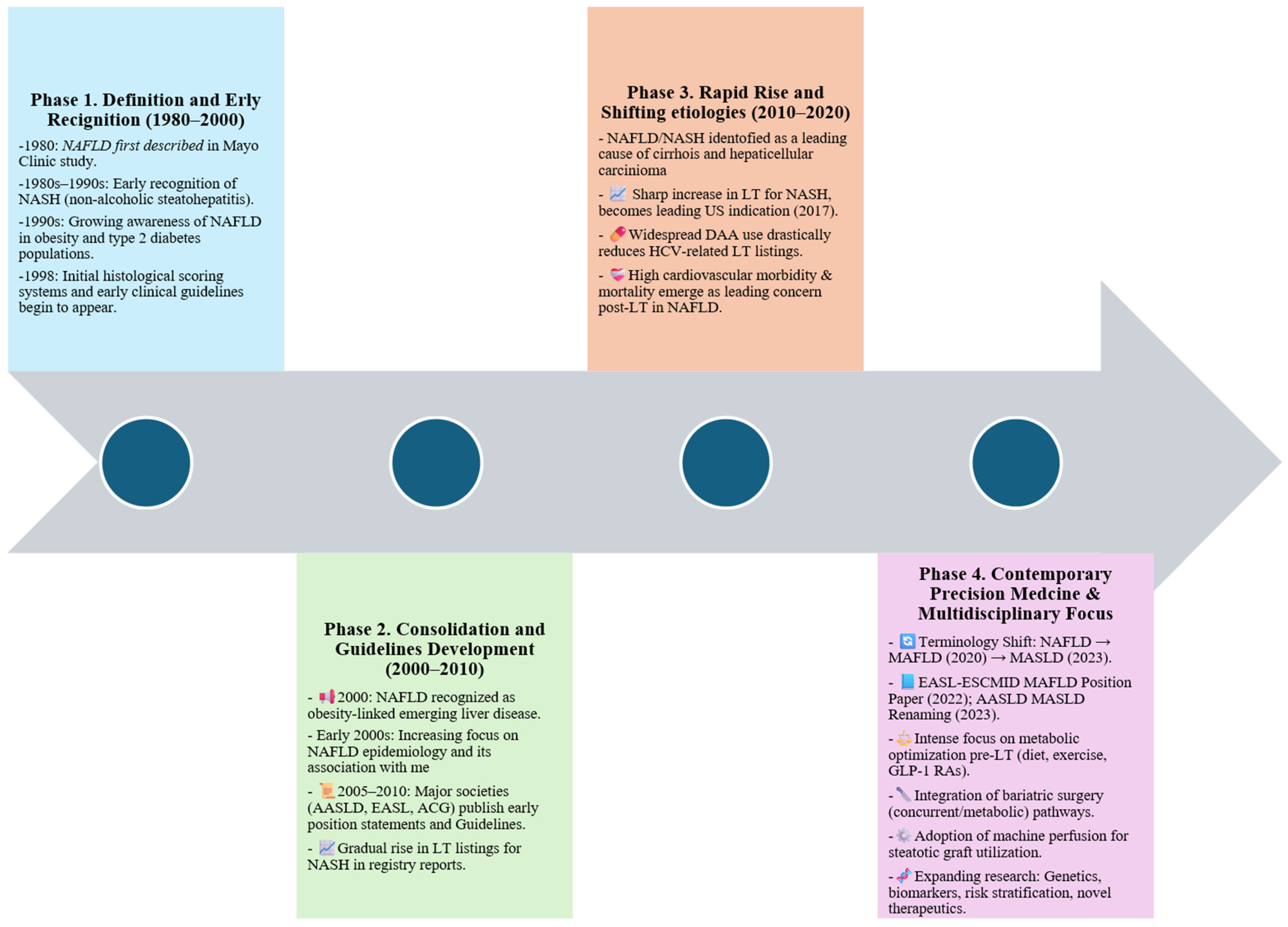
1. Introduction
1.1. From NAFLD to MAFLD/MASLD: Evolving Nomenclature and Diagnostic Criteria
| Feature | NAFLD (1980–2020) | MAFLD (2020–Present) | MASLD (2023–Present) | MetALD (2023–Present) |
|---|---|---|---|---|
| Definition [13,14] | Hepatic steatosis, excluding significant alcohol use and other causes | Hepatic steatosis plus ≥1 of: overweight/obesity, T2DM, or ≥2 metabolic risk factors † | Hepatic steatosis plus ≥1 cardiometabolic risk factor ‡ | MASLD criteria plus moderate alcohol intake |
| Alcohol Threshold * [7,13,15] | <30 g/day (men), <20 g/day (women) | No restriction if metabolic criteria met | ≤140 g/week (women), ≤210 g/week (men) | >140–350 g/week (women), >210–420 g/week (men) |
| Diagnostic Approach [7,13,15] | Diagnosis of exclusion | Positive criteria emphasizing metabolic dysfunction | Positive criteria with broader inclusion | Positive criteria plus specified alcohol intake |
| Key Criticisms [7,13,15,16,17] | Excludes metabolic pathogenesis; stigmatizing term; exclusionary | May underplay role of alcohol | New term may cause confusion; limited criteria for lean individuals | May underestimate alcohol-related liver injury |
1.2. Global Epidemiological Trends
1.3. Rationale for Review: Increasing Burden of MAFLD-Related Liver Failure and Transplantation Needs
1.4. Literature Search and Selection
2. Epidemiology and Burden of MAFLD in the Transplant Setting
2.1. Rising Impact of MAFLD in Cirrhosis and End-Stage Liver Disease
2.2. MAFLD as an Emerging Indication for LT
| Parameter | MAFLD | Alcohol-Related Liver Disease (ALD) | Hepatitis C Virus (HCV) | Other (e.g., Autoimmune, Cholestatic) |
|---|---|---|---|---|
| Primary Risk Factors | Obesity, T2DM, dyslipidemia, metabolic syndrome [12,13] | Chronic alcohol use [40] | Chronic HCV infection [41] | Autoimmune or genetic cholestatic disorders [42] |
| Demographics | Predominantly middle-aged to older adults; higher prevalence in women [17,24] | Younger to middle-aged males [40] | Middle-aged, historically more male-predominant [41] | Variable depending on etiology [43] |
| Trend in LT Listing (2000–2020) ‡ | Rapid increase; leading indication in the US by 2017 [15,44] | Stable to slightly increasing [45] | Sharp decline after DAA * introduction [46,47] | Stable or slowly declining |
| Comorbidities | High burden of cardiovascular disease, obesity, T2DM, metabolic syndrome [25,48] | Alcohol use disorder, malnutrition, and psychiatric comorbidities [49] | Hepatic decompensation; antiviral treatment failure [50] | Disease-specific comorbidities [51] |
| Waitlist Mortality | Higher due to CVD, metabolic syndrome, sarcopenia; often underestimated by MELD [52] | Variable; high risk of decompensation and relapse [53] | Lower in DAA era [50] | Variable by disease |
| MELD at Listing (Median) | Often lower MELD despite significant morbidity; underestimates risk [52] | Moderate MELD; matches severity [49] | Historically higher MELD pre-DAAs; now lower [46] | Variable |
| 1-Year Graft Survival # | 85–90%; comparable to ALD, slightly lower than HCV [54] | ~85%; relapse risk affects outcomes [45] | >90% with viral clearance [46] | ~85–90%, depending on etiology [51] |
| 5-Year Graft Survival # | ~70–75%; limited by cardiovascular mortality and metabolic complications [55] | ~70%; relapse reduces survival [45] | ~80–85% with DAAs [46] | ~75–80%, variable [43] |
| Post-LT Challenges | High recurrence of steatosis, MASH †; increased cardiovascular mortality [56] | Risk of alcohol relapse; infections [45] | Recurrence risk (now low with DAAs) [46] | Variable, depending on underlying disease [51] |
| Special Considerations | Pre-LT metabolic optimization; aggressive cardiovascular risk assessment [57] | Addiction counseling, relapse prevention [40] | Antiviral therapy pre/post-LT [41] | Disease-specific treatments |
2.3. Comparison with Other Etiologies (HCV, ALD)
2.4. Global and Regional Disparities in the Prevalence and Burden of MAFLD
3. Indications and Timing for Liver Transplant in MAFLD
3.1. Indications: Decompensated Cirrhosis, HCC, and ACLF
3.2. Pathogenesis of HCC in MAFLD and Its Implications for Liver Transplantation
3.3. ACLF as an Emerging Indication
3.4. Challenges in Timing Due to Multi-System Comorbidities
3.5. Role and Limitations of the MELD Score in MAFLD
4. Pre-Transplant Evaluation: Unique Considerations in MAFLD
4.1. Diabetes and Metabolic Syndrome
4.2. Cardiovascular Risk Assessment
4.3. Sarcopenia and Frailty
4.4. Obstructive Sleep Apnea and Pulmonary Complications
4.5. Psychosocial and Mental Health Evaluation
4.6. Extrahepatic Malignancy Risk in MAFLD and Implications for Transplant Evaluation
5. Intraoperative and Perioperative Challenges
5.1. Technical Surgical Considerations Due to Obesity
5.2. Anesthetic Risk and Ventilation Difficulties
5.3. Impact on Graft Function and Early Complications
5.4. Role of Novel Surgical Techniques and Technologies
6. Post-Transplant Outcomes in MAFLD Patients
6.1. Patient and Graft Survival Data
6.2. Cancers as Post-Transplant Outcomes
| Outcome | Summary Findings | Key References |
|---|---|---|
| Short-Term Survival (1-Year) * | Generally favorable; some studies report highest 1-year graft survival rates among NASH/MAFLD recipients compared to ALD and HCV | [65] |
| Intermediate-Term Survival (3–5 Years) * | Comparable or slightly better than ALD and HCV; cardiovascular mortality remains a major concern | [38,153] |
| Long-Term Survival (>5 Years) * | Outcomes often limited by cardiovascular events and metabolic complications rather than graft failure | [154,155] |
| Recurrence of MAFLD/MASLD † | High rates: steatosis ~80% at 5 years; steatohepatitis ~60.3%; progression to advanced fibrosis in ~20% | [163] |
| Major Adverse Cardiovascular Events (MACE) | Significant contributor to late mortality; strongly associated with pre- and post-transplant metabolic syndrome | [164,165] |
| New-Onset Diabetes (NODAT) ‡ | Common, driven by pre-existing insulin resistance and immunosuppressive therapy | [166,167] |
| De Novo Malignancies | Increased incidence of gastrointestinal and hormone-related cancers; linked to metabolic dysregulation and immunosuppression; mTOR/MMF-based regimens and lifestyle changes may reduce risk | [135,156,157,158,159,160,161,162] |
| Quality of Life | Significant improvement post-LT, but physical function may remain lower than general population; comorbidities impact outcomes | [168,169] |
6.3. Recurrence of MAFLD Post-Transplant
6.4. Long-Term Metabolic Complications
6.5. Quality of Life (QoL) and Functional Status
7. Immunosuppression Management and MAFLD
7.1. Metabolic Consequences of Immunosuppressants
- Corticosteroids are highly effective but strongly associated with post-transplant weight gain, insulin resistance, hypertension, and dyslipidemia [181,182,183,184]. Early withdrawal or maintenance at low doses reduces these complications while preserving graft integrity, especially in tacrolimus-based regimens [185,186].
- Calcineurin inhibitors (CNIs) such as tacrolimus and cyclosporine remain the backbone of most protocols. Tacrolimus is more diabetogenic, increasing the risk of NODAT, whereas cyclosporine more prominently disrupts lipid metabolism [166,181,187,188]. Both agents contribute to hypertension and nephrotoxicity.
7.2. Tailored Immunosuppression Strategies
- Incorporation of mTOR inhibitors into CNI-based regimens may allow lower CNI exposure, potentially improving blood pressure and kidney function [194]. Their antiproliferative properties may also lower HCC recurrence and slow fibrosis, though careful monitoring for hyperlipidemia and wound complications is essential [193,194]. However, this combination is not without drawbacks. Co-administration of CNIs and mTOR inhibitors has been associated with an increased risk of adverse events, including delayed wound healing, proteinuria, oral ulcers, and dyslipidemia. Furthermore, some studies suggest a potential rise in acute rejection rates and nephrotoxicity when dosing is not properly balanced [195,196]. These limitations underscore the need for careful monitoring and individualized risk-benefit assessment when implementing dual immunosuppressive regimens.
8. Allocation and Ethical Considerations
8.1. Equity in Access: Impact of Obesity, Age, and Comorbidities
8.2. Allocation Policy Debates: BMI and Transplant Eligibility
8.3. Disparities in Access and Outcomes
8.4. Role of Pre-Transplant Interventions in Shaping Allocation Decisions
9. Future Directions and Emerging Research
9.1. Role of Biomarkers and Non-Invasive Testing Pre- and Post-Transplant
9.2. Weight Loss Therapies and Metabolic Interventions Pre-Transplant
9.3. Machine Perfusion and Marginal Grafts in MAFLD Patients
9.4. Genetics and Precision Medicine Approaches
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noureddin, M.; Wei, L.; Castera, L.; Tsochatzis, E.A. Embracing Change: From Nonalcoholic Fatty Liver Disease to Metabolic Dysfunction-Associated Steatotic Liver Disease Under the Steatotic Liver Disease Umbrella. Clin. Gastroenterol. Hepatol. 2024, 22, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Perseghin, G. From NAFLD to MAFLD and MASLD: A tale of alcohol, stigma and metabolic dysfunction. Metab. Target Organ Damage 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Kaya, E.; Yilmaz, Y. Deciphering the implications of MAFLD and MASLD definitions in the NAFLD population: Results from a single-center biopsy study. Chin. Med. J. 2024, 137, 616–618. [Google Scholar] [CrossRef]
- Zhou, X.D.; Targher, G.; Byrne, C.D.; Shapiro, M.D.; Chen, L.L.; Zheng, M.H. Metabolic dysfunction-associated fatty liver disease: Bridging cardiology and hepatology. Cardiol. Plus 2024, 9, 275–282. [Google Scholar] [CrossRef]
- Eslam, M.; Alkhouri, N.; Vajro, P.; Baumann, U.; Weiss, R.; Socha, P.; Marcus, C.; Lee, W.S.; Kelly, D.; Porta, G.; et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: An international expert consensus statement. Lancet Gastroenterol. Hepatol. 2021, 6, 864–873. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; on behalf of theInternational Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Alharthi, J.; Gastaldelli, A.; Cua, I.H.; Ghazinian, H.; Eslam, M. Metabolic dysfunction-associated fatty liver disease: A year in review. Curr. Opin. Gastroenterol. 2022, 38, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Canivet, C.M.; Boursier, J.; Loomba, R. New Nomenclature for Nonalcoholic Fatty Liver Disease: Understanding Metabolic Dysfunction-Associated Steatotic Liver Disease, Metabolic Dysfunction- and Alcohol-Associated Liver Disease, and Their Implications in Clinical Practice. Semin. Liver Dis. 2024, 44, 035–042. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.; Ghazinyan, H.; Alboraie, M.; Al Khatry, M.; Desalegn, H.; Al-Ali, F.; El-Shabrawi, M.H.; Ocama, P.; Derbala, M.; Barakat, S.; et al. Joint position statement from the Middle East and North Africa and sub-Saharan Africa on continuing to endorse the MAFLD definition. J. Hepatol. 2024, 80, e194–e197. [Google Scholar] [CrossRef]
- Kaewdech, A.; Sripongpun, P. Navigating the Nomenclature of Liver Steatosis: Transitioning from NAFLD to MAFLD and MASLD—Understanding Affinities and Differences. Siriraj Med. J. 2024, 76, 234–243. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Ott, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Ekstedt, M.; Wong, G.L.H.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef]
- Hsu, C.L.; Loomba, R. From NAFLD to MASLD: Implications of the new nomenclature for preclinical and clinical research. Nat. Metab. 2024, 6, 600–602. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of Metabolic Dysfunction Associated Steatotic Liver Disease. Clin. Mol. Hepatol. 2024, 31, 32–50. [Google Scholar] [CrossRef]
- van Erpecum, K.J.; van Kleef, L.A.; Beuers, U.; de Knegt, R.J. The new international nomenclature for steatotic liver disease: One step forward towards enhanced awareness for healthier life. Eur. J. Intern. Med. 2023, 117, 1–2. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Suparan, K.; Diaz, L.A.; Fallon, M.B.; Chen, V.L.; Namsathimaphorn, K.; Rakwong, K.; Inkongngam, T.; Kaeosri, C.; Kalligeros, M.; et al. The Rising Global Burden of MASLD and Other Metabolic Diseases (2000–2021). United Eur. Gastroenterol. J. 2025, 13, 1141–1154. [Google Scholar] [CrossRef]
- Li, M.; Xie, W. Are there all-cause mortality differences between metabolic dysfunction-associated steatotic liver disease subtypes? J. Hepatol. 2024, 80, e53–e54. [Google Scholar] [CrossRef]
- Kalligeros, M.; Henry, L.; Younossi, Z.M. Metabolic dysfunction-associated steatotic liver disease and its link to cancer. Metabolism 2024, 160, 156004. [Google Scholar] [CrossRef]
- Wentworth, B.J. Metabolic dysfunction-associated steatotic liver disease throughout the liver transplant cycle: A comprehensive review. Metab. Target Organ Damage 2024, 4, 2. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Zezos, P.; Renner, E.L. Liver transplantation and non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 15532–15538. [Google Scholar] [CrossRef]
- Burra, P.; Becchetti, C.; Germani, G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020, 2, 100192. [Google Scholar] [CrossRef]
- Gill, M.G.; Majumdar, A. Metabolic associated fatty liver disease: Addressing a new era in liver transplantation. World J. Hepatol. 2020, 12, 1168–1181. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Kumar, R.; Priyadarshi, R.N.; Anand, U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J. Clin. Transl. Hepatol. 2019, 8, 76–86. [Google Scholar] [CrossRef]
- Rodas, F.V.; Shankar, N. NAFLD: A pretransplant and post-transplant conundrum. Clin. Liver Dis. 2023, 21, 93–98. [Google Scholar] [CrossRef]
- Patel, Y.A.; Berg, C.L.; Moylan, C.A. Nonalcoholic Fatty Liver Disease: Key Considerations Before and After Liver Transplantation. Dig. Dis. Sci. 2016, 61, 1406–1416. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 204201882211455. [Google Scholar] [CrossRef]
- Habibullah, M.; Jemmieh, K.; Ouda, A.; Haider, M.Z.; Malki, M.I.; Elzouki, A.N. Metabolic-associated fatty liver disease: A selective review of pathogenesis, diagnostic approaches, and therapeutic strategies. Front. Med. 2024, 11, 1291501. [Google Scholar] [CrossRef]
- Yang, R.; Jin, Q.; Fan, J. Metabolic dysfunction-associated fatty liver disease: From basic research to clinical application. Chin. Med. J. 2022, 135, 1138. [Google Scholar] [CrossRef]
- Owrangi, S.; Paik, J.M.; Golabi, P.; de Avila, L.; Hashida, R.; Younossi, Z.M. Prevalence and Mortality of Cirrhosis Related Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Available SSRN 2024. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, X.; Li, G.; Wong, G.L.H.; Wong, V.W.S. Epidemiology and Clinical Outcomes of Metabolic (Dysfunction)-associated Fatty Liver Disease. J. Clin. Transl. Hepatol. 2021, 9, 972. [Google Scholar] [CrossRef]
- Dayal, U.; Soni, U.; Bansal, S.; Aggarwal, K.; Chennupati, C.; Kanagala, S.G.; Gupta, V.; Munjal, R.S.; Jain, R. MAFLD: Exploring the Systemic Effects Beyond Liver. J. Community Hosp. Intern. Med. Perspect. 2025, 15, 8–48. [Google Scholar] [CrossRef]
- Zheng, H.; Sechi, L.A.; Navarese, E.P.; Casu, G.; Vidili, G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: A comprehensive review. Cardiovasc. Diabetol. 2024, 23, 346. [Google Scholar] [CrossRef]
- Sato-Espinoza, K.; Chotiprasidhi, P.; Liza, E.; Placido-Damian, Z.; Diaz-Ferrer, J. Evolution of liver transplantation in the metabolic dysfunction-associated steatotic liver disease era: Tracking impact through time. World J. Transplant. 2024, 14, 98718. [Google Scholar] [CrossRef]
- Wong, R.J. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab. Target Organ Damage 2024, 4, 35. [Google Scholar] [CrossRef]
- Thursz, M.; Gual, A.; Lackner, C.; Mathurin, P.; Moreno, C.; Spahr, L.; Sterneck, M.; Cortez-Pinto, H. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Aronsohn, A.; Price, J.; Re, V.L.; the American Association for the Study of Liver Diseases–Infectious Diseases Society of America HCV Guidance Panel; Heald, J.; Demisashi, G.; Durzy, E.; Davis-Owino, A.; Tynes, S. Hepatitis C Guidance 2023 Update: American Association for the Study of Liver Diseases—Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2023, ciad319. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, J.-H.; Kim, S.-E.; Jung, J.H.; Jang, M.-K.; Park, S.-H.; Lee, M.-S.; Kim, H.-S.; Suk, K.T.; Kim, D.J. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics. Biomedicines 2022, 10, 1288. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Ronca, V.; Ebadi, M.; Hansen, B.E.; Hirschfield, G.; Elwir, S.; Alsaed, M.; Milkiewicz, P.; Janik, M.K.; Marschall, H.-U.; et al. Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J. Hepatol. 2022, 77, 84–97. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Lee, B.P.; Vittinghoff, E.; Dodge, J.L.; Cullaro, G.; Terrault, N.A. National Trends and Long-term Outcomes of Liver Transplant for Alcohol-Associated Liver Disease in the United States. JAMA Intern. Med. 2019, 179, 340–348. [Google Scholar] [CrossRef]
- Belli, L.S.; Perricone, G.; Adam, R.; Cortesi, P.A.; Strazzabosco, M.; Facchetti, R.; Karam, V.; Salizzoni, M.; Andujar, R.L.; Fondevila, C.; et al. Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J. Hepatol. 2018, 69, 810–817. [Google Scholar] [CrossRef]
- Young, K.; Liu, B.; Bhuket, T.; Gish, R.G.; Wong, R.J. Improved liver transplant waitlist mortality and lower risk of disease progression among chronic hepatitis C patients awaiting liver transplantation after the introduction of direct-acting antiviral therapies in the United States. J. Viral Hepat. 2019, 26, 350–361. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Singal, A.K.; Kodali, S.; Vucovich, L.A.; Darley-Usmar, V.; Schiano, T.D. Diagnosis and Treatment of Alcoholic Hepatitis: A Systematic Review. Alcohol. Clin. Exp. Res. 2016, 40, 1390–1402. [Google Scholar] [CrossRef]
- Flemming, J.A.; Kim, W.R.; Brosgart, C.L.; Terrault, N.A. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2017, 65, 804–812. [Google Scholar] [CrossRef]
- Carbone, M.; Neuberger, J.M. Autoimmune liver disease, autoimmunity and liver transplantation. J. Hepatol. 2014, 60, 210–223. [Google Scholar] [CrossRef]
- Allen, A.M.; Therneau, T.M.; Larson, J.J.; Coward, A.; Somers, V.K.; Kamath, P.S. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology 2018, 67, 1726–1736. [Google Scholar] [CrossRef]
- Sundaram, V.; Jalan, R.; Wu, T.; Volk, M.L.; Asrani, S.K.; Klein, A.S.; Wong, R.J. Factors Associated with Survival of Patients with Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology 2019, 156, 1381–1391.e3. [Google Scholar] [CrossRef]
- Agopian, V.G.; Kaldas, F.M.; Hong, J.C.; Whittaker, M.; Holt, C.; Rana, A.; Ali, Z.; Henrik, P.; Douglas, F.; Hasan, Y.; et al. Liver transplantation for nonalcoholic steatohepatitis: The new epidemic. Ann. Surg. 2012, 256, 624–633. [Google Scholar] [CrossRef]
- Haldar, D.; Kern, B.; Hodson, J.; Armstrong, M.J.; Adam, R.; Berlakovich, G.; Fritz, J.; Feurstein, B.; Popp, W.; Karam, V.; et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J. Hepatol. 2019, 71, 313–322. [Google Scholar] [CrossRef]
- Watt, K.D.S.; Charlton, M.R. Metabolic syndrome and liver transplantation: A review and guide to management. J. Hepatol. 2010, 53, 199–206. [Google Scholar] [CrossRef]
- Laish, I.; Braun, M.; Mor, E.; Sulkes, J.; Harif, Y.; Ari, Z.B. Metabolic syndrome in liver transplant recipients: Prevalence, risk factors, and association with cardiovascular events. Liver Transplant. 2011, 17, 15–22. [Google Scholar] [CrossRef]
- Shi, Y.; Taherifard, E.; Saeed, A.; Saeed, A. MASLD-Related HCC: A Comprehensive Review of the Trends, Pathophysiology, Tumor Microenvironment, Surveillance, and Treatment Options. Curr. Issues Mol. Biol. 2024, 46, 5965–5983. [Google Scholar] [CrossRef]
- Portincasa, P.; Baffy, G. Metabolic dysfunction-associated steatotic liver disease: Evolution of the final terminology. Eur. J. Intern. Med. 2024, 124, 35–39. [Google Scholar] [CrossRef]
- Savino, A.; Loglio, A.; Neri, F.; Camagni, S.; Pasulo, L.; Lucà, M.G.; Trevisan, R.; Fagiuoli, S.; Viganò, M. Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) after Liver Transplantation: A Narrative Review of an Emerging Issue. J. Clin. Med. 2024, 13, 3871. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Watt, K.D.; VanWagner, L.B.; Verna, E.C.; Berzigotti, A. Evaluation of recipients with significant comorbidity—Patients with cardiovascular disease. J. Hepatol. 2023, 78, 1089–1104. [Google Scholar] [CrossRef]
- Anyane-Yeboa, A.; Stewart, C.A. A Review of Non-Alcoholic Fatty Liver Disease: From Obesity to Liver Transplant. Can. J. Gen. Intern. Med. 2015, 10, 23–28. [Google Scholar] [CrossRef]
- Choudhury, A.; Adali, G.; Kaewdech, A.; Giri, S.; Kumar, R. Liver Transplantation in chronic liver disease and acute on chronic liver disease- Indication, Timing and Practices. J. Clin. Exp. Hepatol. 2024, 14, 101347. [Google Scholar] [CrossRef]
- Butt, M.F.; Jalan, R. Review article: Emerging and current management of acute-on-chronic liver failure. Aliment. Pharmacol. Ther. 2023, 58, 774–794. [Google Scholar] [CrossRef]
- Moon, G.; Ajayi, T.; Pan, S.; Chen, S.; Oseini, A.; Houchen, C.W. S1524 A National Database Study: NASH Liver Transplant Has Better 1-Year Post-Transplant Graft Survival in Comparison to AC and HCV-Related Liver Transplants. Am. J. Gastroenterol. 2023, 118, S1153–S1154. [Google Scholar] [CrossRef]
- Vitale, A.; Trapani, S.; Russo, F.P.; Miele, L.; Baroni, G.S.; Marchesini, G.; Burra, P.; Ottoveggio, M.S.; Romagnoli, R.; Martini, S.; et al. Waiting list mortality and 5-year transplant survival benefit of patients with MASLD: An Italian liver transplant registry study. JHEP Rep. 2024, 6, 101147. [Google Scholar] [CrossRef]
- Lv, J.J.; Zhang, Y.C.; Li, X.Y.; Guo, H.; Yang, C.H. The burden of non-alcoholic fatty liver disease among working-age people in the Western Pacific Region, 1990–2019: An age–period–cohort analysis of the Global Burden of Disease study. BMC Public Health 2024, 24, 1852. [Google Scholar] [CrossRef]
- Gulati, R.; Moylan, C.A.; Wilder, J.; Wegermann, K. Racial and ethnic disparities in metabolic dysfunction-associated steatotic liver disease. Metab. Target Organ Damage 2024, 4, 9. [Google Scholar] [CrossRef]
- Chen, H.; Zhan, Y.; Zhang, J.; Cheng, S.; Zhou, Y.; Chen, L.; Zeng, Z. The global, regional, and national burden and trends of NAFLD in 204 countries and territories: An analysis from Global Burden of Disease 2019. JMIR Public Heal. Surveill. 2022, 8, e34809. [Google Scholar] [CrossRef]
- Wang, J.; Du, J.; Wang, M.; Jin, M.; Tang, Z.; Mao, Y. Global, Regional, and National Burden of NAFLD in Youths and Young Adults Aged 15–39 Years, 1990–2021, Its Attributable Risk Factors, and Projections to 2035: A Systematic Analysis of the Global Burden of Disease Study 2021. Front. Nutr. 2025, 12, 1509232. [Google Scholar] [CrossRef]
- Herren, O.M.; Gillman, A.S.; Marshall, V.J.; Das, R. Understanding the Changing Landscape of Health Disparities in Chronic Liver Diseases and Liver Cancer. Gastro Hep Adv. 2022, 2, 505–520. [Google Scholar] [CrossRef]
- Kamani, L.; Rahat, A.; Yilmaz, Y. Addressing the looming epidemic of metabolic dysfunction-associated steatotic liver disease in Pakistan: A call for action. Hepatol. Forum 2024, 5, 1–2. [Google Scholar] [CrossRef]
- Allen, A.M.; Pose, E.; Reddy, K.R.; Russo, M.W.; Kamath, P.S. NAFLD Gets Renamed as MASLD: Progress but with Challenges. Gastroenterology 2023, 166, 229–234. [Google Scholar] [CrossRef]
- Romeo, S.; Valenti, L. African genetic ancestry and protection against fatty liver disease. Liver Int. 2022, 42, 2122–2123. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wang, K.; Wang, Z.; Sun, X.; Zhong, L.; Deng, G.; Song, G.; Sun, B.; Peng, Z.; et al. Additive effects of the risk alleles of PNPLA3 and TM6SF2 on non-alcoholic fatty liver disease (NAFLD) in a Chinese population. Front. Genet. 2016, 7, 140. [Google Scholar] [CrossRef]
- Wang, J.; Conti, D.V.; Bogumil, D.; Sheng, X.; Noureddin, M.; Wilkens, L.R.; Le Marchand, L.; Rosen, H.R.; Haiman, C.A.; Setiawan, V.W. Association of Genetic Risk Score with NAFLD in An Ethnically Diverse Cohort. Hepatol. Commun. 2021, 5, 1689–1703. [Google Scholar] [CrossRef]
- Alharthi, J.; Bayoumi, A.; Thabet, K.; Pan, Z.; Gloss, B.S.; Latchoumanin, O.; Lundberg, M.; Twine, N.A.; McLeod, D.; Alenizi, S.; et al. A metabolic associated fatty liver disease risk variant in MBOAT7 regulates toll like receptor induced outcomes. Nat. Commun. 2022, 13, 7430. [Google Scholar] [CrossRef]
- Pan, Z.; El Sharkway, R.; Bayoumi, A.; Metwally, M.; Gloss, B.S.; Brink, R.; Lu, D.B.; Liddle, C.; Alqahtani, S.A.; Yu, J.; et al. Inhibition of MERTK reduces organ fibrosis in mouse models of fibrotic disease. Sci. Transl. Med. 2024, 16, eadj0133. [Google Scholar] [CrossRef]
- Phoolchund, A.G.S.; Khakoo, S.I. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers 2024, 16, 259. [Google Scholar] [CrossRef]
- Chrysavgis, L.; Giannakodimos, I.; Diamantopoulou, P.; Cholongitas, E. Non-alcoholic fatty liver disease and hepatocellular carcinoma: Clinical challenges of an intriguing link. J. Gastroenterol. 2022, 28, 310. [Google Scholar] [CrossRef]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e2. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Sapisochin, G.; Bruix, J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 203–217. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021, 161, 1887–1895.e4. [Google Scholar] [CrossRef]
- Ganakumar, V.; Halebidu, T.; Goroshi, M.; Ghatnatti, V. Diagnosis and Management of MASLD: An Metabolic Perspective of a Multisystem Disease. Int. J. Clin. Metab. Diabetes 2024, 1, 45–57. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef]
- Jeeyavudeen, M.S.; Khan, S.K.A.; Fouda, S.; Pappachan, J.M. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J. Gastroenterol. 2023, 29, 126–143. [Google Scholar] [CrossRef]
- Tang, A.S.P.; Tan, C.M.; Ng, C.H.M.; Tan, D.J.H.M.; Zeng, R.M.; Xiao, J.M.; Ong, E.Y.H.M.; Cho, E.M.; Chung, C.M.; Lim, W.S.M.; et al. Impact of Pretransplant Diabetes on Outcomes After Liver Transplantation: An Updated Meta-analysis with Systematic Review. Transplantation 2023, 108, 1157–1165. [Google Scholar] [CrossRef]
- Berkovic, M.C.; Virovic-Jukic, L.; Bilic-Curcic, I.; Mrzljak, A. Post-transplant diabetes mellitus and preexisting liver disease—A bidirectional relationship affecting treatment and management. World J. Gastroenterol. 2020, 26, 2740–2757. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Huttasch, M.; Roden, M.; Kahl, S. Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease? Metabolism 2024, 157, 155937. [Google Scholar] [CrossRef] [PubMed]
- Brosnihan, P.; Luce, M.S.; Yetasook, A.K.; Perez, C.; Scharf, K.R.; Aly, S. Great Debates: Undergoing the Knife versus Pill-Popping—The Comparative Efficacy and Cost-Effectiveness of Bariatric Surgery and GLP-1 Receptor Agonists in the Management of Obesity. Am. Surg. 2025, 91, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Bołdys, A.; Bułdak, Ł.; Nicze, M.; Okopień, B. Liraglutide Reduces Liver Steatosis and Improves Metabolic Indices in Obese Patients Without Diabetes: A 3-Month Prospective Study. Int. J. Mol. Sci. 2025, 26, 5883. [Google Scholar] [CrossRef]
- Wang, M.W.; Lu, L.G. Current Status of Glucagon-like Peptide-1 Receptor Agonists in Metabolic Dysfunction-associated Steatotic Liver Disease: A Clinical Perspective. J. Clin. Transl. Hepatol. 2024, 13, 47–61. [Google Scholar] [CrossRef]
- Njei, B.; Al-Ajlouni, Y.A.; Lemos, S.Y.; Ugwendum, D.; Ameyaw, P.; Njei, L.P.; Boateng, S. Efficacy and Safety of GLP-1 Receptor Agonists in Patients with MASLD: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Authorea, 2024; preprints. [Google Scholar] [CrossRef]
- Gonzalez, H.C.; Myers, D.T.; Venkat, D. Successful Implementation of a Multidisciplinary Weight Loss Program Including GLP1 Receptor Agonists for Liver Transplant Candidates with High Body Mass Index. Transplantation 2024, 108, 2233–2237. [Google Scholar] [CrossRef]
- Ismaiel, A.; Dumitraşcu, D.L. Cardiovascular Risk in Fatty Liver Disease: The Liver-Heart Axis—Literature Review. Front. Med. 2019, 6, 202. [Google Scholar] [CrossRef]
- Platek, A.E.; Szymanska, A. Metabolic dysfunction-associated steatotic liver disease as a cardiovascular risk factor. Clin. Exp. Hepatol. 2023, 9, 187–192. [Google Scholar] [CrossRef]
- Martinez-Perez, S.; McCluskey, S.A.; Davierwala, P.M.; Kalra, S.; Nguyen, E.; Bhat, M.; Borosz, C.; Luzzi, C.; Jaeckel, E.; Neethling, E. Perioperative Cardiovascular Risk Assessment and Management in Liver Transplant Recipients: A Review of the Literature Merging Guidelines and Interventions. J. Cardiothorac. Vasc. Anesthesia 2023, 38, 1015–1030. [Google Scholar] [CrossRef]
- Wray, C.; Findlay, J.Y. Cardiac Evaluation and Management. In Critical Care for Potential Liver Transplant Candidates; Springer: Cham, Switzerland, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Meurer, L.; Vanwagner, L.B. Preexisting Coronary Artery Disease in Liver Transplant Candidates: Risk Factor or Risk Marker? Transplantation 2022, 107, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Nagraj, S.; Peppas, S.; Guerrero, M.G.R.; Kokkinidis, D.G.; Contreras-Yametti, F.I.; Murthy, S.; Jorde, U.P. Cardiac risk stratification of the liver transplant candidate: A comprehensive review. World J. Transplant. 2022, 12, 142–156. [Google Scholar] [CrossRef]
- Doycheva, I.; Izzy, M.; Watt, K.D. Cardiovascular assessment before liver transplantation. In Cardio-Hepatology: Connections Between Hepatic and Cardiovascular Disease; Academic Press: Cambridge, MA, USA, 2023; pp. 309–326. [Google Scholar] [CrossRef]
- Saraswat, V.A.; Kumar, K. Untangling the Web of Malnutrition, Sarcopenia, and Frailty in Chronic Liver Disease. J. Clin. Exp. Hepatol. 2022, 12, 268–271. [Google Scholar] [CrossRef]
- Warner, E.R.; Satapathy, S.K. Sarcopenia in the Cirrhotic Patient: Current Knowledge and Future Directions. J. Clin. Exp. Hepatol. 2023, 13, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, M.; El Sabagh, A.; Mohamed, I.B.; Bhongade, M.; Jalal, P.K.; Hassan, M.M. Frailty in end-stage liver disease: Understanding pathophysiology, tools for assessment, and strategies for management. World J. Gastroenterol. 2023, 29, 6028–6048. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Williams, F.R.; El-sherif, O.; Armstrong, M.J. Sarcopenia in Liver Transplantation: An Update. Curr. Hepatol. Rep. 2020, 19, 128–137. [Google Scholar] [CrossRef]
- Prokopidis, K.; Affronti, M.; Testa, G.D.; Ungar, A.; Cereda, E.; Smith, L.; Pegreffi, F.; Barbagallo, M.; Veronese, N. Sarcopenia increases mortality risk in liver transplantation: A systematic review and meta-analysis. Panminerva Medica 2024, 66, 47–54. [Google Scholar] [CrossRef]
- Sonnenday, C.J. Frailty and Sarcopenia in the Selection of Candidates for Liver Transplantation. In Frailty and Sarcopenia in Cirrhosis: The Basics, the Challenges, and the Future; Springer: Cham, Switzerland, 2020; pp. 161–168. [Google Scholar] [CrossRef]
- Leunis, S.; Vandecruys, M.; Van Craenenbroeck, A.; Cornelissen, V.; Bogaerts, S.; De Smet, S.; Monbaliu, D. Sarcopenia in end-stage liver disease and after liver transplantation. Acta Gastro Enterol. Belg. 2023, 86, 323–334. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, D.; Xu, S.; Zhang, M.; Li, J. Study of the occurrence of metabolic dysfunction-associated fatty liver disease in obstructive sleep apnea hypopnea syndrome and its risk factors. Chin. J. Intern. Med. 2025, 64, 128–133. [Google Scholar] [CrossRef]
- Bettini, S.; Serra, R.; Fabris, R.; Prà, C.D.; Favaretto, F.; Dassie, F.; Duso, C.; Vettor, R.; Busetto, L. Association of obstructive sleep apnea with non-alcoholic fatty liver disease in patients with obesity: An observational study. Eat. Weight. Disord.-Stud. Anorexia Bulim. Obes. 2021, 27, 335–343. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir. Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Olivetti, C.; Rosina, F.; Carbone, G.; Gambino, R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes. Rev. 2013, 14, 417–431. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E.; Polotsky, V.Y. Obstructive sleep apnea and non-alcoholic fatty liver disease: Is the liver another target? Front. Neurol. 2012, 3, 149. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Meng, H.; Li, Y.; Han, T.; Wang, C. Obstructive sleep apnea and liver injury in severely obese patients with nonalcoholic fatty liver disease. Sleep Breath. 2020, 24, 1515–1521. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Lin, P.; Qian, T.; Xu, L. Causal relationships between neuropsychiatric disorders and nonalcoholic fatty liver disease: A bidirectional Mendelian randomization study. BMC Gastroenterol. 2024, 24, 299. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.; Lionis, C.; Kite, C.; Lagojda, L.; Uthman, O.A.; Dallaway, A.; Atkinson, L.; Chaggar, S.S.; Randeva, H.S.; Kyrou, I. Non-alcoholic fatty liver disease and coexisting depression, anxiety and/or stress in adults: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1357664. [Google Scholar] [CrossRef] [PubMed]
- Funuyet-Salas, J.; Martín-Rodríguez, A.; Conrad, R.; Pérez-San-Gregorio, M.Á. Psychological Biomarker Profile in NAFLD/NASH with Advanced Fibrosis. In NAFLD and NASH: Biomarkers in Detection, Diagnosis and Monitoring; Springer: Cham, Switzerland, 2020; pp. 205–223. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Asai, T.; Tsuji, A.; Matsuda, S. Metabolic Associated Fatty Liver Disease as a Risk Factor for the Development of Central Nervous System Disorders. Livers 2023, 3, 21–32. [Google Scholar] [CrossRef]
- DiMartini, A.F.; Golden, E.; Matz, A.; Dew, M.A.; Crone, C. Post-transplant Psychosocial and Mental Health Care of the Liver Recipient. In Psychosocial Care of End-Stage Organ Disease and Transplant Patients; Springer: Cham, Switzerland, 2019; pp. 181–191. [Google Scholar] [CrossRef]
- Bush, B.A. Psychosocial, emotional, and neuropsychologic factors influencing compliance and liver transplantation outcomes. Curr. Opin. Organ Transplant. 2004, 9, 104–109. [Google Scholar] [CrossRef]
- García-Alanís, M.; Toapanta-Yanchapaxi, L.; Vilatobá, M.; Cruz-Martínez, R.; Contreras, A.; López-Yáñez, S.; Flores-García, N.; Marquéz-Guillén, E.; García-Juárez, I. Psychosocial evaluation for liver transplantation: A brief guide for gastroenterologists. Rev. Gastroenterol. Mex. (Engl. Ed.) 2021, 86, 172–187. [Google Scholar] [CrossRef]
- Bailey, P.; Vergis, N.; Allison, M.; Riddell, A.; Massey, E. Psychosocial Evaluation of Candidates for Solid Organ Transplantation. Transplantation 2021, 105, E292–E302. [Google Scholar] [CrossRef]
- Matthews, L.A.; Lucey, M.R. Psychosocial Evaluation in Liver Transplantation for Patients with Alcohol-Related Liver Disease. Clin. Liver Dis. 2022, 19, 17–20. [Google Scholar] [CrossRef]
- Zanatta, E.; Patron, E.; Benvenuti, S.M.; Pelizzaro, F.; Russo, F.P.; Gambato, M.; Germani, G.; Ferrarese, A.; Zanetto, A.; Battermann, F.; et al. Alcoholic Etiology, Severity of Liver Disease, and Post-Transplant Adherence Are Correlated with Worse Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT) in Liver Transplant Candidates. Stomatology 2024, 13, 3807. [Google Scholar] [CrossRef]
- Wang, R.X.; Lee, J.J.; Mirda, D.; Hao, J.; Goebel, A.M.; Deutsch-Link, S.; Serper, M.; Bittermann, T. Association of psychosocial risk factors and liver transplant evaluation outcomes in metabolic dysfunction-associated steatotic liver disease. Liver Transplant. 2024, 30, 1226–1237. [Google Scholar] [CrossRef]
- Becker, U. The influence of ethanol and liver disease on sex hormones and hepatic estrogen receptors in women. Acta Obstet. Gynecol. Scand. 1994, 73, 437–440. [Google Scholar] [CrossRef]
- Weiss, E.; Kabacam, G.; Gorvin, L.; Spiro, M.; Raptis, D.A.; Keskin, O.; Orloff, S.; Belghiti, J.; the ERAS4OLT.org Working Group. The role of preoperative psychosocial counseling on the improvement of the recipient compliance and speed of recovery after liver transplantation—A systematic review of the literature and expert panel recommendations. Clin. Transplant. 2022, 36, e14632. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Wei, S.; Hao, Y.; Dong, X.; Huang, J.; Huang, K.; Xie, Y.; Liu, H.; Wei, C.; Xu, J.; Huang, W.; et al. The relationship between metabolic dysfunction-associated fatty liver disease and the incidence rate of extrahepatic cancer. Front. Endocrinol. 2023, 14, 985858. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.C.; Lyssiotis, C.A.; Cantley, L.C. Metabolic Reprogramming by the PI3K-Akt-mTOR Pathway in Cancer. Recent Results Cancer Res. 2016, 207, 39–72. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Odden, M.C.; Nguyen, M.H. Statin Use and Reduced Hepatocellular Carcinoma Risk in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 435–444.e6. [Google Scholar] [CrossRef]
- Thuluvath, P.J. Obesity and liver transplantation. World J. Transplant. 2015, 5, 95–101. [Google Scholar] [CrossRef]
- Ahmed, Z.; Khan, M.A.; Vazquez-Montesino, L.M.; Ahmed, A. Bariatric surgery, obesity and liver transplantation. Transl. Gastroenterol. Hepatol. 2021, 7, 25. [Google Scholar] [CrossRef]
- Tejedor-Tejada, J.; Garcia-Pajares, F.; Safadi, R.; Mauriz-Barreiro, V.; Molina, E.; Juan-Casamayor, L.; Fernández-Prada, S.; Helal, A.; Fuentes-Valenzuela, E.; Alonso-Martin, C.; et al. The impact of obesity on postoperative complications and short-term survival after liver transplantation. Eur. J. Gastroenterol. Hepatol. 2023, 35, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Berkovic, M.C.; Šeša, V.; Balen, I.; Lai, Q.; Silovski, H.; Mrzljak, A. Key challenges of post-liver transplant weight management. World J. Transplant. 2024, 14, 95033. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Brown, R.S. Management and Risks Before, During, and After Liver Transplant in Individuals with Obesity. Gastroenterol. Hepatol. 2023, 19, 20. [Google Scholar]
- Vogel, A.S.; Roediger, R.; von Ahrens, D.; Fortune, B.E.; Schwartz, J.M.; Frager, S.; Chacko, K.R.; Tow, C.Y. The Impact of Metabolic Health and Obesity on Liver Transplant Candidates and Recipients. Reprod. Dev. Biol. 2024, 14, 685. [Google Scholar] [CrossRef]
- Jetani, V.; Vaghani, U.; Lakhani, D.; Jogani, V.; Ghevariya, N.; Sanghani, I.; Patel, A. Cardiovascular Considerations in Liver TransplantationA Review of Risks, Diagnostics, and Management. J. Gastroenterol. Dig. Syst. 2024, 8, 1–5. [Google Scholar] [CrossRef]
- Brezeanu, L.N.; Brezeanu, R.C.; Diculescu, M.; Droc, G. Anaesthesia for Liver Transplantation: An Update. J. Crit. Care Med. 2020, 6, 91–100. [Google Scholar] [CrossRef]
- Milliken, D.M.; Davidson, B.R.; Spiro, M.D. Anaesthesia for Liver Transplantation. In Liver Diseases; Springer: Cham, Switzerland, 2020; pp. 757–767. [Google Scholar] [CrossRef]
- Yuan, G.; Li, S.; Liang, P.; Chen, G.; Luo, Y.; Shen, Y.; Hu, X.; Hu, D.; Li, J.; Li, Z. High visceral adipose tissue area is independently associated with early allograft dysfunction in liver transplantation recipients: A propensity score analysis. Insights Imaging 2022, 13, 165. [Google Scholar] [CrossRef]
- Nicolau-Raducu, R.; Cohen, A.J.; Bokhari, A.; Bohorquez, H.; Bruce, D.; Carmody, I.; Bugeaud, E.; Seal, J.; Sonnier, D.; Nossaman, B.; et al. Predictive model and risk factors associated with a revised definition of early allograft dysfunction in liver transplant recipients. Clin. Transplant. 2017, 31, e13097. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Bianco, F.M.; Daskalaki, D.; Gonzalez-Ciccarelli, L.F.; Kim, J.; Benedetti, E. Robotic liver surgery: Technical aspects and review of the literature. HepatoBiliary Surg. Nutr. 2016, 5, 311–321. [Google Scholar] [CrossRef]
- Sandri, G.B.L.; de Werra, E.; Mascianà, G.; Guerra, F.; Spoletini, G.; Lai, Q. The use of robotic surgery in abdominal organ transplantation: A literature review. Clin. Transplant. 2017, 31, e12856. [Google Scholar] [CrossRef]
- Lai, Q.; Ruberto, F.; Pawlik, T.M.; Pugliese, F.; Rossi, M. Use of machine perfusion in livers showing steatosis prior to transplantation: A systematic review. Updat. Surg. 2020, 72, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Banker, A.; Bhatt, N.; Rao, P.S.; Agrawal, P.; Shah, M.; Nayak, M.; Mohanka, R. A Review of Machine Perfusion Strategies in Liver Transplantation. J. Clin. Exp. Hepatol. 2022, 13, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Paklar, N.; Mijic, M.; Filipec-Kanizaj, T. The Outcomes of Liver Transplantation in Severe Metabolic Dysfunction-Associated Steatotic Liver Disease Patients. Biomedicines 2023, 11, 3096. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Husain, M.; Diab, C.; Mangla, K.K.; Shoeb, A.; Lingvay, I.; Tapper, E.B. Cardiovascular disease in patients with metabolic dysfunction-associated steatohepatitis compared with metabolic dysfunction-associated steatotic liver disease and other liver diseases: A systematic review. Am. Hear. J. Plus Cardiol. Res. Pr. 2024, 41, 100386. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Gadour, E.; Miutescu, B.; Abufarhaneh, E.; Kuriry, H.; Nica, C.; Alsheekh, L.; Taheri, E.; Al Saeed, Z.A.; Koppandi, O.; Abaalkhail, F.; et al. Calcineurin inhibitor exposure and de novo malignancy risk in liver transplant recipients: A narrative review of dose-dependent effects, risk factors and minimisation strategiesFrontline. Gastroenterology 2025. [CrossRef]
- Gutierrez-Dalmau, A.; Campistol, J.M. Immunosuppressive therapy and malignancy in organ transplant recipients: A systematic review. Drugs 2007, 67, 1167–1198. [Google Scholar] [CrossRef]
- Campistol, J.M. Minimizing the risk of posttransplant malignancy. Transplant. Proc. 2008, 40, S40–S43. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Duan, J.; Chen, S.; Wang, Z.; Ren, J.; Lu, J.; Chen, T.; Song, Z.; Wu, D.; Chang, Y.; et al. Transplant oncology and anti-cancer immunosuppressants. Front. Immunol. 2025, 15, 1520083. [Google Scholar] [CrossRef]
- De Fijter, J.W. Cancer and mTOR Inhibitors in Transplant Recipients. Transplantation 2017, 101, 45–55. [Google Scholar] [CrossRef]
- Vanlerberghe, B.T.K.; van Malenstein, H.; Sainz-Barriga, M.; Jochmans, I.; Cassiman, D.; Monbaliu, D.; van der Merwe, S.; Pirenne, J.; Nevens, F.; Verbeek, J. Tacrolimus Drug Exposure Level and Smoking Are Modifiable Risk Factors for Early De Novo Malignancy After Liver Transplantation for Alcohol-Related Liver Disease. Transpl. Int. 2024, 37, 12055. [Google Scholar] [CrossRef]
- Dantal, J.; Campone, M. Daunting but Worthy Goal: Reducing the De Novo Cancer Incidence After Transplantation. Transplantation 2016, 100, 2569–2583. [Google Scholar] [CrossRef]
- Villeret, F.; Dharancy, S.; Erard, D.; Abergel, A.; Barbier, L.; Besch, C.; Boillot, O.; Boudjema, K.; Coilly, A.; Conti, F.; et al. Disease recurrence after liver transplantation for NAFLD cirrhosis is ineluctable. JHEP Rep. 2023, 5, 100668. [Google Scholar] [CrossRef]
- Kim, N.G.; Sharma, A.; Saab, S. Cardiovascular and metabolic disease in the liver transplant recipient. Best Pr. Res. Clin. Gastroenterol. 2020, 46–47, 101683. [Google Scholar] [CrossRef]
- Chauhan, K.; Khan, A.; Chowdhury, S.; Ross, H.M.; Parra, N.S.; Halegoua-DeMarzio, D. A Comprehensive Review on the Risk of Metabolic Syndrome and Cardiovascular Disease after Liver Transplantation. Livers 2022, 2, 85–96. [Google Scholar] [CrossRef]
- Bhat, M.; Usmani, S.E.; Azhie, A.; Woo, M. Metabolic Consequences of Solid Organ Transplantation. Endocr. Rev. 2021, 42, 171–197. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Westbrook, R.; Tsochatzis, E.A. Metabolic and cardiovascular complications in the liver transplant recipient. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 183. [Google Scholar]
- Onghena, L.; Develtere, W.; Poppe, C.; Geerts, A.; Troisi, R.; Vanlander, A.; Berrevoet, F.; Rogiers, X.; Van Vlierberghe, H.; Verhelst, X. Quality of life after liver transplantation: State of the art. World J. Hepatol. 2016, 8, 749–756. [Google Scholar] [CrossRef]
- Åberg, F. mQuality of life after liver transplantation. Best Pr. Res. Clin. Gastroenterol. 2020, 46–47, 101684. [Google Scholar] [CrossRef]
- Zatta, R.; da Silva, L.S.; Felga, G.; Pimentel, C.F.M.G. Are we standing on the shifting sands of post-transplant metabolic-associated steatotic liver disease? World J. Hepatol. 2025, 17, 107837. [Google Scholar] [CrossRef]
- Jadaun, S.S.; Saigal, S. Post-transplant complications in alcohol- and metabolic-associated steatotic liver disease. Metab. Target Organ Damage 2024, 5, 1. [Google Scholar] [CrossRef]
- Taneja, S.; Roy, A. Nonalcoholic steatohepatitis recurrence after liver transplant. Transl. Gastroenterol. Hepatol. 2020, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Batisti, J.; Mehal, W.Z. Nonalcoholic Fatty Liver Disease in the Post Liver Transplant Patient. Curr. Transplant. Rep. 2020, 7, 332–339. [Google Scholar] [CrossRef]
- Narayanan, P.; Mara, K.; Izzy, M.; Dierkhising, R.; Heimbach, J.; Allen, A.M.; Watt, K.D. Recurrent or De Novo Allograft Steatosis and Long-term Outcomes After Liver Transplantation. Transplantation 2019, 103, E14–E21. [Google Scholar] [CrossRef]
- Barrera, F.; Uribe, J.; Olvares, N.; Huerta, P.; Cabrera, D.; Romero-Gómez, M. The Janus of a disease: Diabetes and metabolic dysfunction-associated fatty liver disease. Ann. Hepatol. 2024, 29, 101501. [Google Scholar] [CrossRef]
- Czarnecka, K.; Czarnecka, P.; Tronina, O.; Bączkowska, T.; Durlik, M. MASH Continues as a Significant Burden on Metabolic Health of Liver Recipients. Transplant. Proc. 2024, 56, 822–831. [Google Scholar] [CrossRef]
- Bryan, S.; Ratcliffe, J.; Neuberger, J.M.; Burroughs, A.K.; Gunson, B.K.; Buxton, M.J. Health-related quality of life following liver transplantation. Qual. Life Res. 1998, 7, 115–120. [Google Scholar] [CrossRef]
- Braun, F.; Teren, K.; Wilms, P.; Günther, R.; Allmann, J.; Broering, D.; Küchler, T. Quality of Life After Liver Transplantation. Transplant. Proc. 2009, 41, 2564–2566. [Google Scholar] [CrossRef]
- Dąbrowska-Bender, M.; Michałowicz, B.; Pączek, L. Assessment of the Quality of Life in Patients After Liver Transplantation as an Important Part of Treatment Results. Transplant. Proc. 2016, 48, 1697–1702. [Google Scholar] [CrossRef]
- Yang, L.S.; Shan, L.L.; Saxena, A.; Morris, D.L. Liver transplantation: A systematic review of long-termquality of life. Liver Int. 2014, 34, 1298–1313. [Google Scholar] [CrossRef]
- Stegall, M.D.; Everson, G.; Schroter, G.; Bilir, B.; Karrer, F.; Kam, I. Metabolic complications after liver transplantation: Diabetes, hypercholesterolemia, hypertension, and obesity. Transplantation 1995, 60, 1057–1060. [Google Scholar]
- Gabrielli, F.; Golfieri, L.; Nascimbeni, F.; Andreone, P.; Gitto, S. Metabolic Disorders in Liver Transplant Recipients: The State of the Art. J. Clin. Med. 2024, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Marzbani, C.; Bhimaraj, A. Corticosteroids in Immunosuppression. In Pharmacology of Immunosuppression; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2022; Volume 272, pp. 73–84. [Google Scholar] [CrossRef]
- Correia, M.I.T.D.; Rego, L.O.; Lima, A.S. post-liver transplant obesity and diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 457–460. [Google Scholar] [CrossRef]
- Gu, J.; Wu, X.; Lu, L.; Zhang, S.; Bai, J.; Wang, J.; Li, J.; Ding, Y. Role of steroid minimization in the tacrolimus-based immunosuppressive regimen for liver transplant recipients: A systematic review and meta-analysis of prospective randomized controlled trials. Hepatol. Int. 2014, 8, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Zaydfudim, V.M.; Pelletier, S.J. Towards Steroid-Free Immunosuppression after Liver Transplantation. Gut Liver 2016, 10, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.J.; Dousset, B.; Mayer, D.; McMaster, P.; Buckels, J.; Cramb, R.; Smith, J.M.; Nattrass, M. Metabolic effects of cyclosporin A and FK 506 in liver transplant recipients. Diabetes 1993, 42, 1753–1759. [Google Scholar] [CrossRef]
- Ascha, M.S.; Ascha, M.L.; Hanouneh, I.A. Management of immunosuppressant agents following liver transplantation: Less is more. World J. Hepatol. 2016, 8, 148–161. [Google Scholar] [CrossRef]
- Krentz, A.J.; Dmitrewski, J.; Mayer, D.; Nattrass, M. Effects of Immunosuppressive Agents on Glucose Metabolism. Clin. Immunother. 1995, 4, 103–123. [Google Scholar] [CrossRef]
- Monostory, K. Metabolic Drug Interactions with Immunosuppressants. In Organ Donation and Transplantation—Current Status and Future Challenges; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Charlton, M.; Levitsky, J.; Aqel, B.; O’Grady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef]
- Manzia, T.M.; Antonelli, B.; Carraro, A.; Conte, G.; Guglielmo, N.; Lauterio, A.; Mameli, L.; Cillo, U.; De Carlis, L.; Del Gaudio, M.; et al. Immunosuppression in adult liver transplant recipients: A 2024 update from the Italian Liver Transplant Working Group. Hepatol. Int. 2024, 18, 1416–1430. [Google Scholar] [CrossRef]
- De Simone, P.; Carrai, P.; Coletti, L.; Ghinolfi, D.; Petruccelli, S.; Filipponi, F. Modification of immunosuppressive therapy as risk factor for complications after liver transplantation. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 199–209. [Google Scholar] [CrossRef]
- Han, J.W.; Park, S.H. Advancing immunosuppression in liver transplantation: The role of regulatory T cells in immune modulation and graft tolerance. Clin. Transplant. Res. 2024, 38, 257–272. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Howard, R.J.; Fujita, S.; Kaplan, B. Sirolimus in combination with tacrolimus is associated with worse renal allograft survival compared to mycophenolate mofetil combined with tacrolimus. Am. J. Transplant. 2005, 5, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kriesche, H.U.; Steffen, B.J.; Chu, A.H.; Loveland, J.J.; Gordon, R.D.; Morris, J.A.; Kaplan, B. Sirolimus with neoral versus mycophenolate mofetil with neoral is associated with decreased renal allograft survival. Am. J. Transplant. 2004, 4, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Spengler, E.K.; O’lEary, J.G.; Te, H.S.; Rogal, S.; Pillai, A.A.; Al-Osaimi, A.; Desai, A.; Fleming, J.N.; Ganger, D.; Seetharam, A.; et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation 2017, 101, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Emamaullee, J.; Lian, T.B.; Lo, M.; Ender, P.; Kahn, J.; Sher, L. Impact of Morbid Obesity on Liver Transplant Candidacy and Outcomes: National and Regional Trends. Transplantation 2021, 105, 1052–1060. [Google Scholar] [CrossRef]
- Burra, P.; Ferrarese, A. Transplanting severely obese cirrhotic patients: Heavy clouds still on the horizon. United Eur. Gastroenterol. J. 2022, 10, 445–446. [Google Scholar] [CrossRef]
- Lin, J.S.; Muhammad, H.; Lin, T.; Kamel, I.; Baghdadi, A.; Rizkalla, N.; Ottmann, S.E.; Wesson, R.; Philosophe, B.; Gurakar, A. Donor BMI and Post–living Donor Liver Transplantation Outcomes: A Preliminary Report. Transplant. Direct 2023, 9, e1431. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J. Ethnic-specific cutpoints for obesity vs country-specific guidelines for action. Int. J. Obes. 2003, 27, 287–288. [Google Scholar] [CrossRef]
- Ayuk-Arrey, A.T.; Nephew, L.; Caicedo, J.C.; Ross-Driscoll, K. Racial and ethnic disparities in liver transplant access vary within and across transplant referral regions. Liver Transplant. 2025, 31, 857–869. [Google Scholar] [CrossRef]
- Althoff, A.L.; Ali, M.S.; O’SUllivan, D.M.; Dar, W.; Emmanuel, B.; Morgan, G.; Einstein, M.; Richardson, E.; Sotil, E.; Swales, C.; et al. Short- and Long-Term Outcomes for Ethnic Minorities in the United States After Liver Transplantation: Parsing the Hispanic Paradox. Transplant. Proc. 2022, 54, 2263–2269. [Google Scholar] [CrossRef]
- Sakowitz, S.; Bakhtiyar, S.S.; Mallick, S.; Kaldas, F.; Benharash, P. Association of Community Socioeconomic Distress with Waitlist and Survival Outcomes in Liver Transplantation. Transplantation 2025, 109, 976–984. [Google Scholar] [CrossRef]
- Srinivas, N.G.; Chen, Y.; Rodday, A.M.; Ko, D. Disparities in Liver Transplant Outcomes: Race/Ethnicity and Individual- and Neighborhood-Level Socioeconomic Status. Clin. Nurs. Res. 2024, 33, 509–518. [Google Scholar] [CrossRef]
- Martins, P.N.; Kim, I.K. Editorial: Disparities in transplantation access and outcomes: Mind the gap! Curr. Opin. Organ Transplant. 2021, 26, 498–500. [Google Scholar] [CrossRef]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2018, 29, 3. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.C.; Tavakol, M.M.; Sarin, A.; Amirkiai, S.M.; Rogers, S.J.; Carter, J.T.; Posselt, A.M. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg. Obes. Relat. Dis. 2013, 9, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Sarno, G.; Schiavo, L.; Calabrese, P.; Córdova, L.Á.; Frias-Toral, E.; Cucalón, G.; Garcia-Velasquez, E.; Fuchs-Tarlovsky, V.; Pilone, V. The Impact of Bariatric-Surgery-Induced Weight Loss on Patients Undergoing Liver Transplant: A Focus on Metabolism, Pathophysiological Changes, and Outcome in Obese Patients Suffering NAFLD-Related Cirrhosis. Stomatology 2022, 11, 5293. [Google Scholar] [CrossRef]
- D’Amico, G.; Tulla, K.; Tzvetanov, I. Bariatric Surgery and Transplantation. In Global Bariatric Surgery: The Art of Weight Loss across the Borders; Springer: Cham, Switzerland, 2018; pp. 471–478. [Google Scholar] [CrossRef]
- Lee, Y.; Anvari, S.; Soon, M.B.S.; Tian, C.B.; Wong, J.A.; Hong, D.M.M.; Anvari, M.M.; Doumouras, A.G. Bariatric Surgery as a Bridge to Heart Transplantation in Morbidly Obese Patients: A Systematic Review and Meta-Analysis. Cardiol. Rev. 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Dawod, S.; Brown, K. Non-invasive testing in metabolic dysfunction-associated steatotic liver disease. Front. Med. 2024, 11, 1499013. [Google Scholar] [CrossRef]
- Cho, Y. Evaluation of Liver Fibrosis through Noninvasive Tests in Steatotic Liver Disease. Korean J. Gastroenterol. 2024, 84, 215–222. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.J.; Jiang, Y.; Lai, J.C.-T.; Wong, G.L.-H.; Wong, V.W.-S.; Yip, T.C.-F. Role of noninvasive tests in the prognostication of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2024, 31, 51–75. [Google Scholar] [CrossRef]
- Singh, A.; Sohal, A.; Batta, A. Recent developments in non-invasive methods for assessing metabolic dysfunction-associated fatty liver disease. World J. Gastroenterol. 2024, 30, 4324–4328. [Google Scholar] [CrossRef]
- Merola, J.; Emond, J.C.; Levitsky, J. Novel Noninvasive Biomarkers in Liver Transplantation: A Tool on the Doorstep of Clinical Utilization. Transplantation 2023, 107, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Marano, M.; Gjini, K.; Vaira, L.; Bugianesi, E.; Armandi, A. Novel dynamic and static biomarkers for the assessment of liver disease severity in MASLD. Minerva Biotechnol. Biomol. Res. 2024, 36, 203–216. [Google Scholar] [CrossRef]
- Babu, K.S.T.J.; Khan, I.A. Role of GLP-1 Agonists in Obesity: A Comprehensive Review. Preprint 2024. [Google Scholar] [CrossRef]
- Myerson, M.; Paparodis, R.D. Pharmacotherapy of Weight-loss and Obesity with a Focus on GLP 1-Receptor Agonists. J. Clin. Pharmacol. 2024, 64, 1204–1221. [Google Scholar] [CrossRef]
- Velji-Ibrahim, J.; Radadiya, D.; Devani, K. S1628 GLP-1 Agonists and Their Efficacy for Weight Loss: Unraveling the Best Option Through Network Meta-Analysis. Am. J. Gastroenterol. 2023, 118, S1220. [Google Scholar] [CrossRef]
- Raigani, S.; Acun, A.; Uygun, B.; Uygun, K.; Yeh, H. Steatotic livers for transplantation: Improving utilization of a prevalent resource through organ repair. In Organ Repair and Regeneration: Preserving Organs in the Regenerative Medicine Era; Academic Press: Cambridge, MA, USA, 2021; pp. 247–256. [Google Scholar] [CrossRef]
- Patrono, D.; De Stefano, N.; Rigo, F.; Cussa, D.; Romagnoli, R. Some like it hot. utility and mechanisms of ex-situ normothermic machine perfusion of the liver. Eur. J. Transplant. 2023, 1, 92–112. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Afford, S.C.; Mergental, H. Pushing the Limits: Machine Preservation of the Liver as a Tool to Recondition High-Risk Grafts. Curr. Transplant. Rep. 2018, 5, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cywes, C.; Banker, A.; Muñoz, N.; Levine, M.; Abu-Gazala, S.; Bittermann, T.M.; Abt, P. The Potential Utilization of Machine Perfusion to Increase Transplantation of Macrosteatotic Livers. Transplantation 2024, 108, e370–e375. [Google Scholar] [CrossRef]
- Nguyen, M.C.; Li, X.; Linares, N.; Jadlowiec, C.; Moss, A.; Reddy, K.S.; Mathur, A.K. Ex-situ machine perfusion in clinical liver transplantation: Current practices and future directions. Liver Transplant. 2024, 31, 531–544. [Google Scholar] [CrossRef]
- Da Silva, R.X.S.; Borrego, L.B.; Lenggenhager, D.; Huwyler, F.; Binz, J.; Mancina, L.; Breuer, E.; Wernlé, K.; Hefti, M.; Mueller, M.; et al. Defatting of Human Livers during Long-Term ex situ Normothermic Perfusion. Novel Strategy to Rescue Discarded Organs for Transplantation. Ann. Surg. 2023, 278, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Shaked, A.; Loza, B.; Olthoff, K.; Keating, B. Testing the application of polygenic risk scores in the transplant setting—Relevance for precision medicine. Clin. Transl. Med. 2022, 12, e1009. [Google Scholar] [CrossRef]
- Ajmera, V.; Loomba, R. Advances in the genetics of nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 2023, 39, 150–155. [Google Scholar] [CrossRef]
- Souza, M.; Al-Sharif, L.; Diaz, I.; Mantovani, A.; Villela-Nogueira, C.A. Global epidemiology and implications of PNPLA3 I148M variant in MASLD: A systematic review and meta-analysis. J. Clin. Exp. Hepatol. 2024, 15, 102495. [Google Scholar] [CrossRef]
- Tulone, A.; Pennisi, G.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Cannella, R.; Mercurio, F.; Petta, S. Are we ready for genetic testing in metabolic dysfunction-associated steatotic liver disease? United Eur. Gastroenterol. J. 2024, 12, 638–648. [Google Scholar] [CrossRef]
- Addissouky, T.A. Transforming Screening, Risk Stratification, and Treatment Optimization in Chronic Liver Disease Through Data Science and translational Innovation. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2024, 25, 53–62. [Google Scholar] [CrossRef]
- Cespiati, A.; Youngson, N.A.; Tourna, A.; Valenti, L. Genetics and Epigenetics in the Clinic: Precision Medicine in the Management of Fatty Liver Disease. Curr. Pharm. Des. 2020, 26, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chang, K.M.; Yu, J.; Loomba, R. Unraveling Mechanisms of Genetic Risks in Metabolic Dysfunction-Associated Steatotic Liver Diseases: A Pathway to Precision Medicine. Annu. Rev. Pathol. Mech. Dis. 2025, 20, 375–403. [Google Scholar] [CrossRef] [PubMed]
- Moretti, V.; Romeo, S.; Valenti, L. The contribution of genetics and epigenetics to MAFLD susceptibility. Hepatol. Int. 2024, 18 (Suppl. 2), 848–860. [Google Scholar] [CrossRef] [PubMed]


| Immunosuppressant Class | Key Metabolic Effects | Management Strategies † |
|---|---|---|
| Corticosteroids | Weight gain, insulin resistance, hypertension, dyslipidemia | Early withdrawal or taper to low dose; use steroid-minimization protocols |
| Calcineurin Inhibitors (Tacrolimus, Cyclosporine) | Hypertension, nephrotoxicity, hyperglycemia; tacrolimus → higher risk of NODAT, cyclosporine → greater dyslipidemia; increased risk of de novo malignancies, especially in metabolically at-risk recipients | CNI minimization strategies (dose reduction, combination with antimetabolites or mTOR inhibitors); careful monitoring of glucose and renal function; consider mTOR/MMF-based regimens to reduce long-term oncologic risk |
| mTOR Inhibitors (Sirolimus, Everolimus) | Hyperlipidemia, insulin resistance, proteinuria, impaired wound healing | Use as CNI-sparing agents; monitor lipid profile and renal function; consider benefits for fibrosis delay and reduced HCC recurrence |
| Antimetabolites (Mycophenolate mofetil, Azathioprine) | Largely metabolically neutral * | Adjunct therapy to reduce CNI/corticosteroid exposure; valuable in high-risk metabolic profiles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Busafi, S.A.; Eslam, M. Liver Transplantation in the Era of Metabolic Dysfunction–Associated Fatty Liver Disease: Challenges, Ethical Dilemmas, and Future Directions. Transplantology 2025, 6, 35. https://doi.org/10.3390/transplantology6040035
Al-Busafi SA, Eslam M. Liver Transplantation in the Era of Metabolic Dysfunction–Associated Fatty Liver Disease: Challenges, Ethical Dilemmas, and Future Directions. Transplantology. 2025; 6(4):35. https://doi.org/10.3390/transplantology6040035
Chicago/Turabian StyleAl-Busafi, Said A., and Mohammed Eslam. 2025. "Liver Transplantation in the Era of Metabolic Dysfunction–Associated Fatty Liver Disease: Challenges, Ethical Dilemmas, and Future Directions" Transplantology 6, no. 4: 35. https://doi.org/10.3390/transplantology6040035
APA StyleAl-Busafi, S. A., & Eslam, M. (2025). Liver Transplantation in the Era of Metabolic Dysfunction–Associated Fatty Liver Disease: Challenges, Ethical Dilemmas, and Future Directions. Transplantology, 6(4), 35. https://doi.org/10.3390/transplantology6040035







