1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic liver condition characterized by excessive fat accumulation (hepatic steatosis) in individuals without significant alcohol consumption [
1]. It represents a growing global health concern, closely linked to the rising prevalence of obesity, type 2 diabetes mellitus, and metabolic syndrome [
2]. MASLD encompasses a spectrum of liver abnormalities, ranging from simple steatosis to progressive forms—metabolic dysfunction-associated steatohepatitis (MASH) and MASH with advanced fibrosis [
1]. MASH is defined by hepatic steatosis accompanied by inflammation and hepatocellular injury (ballooning), with or without the presence of fibrosis [
3]. It carries a substantial risk of adverse liver-related outcomes, including progression to advanced fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma (HCC) [
3].
Currently, treatment options for MASH are limited, with lifestyle modification being the cornerstone of management. This includes weight loss through caloric restriction and adoption of healthy dietary patterns (such as the Mediterranean diet), increased physical activity, and avoidance of alcohol and other hepatotoxic agents [
4]. Sustained weight loss of ≥9% has been shown to improve liver histology, including reductions in steatosis, inflammation, and fibrosis [
4]. Additionally, aggressive management of metabolic comorbidities such as hyperglycemia, dyslipidemia, and hypertension is critical [
4]. However, achieving and maintaining these lifestyle changes remains challenging for many patients.
Recently, resmetirom became the first FDA-approved pharmacologic therapy for MASLD/MASH. It is a selective thyroid hormone receptor-beta (THR-β) agonist that has demonstrated efficacy in resolving MASH and improving fibrosis in patients with stage F2–F3 disease in clinical trials [
5]. Until this approval, no specific pharmacologic therapies were available for MASH, though several agents have been used off-label or are currently under investigation with promising results. For instance, vitamin E, an antioxidant, has shown histological benefits in non-diabetic adults with MASH without cirrhosis [
6]. However, concerns over long-term safety, including a potential increased risk of prostate cancer in men, limit its widespread use [
7]. Pioglitazone, a thiazolidinedione, improves insulin sensitivity and has demonstrated histological improvement in patients with MASH, particularly those with type 2 diabetes [
6]. Nonetheless, its adverse effects—including weight gain, fluid retention, and potential cardiovascular risks—restrict its broad applicability. Glucagon-like peptide-1 (GLP-1) receptor agonists, originally developed for type 2 diabetes and weight management, have shown significant improvements in liver enzymes, steatosis, inflammation, and fibrosis in MASLD/MASH patients in clinical trials [
8]. Dual GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists, such as tirzepatide, have demonstrated significant reductions in liver fat and favorable histological changes in MASH [
9,
10,
11]. Despite this progress, the off-label use of these agents is often guided by clinical judgment and limited by potential side effects and contraindications. Long-term data on their safety and sustained efficacy remain limited, particularly regarding hard clinical outcomes such as progression to cirrhosis, liver failure, or HCC. Furthermore, many clinical trials exclude patients with cirrhosis due to safety concerns, leaving a significant therapeutic gap for patients already at advanced disease stages.
Although lifestyle modification remains the foundation of MASLD/MASH management, the therapeutic landscape is rapidly evolving. The approval of resmetirom and promising results of GLP-1 and GLP1/GIP RAs signal a new era in treatment. However, challenges remain, including the need for long-term safety data, therapies suitable for patients with advanced disease, and interventions that can address the full heterogeneity of MASLD/MASH.
This case highlights a remarkable response to tirzepatide in a patient with MASH-related cirrhosis. Not only was the medication well tolerated without hepatic decompensation, but the patient also exhibited resolution of steatohepatitis and regression of liver fibrosis, suggesting that agents like tirzepatide may be safe in individuals with stage F4 fibrosis and could even reverse liver damage previously considered irreversible.
2. Case Presentation
A 46-year-old female with a past medical history of obesity (BMI > 40), alcohol use disorder in remission, pre-diabetes, and cirrhosis secondary to alcohol and MASH (metALD) decompensated by ascites and hepatic encephalopathy underwent a successful deceased donor liver transplant in 2019 at the Mayo Clinic in Phoenix, Arizona. Her liver biopsy at the time of transplant noted no macro-vesicular steatosis with her NAFLD Activity Score (NAS) 1/8 and minimal fibrosis (F1).
One year following her liver transplant, she reported increased abdominal distension, concerning for the return of ascites. Her liver enzymes were found to be elevated with aspartate aminotransferase (AST) at 157 U/L, alanine aminotransferase (ALT) at 51 U/L, alkaline phosphatase (ALP) at 255 U/L, and total bilirubin (TB) at 2.9 mg/dL. She reported that she remained abstinent from alcohol, supported with a series of negative phostphatidylethanol (PEth), but gained approximately 36 kg. Her MR Abdomen/MR cholangiopancreatography demonstrated hepatomegaly and marked diffuse hepatic steatosis of her liver allograft. Her calculated FIB-4 score was 0.89, suggesting a low risk of advanced fibrosis, but her SAFE score was 71, identifying her as intermediate risk for stage F2 or higher. She underwent further evaluation with transient elastography and liver biopsy. Results revealed a median controlled attenuation parameter (CAP) of 400 dB/m, consistent with S3 steatosis, and a measured median liver stiffness (LSM) of 61.2 kPa, indicating stage F4/cirrhosis. Her liver biopsy confirmed severe steatohepatitis with diffuse mixed macrovesicular and microvesicular steatosis involving 80% of the hepatic parenchyma, marked ballooning degeneration, and patchy lobular inflammation consistent with NAS at 7/8. Periportal and pericentral sub-sinusoidal fibrosis with areas of portal-to-portal bridging was observed, indicating a Brunt Fibrosis Score of 3/4. There was no relevant portal-based inflammation to suggest acute rejection. Transjugular portosystemic pressure measurements at the time of liver biopsy revealed an elevated portosystemic pressure gradient of 10 mmHg, consistent with clinically significant portal hypertension. Therefore, unsurprisingly, in addition to her development of ascites, she had large esophageal varices seen on esophagogastroduodenoscopy (EGD) requiring banding, and reduced kidney function. Her hepatic decompensation prompted her to be re-listed for a simultaneous liver and kidney transplant with a calculated MELD-Na of 29 in August 2020.
Aside from medical management of her hepatic decompensation, extensive lifestyle modification and consideration of bariatric surgery with sleeve gastrectomy at the time of re-transplantation were recommended. She underwent regular dietitian visits focused on advice regarding balanced eating, portion control, carbohydrate counting, and calorie deficit planning. She also advised on guided exercise plans with the goal of 300 min per week of moderate cardio-type exercise. Intensive lifestyle interventions led to a weight loss of 17 kg and gradual recompensation over the following 2 years. Her repeat portosystemic pressure measurements eventually demonstrated resolution of portal hypertension with return of her portosystemic pressure gradient back to 5 mmHg. Her kidney function also recovered to where her transplant re-listing was altered to just a liver transplant; however, due to her eventual recompensation, she was ultimately made inactive on the waitlist.
Despite her clinical improvement with weight loss, her follow-up liver fibrosis evaluation in 2022 did not improve. Both serologic fibrosis risk scores, with FIB-4 at 3.10 and SAFE at 128, indicated her progression to high risk for F2 fibrosis or higher. Her follow-up elastography demonstrated persistent S3 steatosis with a CAP of 361 dB/m and F4/cirrhosis with an LSM of 13 kPa. These findings from her non-invasive tests were confirmed on liver biopsy, which revealed bridging perisinusoidal fibrosis away from fibrous septa, confirming the Brunt Fibrosis Score of 4/4. There was evidence of ongoing mild steatohepatitis with macrovesicular steatosis occupying 5% of the hepatic parenchyma and scattered balloon hepatocytes (NAS 3/8).
By June 2022, she also developed uncontrolled type 2 diabetes, attributed to the progression of her pre-diabetes before transplant to both steroid use for immunosuppression and worsening overall metabolic dysfunction. Discussion of initiating a GLP-1 agonist was raised as an adjunctive medication to attain tighter glucose control and for potential weight loss benefits. She ultimately started on tirzepatide in 2023 and was titrated up to a weekly dose of 10 mg. This helped facilitate another 13.6 kg weight loss over the next year. Her interval non-invasive fibrosis testing in 2024 revealed notable improvement. Both her calculated FIB-4 and SAFE scores improved to the low risk of advanced fibrosis category at 1.1 and −28, respectively. While her elastography revealed persistent steatosis with a CAP of 323 dB/m, her measured median LSM decreased to 10.3 kPa, aligning with F3 fibrosis and suggesting possible regression of her liver fibrosis.
Despite a reduction in the tirzepatide dose to 7.5 mg, she continued to lose another 29.5 kg. Her follow-up March 2025 non-invasive fibrosis testing revealed a mild rise in her FIB-4 and SAFE scores to the intermediate risk category at 1.34 and 7, respectively. However, her elastography indicated resolution of her steatohepatitis with a CAP of 204 dB/m (S0 steatosis), and a notable further decrease in measured median LSM to 5.5 kPa, consistent with F1 fibrosis. MR elastography corroborated the results, revealing a low estimated hepatic fat fraction of 4.9% and a mean hepatic stiffness of 3.45 kPa, corresponding with stage F1–F2 fibrosis. A liver biopsy was pursued for histologic confirmation. The biopsy demonstrated resolution of hepatic steatosis with only scattered steatotic hepatocytes occupying 5% of parenchyma without ballooned hepatocytes and a NAS of 1/8. While her biopsy did not demonstrate the same degree of fibrosis resolution suggested by her non-invasive tests, it did support a clear improvement of her periportal, perisinusoidal, and bridging fibrosis, indicating that her previous Brunt Fibrosis Score of 4/4 regressed to a Brunt Fibrosis Score of 2–3/4.
3. Discussion
A 46 y.o. female with a history of obesity (BMI > 40) and metALD underwent a successful deceased donor liver transplant, but rapidly developed MASH cirrhosis within a year following transplant despite weight loss through intensive lifestyle modification. Initiation of tirzepatide led to additional weight loss over the subsequent two years with documented fibrosis regression via multiple non-invasive fibrosis evaluation modalities and on liver biopsy. This case highlights several key observations: (1) although intensive lifestyle modifications led to significant weight loss and improvement in hepatic steatosis, this alone was insufficient to halt the progression of liver fibrosis, (2) end-stage liver fibrosis (F4/cirrhosis) may not be as irreversible as once believed, and (3) tirzepatide did not induce hepatic decompensation in a patient with biopsy-proven stage 4 fibrosis (cirrhosis) suggesting safety and a significant clinical benefit.
The predominant theory regarding how GLP-1/GIP RAs benefit MASLD/MASH is through reduction in hepatic inflammation by decreasing fat accumulation with weight loss and improving insulin sensitivity. As visualized on the clinical timeline and corresponding liver biopsies seen in
Figure 1, this patient’s lifestyle modifications led to significant weight loss and marked improvement in hepatic steatosis, as evidenced by a decrease in the NAS from 7/8 in 2020 to 3/8 in 2022 on biopsy. However, despite this improvement, fibrosis progression was still observed. After initiating tirzepatide, the patient continued to lose weight, and notably, her liver biopsy demonstrated not only complete resolution of hepatic steatosis but also regression of fibrosis. These findings suggest that GLP-1/GIP RAs may exert protective effects against steatohepatitis and fibrosis through additional mechanisms beyond weight loss and insulin sensitization.
One proposed hepatoprotective mechanism of GLP-1/GIP RAs is their ability to prevent hepatocyte death. GLP-1 RAs have been found to upregulate genes that mitigate dysfunctional endoplasmic reticulum stress responses, which can trigger apoptosis [
12,
13]. Another key mechanism involves the reduction in both intrahepatic inflammations caused by steatosis and the low-grade chronic systemic inflammation characteristic of metabolic syndrome [
14]. GLP-1 RAs have been shown to activate the liver X receptor/retinoid X receptor (LXR/RXR) pathway, a critical regulator of inflammation as well as lipid and glucose metabolism [
15]. LXR activation improves hepatic inflammation and protects against lipopolysaccharide-induced liver injury [
16]. Additionally, oxidative stress—a major contributor to systemic inflammation in metabolic syndrome—results from increased free radical production leading to cellular damage. GLP-1 RAs have been found to reduce oxidative stress markers by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, thereby enhancing cellular antioxidant defenses [
17].
Emerging evidence also suggests that GLP-1/GIP RAs may help prevent fibrosis progression. Semaglutide, a GLP-1 RA, has shown to reduce the intrahepatic expression of collagen types I, II, and III genes in mouse models after a 17-week treatment course [
18]. Notably, this reduction was observed only in the development of new (de novo) fibrosis and did not affect existing fibrosis prior to treatment. This limited effect on pre-existing fibrosis may be due to the relatively short duration of therapy, highlighting the need for longer-term studies to better understand the potential role of GLP-1 RAs in reversing established fibrosis.
A review by Desmet and Roskams highlighted that although several prior studies have demonstrated regression of fibrosis in established cirrhosis—due to alcohol use, chronic biliary obstruction, chronic viral hepatitis, and autoimmune liver diseases—these studies did not evaluate the accompanying vascular abnormalities that are integral to cirrhosis [
19]. According to the diagnostic criteria set by the Fifth Pan-American Congress of Gastroenterology (1956), cirrhosis is defined by the presence of septal fibrosis with portal-central bridging containing shunting vessels, along with nodular parenchymal regeneration. Desmet and Roskams argue that while fibrosis may regress with appropriate treatment, the associated vascular alterations often persist and are thought to be largely irreversible. This vascular persistence challenges the notion that cirrhosis, as classically defined, is fully reversible.
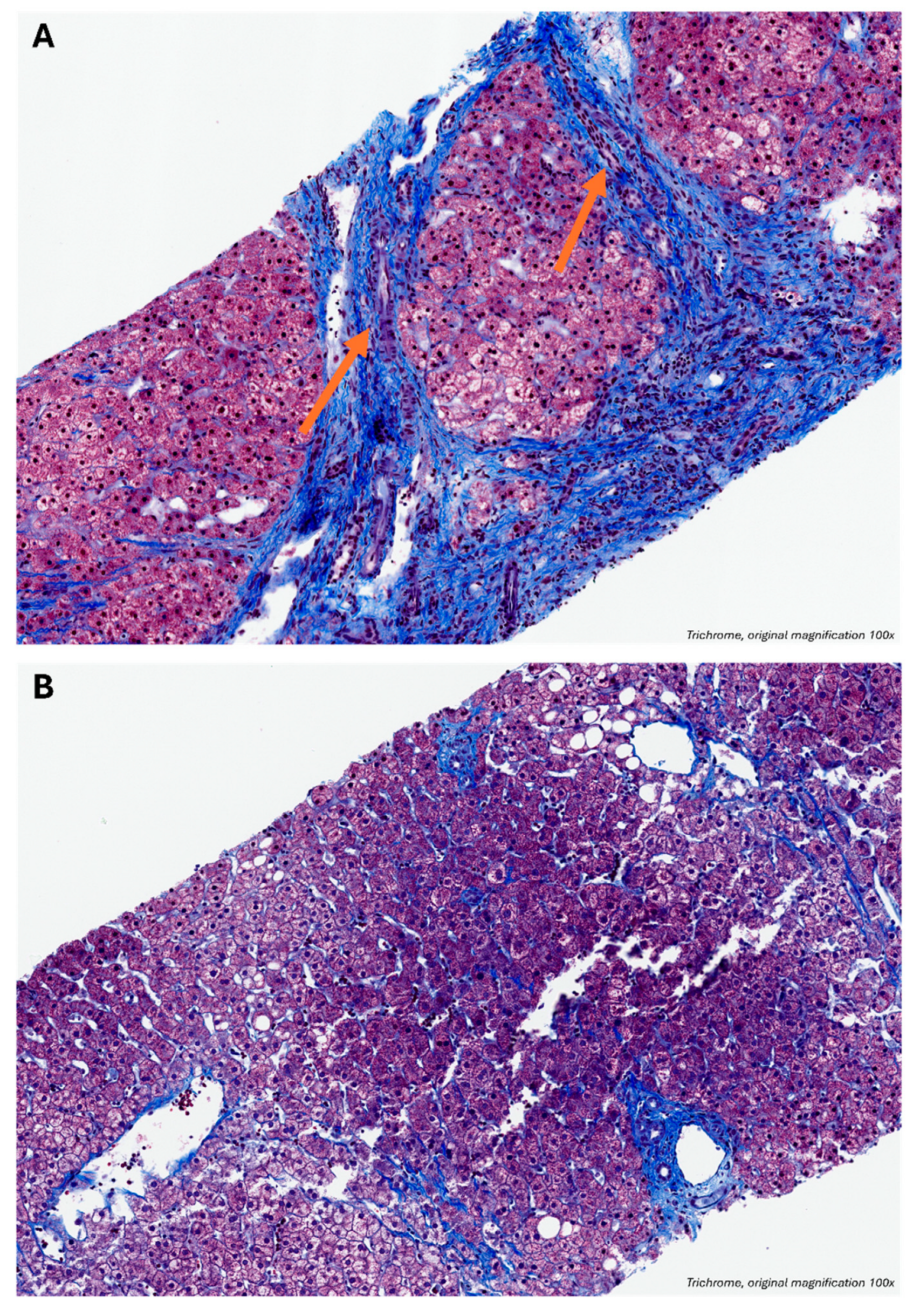
As shown in
Figure 2, the patient’s 2022 liver biopsy (
Figure 2A) met established criteria for cirrhosis, demonstrating vascularized fibrous septa bridging portal tracts and central veins, with hepatocyte islands encased in fibrotic tissue and lacking central veins. In contrast, the 2025 biopsy (
Figure 2B), after two years of tirzepatide therapy, shows distinct portal and central vein structures without bridging septa. While residual delicate pericellular fibrosis and incomplete septa remain, the vascular structures embedded in fibrotic bands—the hallmarks of cirrhosis—are notably absent. These findings suggest regression of both fibrotic and vascular components of cirrhosis. Despite the potential for sampling error with needle biopsies, the patient also exhibited radiographic and elastographic evidence of cirrhosis regression, supporting the histological findings as representative of her overall liver architecture.
Shunting vessels within fibrous septa divert blood from functional parenchyma, reducing hepatic perfusion and impairing filtration. These vessels are thought to arise from two primary mechanisms: (1) persistence of sinusoidal vessels in areas of post-necrotic collapse, and (2) angiogenesis driven by localized hypoxia [
19]. Tirzepatide-induced fibrosis regression may mitigate these stimuli, leading to the attenuation or disappearance of these aberrant vessels. Nonetheless, while vascular remodeling has long been considered irreversible, fibrosis and ongoing inflammation remain the primary drivers of cirrhosis-related complications—such as portal hypertension, hepatic insufficiency, and HCC. Therefore, therapeutic strategies targeting fibrosis regression and inflammation control may substantially reduce morbidity and mortality, even in advanced cirrhosis.
Although GLP-1 and GLP1/GIP RAs are not hepatically cleared, data on their use in patients with cirrhosis remain limited. One key concern with GLP-1 RA-based therapies in this population is the potential for loss of lean muscle mass. Patients with cirrhosis are already at increased risk for sarcopenia, defined as the progressive loss of skeletal muscle mass and function, due to a combination of malnutrition and a chronic inflammatory state that drives hypercatabolism. Sarcopenia is associated with significantly worse clinical outcomes, including increased morbidity and mortality, both pre- and post-liver transplantation. Given the weight-reducing effects of GLP-1 and GLP-1/GIP RAs, there is concern that these agents may further contribute to sarcopenia by promoting loss of lean body mass.
A meta-analysis of 19 randomized controlled trials demonstrated that semaglutide (1.0 mg and 2.4 mg weekly), tirzepatide (15 mg weekly), and liraglutide (3.0 mg daily) were all associated with statistically significant reductions in lean mass compared to placebo [
20]. Notably, lean mass loss accounted for approximately 25% of total weight loss and was independent of baseline body weight. However, a 2024 review by Neeland cautions that lean mass loss does not equate solely to muscle loss, as it also includes reductions in organ mass, bone, fluid, and water content within adipose tissue [
21]. Moreover, emerging evidence suggests that GLP-1–based therapies may preserve or even improve muscle quality through adaptive metabolic mechanisms. Therefore, implementing supportive strategies—such as increased intake of high-quality protein and incorporation of resistance training—may help mitigate lean mass loss and preserve muscle function during GLP-1–based therapy [
22].
In this case, the patient had biopsy-confirmed cirrhosis and tolerated tirzepatide well, without experiencing adverse effects typically associated with GLP-1 or GLP-1/GIP RAs. Notably, she did not develop hepatic decompensation during the two-year course of therapy. It is important to note that when she previously experienced hepatic decompensation in 2020, her Bone Mineral Analysis (BMA) of the lumbar spine and bilateral femoral necks was normal, with Bone Mineral Density (BMD) values ranging from 0.99 to 1.175 g/cm2. These findings suggest that even at her most clinically decompensated state, there was no evidence of sarcopenia. Tirzepatide was initiated after she had recompensated and had already adopted significant lifestyle modifications, including dietary improvements and regular physical activity. Initiating tirzepatide during a compensated stage of cirrhosis—rather than during active decompensation or in the presence of sarcopenia—may have helped reduce the risk of developing sarcopenia. Furthermore, her ongoing commitment to lifestyle interventions likely contributed to the preservation of muscle mass and helped prevent the onset or progression of sarcopenia.
We acknowledge that this case reflects the response of a single patient with MASH-related cirrhosis to a dual GLP-1/GIP RA. As such, the findings may not be generalizable to the broader population with fibrotic MASH, particularly given the presence of potential confounding factors that were not fully explored. However, this case helps address several limitations commonly seen in current studies evaluating the effects of GLP1/GIP RAs. Notably, it includes a longer follow-up period of two years (as opposed to the more typical one-year duration), incorporates serial data from multiple non-invasive fibrosis assessment methods, and provides serial histologic confirmation—offering a more comprehensive and longitudinal view of disease progression and treatment response.
Therefore, future studies investigating GLP-1 RAs and dual GLP-1/GIP RAs should be designed as longitudinal prospective cohort trials with biopsy-controlled surveillance over extended treatment durations beyond one year. This approach is necessary to capture potential long-term benefits that may not be evident in shorter studies, including the degree of fibrosis regression or even cirrhosis reversal. Additionally, these investigations should be powered to assess the safety and efficacy of these therapies specifically in patients with cirrhosis, with an emphasis on identifying patient-specific factors that may influence therapeutic response and risk of adverse outcomes.