1. Introduction
Graft-versus-host disease (GVHD) is a well-recognized complication of allogeneic hematopoietic stem cell transplantation, but it remains an underdiagnosed and often fatal entity in solid organ transplant (SOT) recipients. First described in a liver transplant patient by Burdick et al. in 1988 [
1], SOT-associated GVHD (SOT-GVHD) occurs when immunocompetent donor lymphocytes transferred with the allograft survive within the recipient and initiate an alloimmune response against host tissues [
2,
3]. The likelihood of GVHD is greater with organs that contain abundant lymphoid tissue, such as the liver, intestine, and pancreas [
4,
5].
Although rare, SOT-GVHD carries a high mortality rate, often exceeding 80% and typically presents within the first two to twelve weeks after transplantation [
6]. Clinical manifestations are heterogeneous and may include cutaneous eruptions, liver enzyme abnormalities, diarrhea, cytopenias, and bone marrow aplasia [
4,
6,
7]. Cutaneous involvement is the most common and often earliest presenting feature [
8], with lichenoid skin eruptions more commonly reported in SOT-GVHD than in hematopoietic stem cell transplant-associated GVHD [
9,
10]. Diagnosis is frequently delayed due to symptoms overlapping with drug reactions, infections, and acute rejection [
4]. Histopathological confirmation via tissue biopsy remains the gold standard, supported by chimerism assays and fluorescence in situ hybridization (FISH) to detect donor cell persistence.
Herein, we report a rare and fatal case of biopsy-proven SOT-GVHD in a simultaneous pancreas–kidney transplant recipient, with involvement of the skin, liver, and bone marrow. This case highlights the importance of early clinical suspicion, tissue diagnosis, and interdisciplinary management in improving detection and outcomes in this underrecognized condition.
2. Case Report
Our patient was a 54-year-old gentleman with long-standing type 1 diabetes complicated by diabetic nephropathy, neuropathy, and retinopathy as well as hypertension, dyslipidemia, and peripheral vascular disease with remote right great toe amputation.
He underwent simultaneous pancreas and pre-emptive kidney transplantation from a deceased donor following neurological determination of death (NDD). A 12–16 cm section of duodenum was transplanted alongside the donor pancreas. Serostatus was CMV donor seronegative and recipient seronegative, EBV donor seropositive and recipient seropositive. Kidney donor profile index (KDPI) score was 18%. Donor specific antibodies were not detected at the time of transplantation. Induction of immunosuppression was with rabbit anti-thymocyte globulin (ATG) and methylprednisolone. He received 50 mg of ATG and 250 mg of methylprednisolone IV at the time of surgery followed by a prednisone taper. In total, he received 350 mg (5.8 mg/kg) of rabbit ATG. Mycophenolate sodium 720 mg oral q. 12 h was initiated on post-transplant day (PTD) 1. Tacrolimus 10 mg oral daily was initiated on PTD 5 and the dose was titrated based on levels.
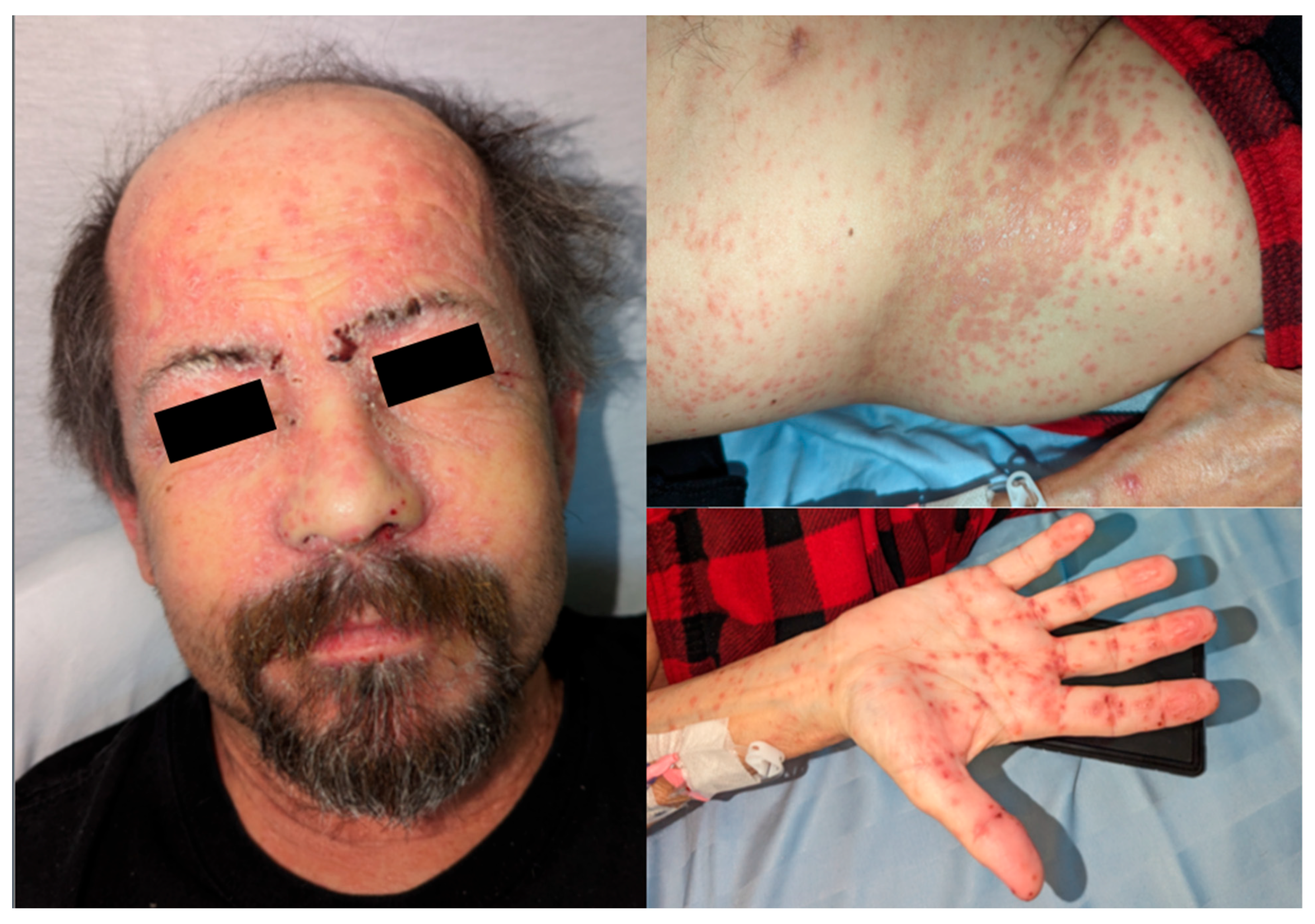
Approximately 3 months post-transplant, the patient developed an erythematous papular eruption, starting on his face and scalp. Over the subsequent 1–2 days, he noted craniocaudal spread of these lesions down the torso and all four extremities, including the right palm. There was no oral ulceration or any other mucosal involvement. The lesions were mildly pruritic but not painful, and at no point did the patient recall any vesicles or pustules. When the rash did not resolve, he notified his surgical team, who suspected disseminated herpes zoster infection and prescribed acyclovir 200 mg oral five times per day for 5 days. He was admitted to hospital on PTD 87 and assessed by the Transplant Infectious Diseases service the following day. Photographs of his lichenoid eruption at that time are shown in
Figure 1. Characteristic lesions were noted to be well-demarcated, non-tender, shiny, reddish-purple polygonal flat-topped papules, some with fine overlying scale. Admission of blood work on PTD 87 was notable for elevated transaminases and peripheral eosinophilia, as shown in
Table 1. An extensive infectious workup was also undertaken at the same time, which was non-contributory. Dermatology was also consulted and suspected lichen planus, lichenoid drug eruption, or lichenoid chronic GVHD at that time. A skin punch biopsy was performed on PTD 88, which showed epidermis with focal hyperkeratosis and no parakeratosis, interface lichenoid chronic inflammatory cells with focal vacuolar interface changes and scattered dyskeratotic keratinocytes, with inflammation extending to the upper dermis, overall favoring graft-versus-host disease of the skin (
Figure 2). He was started on betamethasone valerate 0.05% topical ointment with significant improvement in his skin lesions.
He was seen in the outpatient Transplant Infectious Diseases clinic on PTD 106 and noted at that time to have worsening liver enzyme elevation and new neutropenia, as shown in
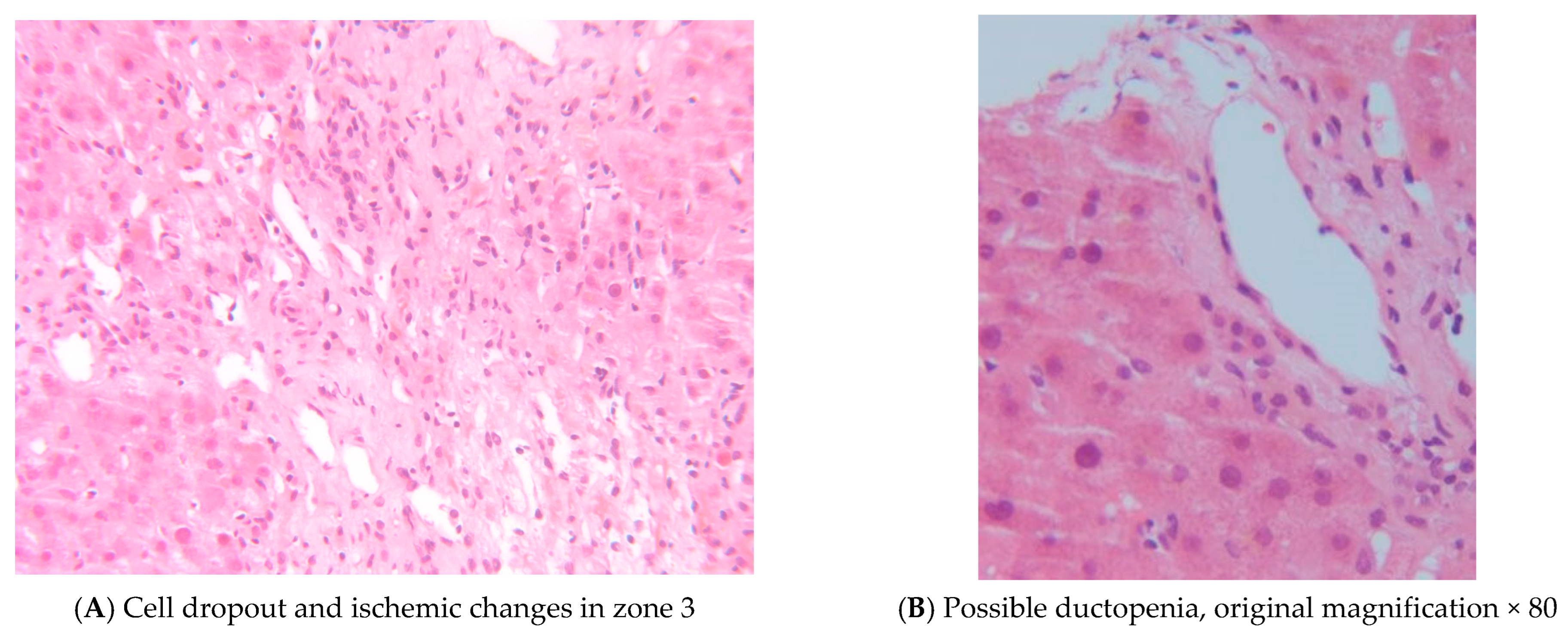
Table 1. Given this, a percutaneous liver biopsy was performed on PTD 113. This showed cell dropout and ischemic changes in zone 3 with duct injury and early ductopenia, with these changes felt to be consistent with SOT-GVHD (
Figure 3).
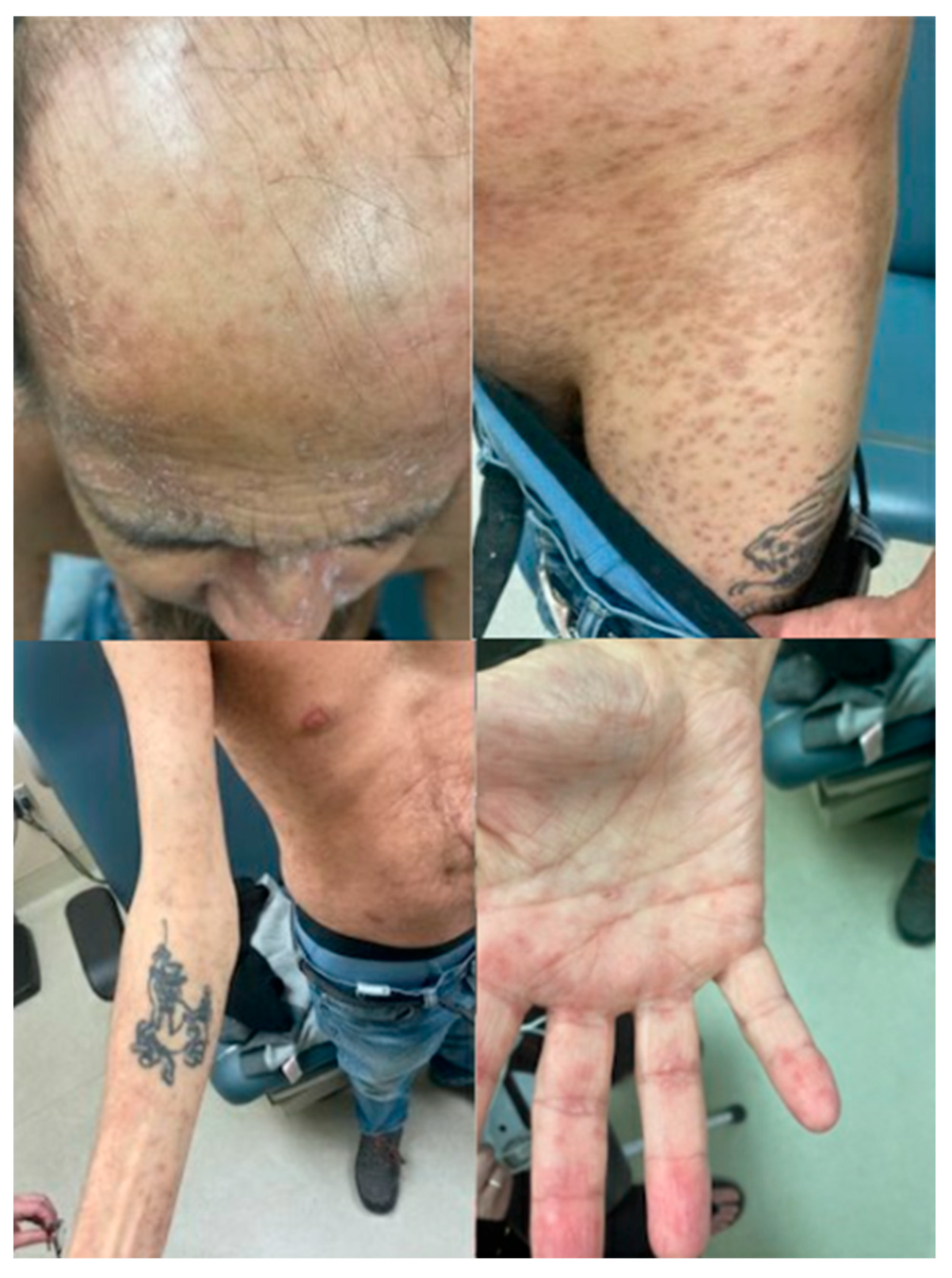
Outpatient follow-up with Dermatology took place on PTD 111, at which time he had few active lesions and mainly post-inflammatory hyperpigmentation changes to the previous lesions. The appearance of the skin lesions at that time is shown in
Figure 4. Another skin punch biopsy was performed again showing a superficial vacuolar interface dermatitis with a predominantly lymphohistiocytic inflammatory infiltrate as well as some melanophages in keeping with post-inflammatory hyperpigmentation. Immunofluorescence was negative and unfortunately the sample was lost before microcherism testing could be performed.
A bone marrow biopsy performed on PTD 115 demonstrated normocellular marrow with relative erythroid predominance, immature myeloid cells present with normal blast percentage, rare mature granulocytes, and normal-appearing megakaryocytes. Microchimerism assay was performed using multiplex PCR amplification of Amelogenin locus and 15 micro-satellite markers (D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, D5S818 and FGA) to compare the recipient bone marrow aspirate to a banked DNA sample from the organ donor. This showed 43% of the CD3 + T-cells, 2% of the CD19 + B-cells, and <2% of the myeloid cells were of donor origin.
Given all of these findings, a diagnosis of SOT-GVHD was made. On PTD 125, he started on prednisone 30 mg oral daily (0.5 mg/kg) for 4 weeks. As the mortality from SOT-GVHD is primarily driven by the associated bone marrow suppression and secondary infections, he was also started on granulocyte colony stimulating factor (G-CSF) as well as prophylactic acyclovir 400 mg oral twice daily and levofloxacin 750 mg oral daily. The patient responded very well to this treatment, with significant improvement in his transaminitis and cytopenias by PTD 153, as shown in
Table 1. The G-CSF, acyclovir, and levofloxacin were therefore discontinued on PTD 153, and a slow prednisone taper was also initiated at that time, reducing the daily dose by 5 mg every 14 days until 5 mg oral daily, then continuing on this dose indefinitely.
The patient remained clinically stable until PTD 425, when he developed progressive cytopenia. At that time, he was evaluated by Urology, which revealed significant leukopenia (WBC < 0.5), hemoglobin of 94, and a platelet count of 18. Due to concerns about possible myelosuppression, the hematology team advised reducing Myfortic from 750 mg BID to 360 mg PO BID.
However, four days later, he presented to the Emergency Department with fever (40 °C), rigors, hypotension (BP 90/30), tachycardia (HR 106/min) left thigh pain, tongue and lip swelling, progressive weakness, poor appetite, nausea, and vomiting. He denied any rash or lymphadenopathy. Examination revealed a small hematoma on the right lateral tongue and hyperpigmented skin lesions consistent with prior GVHD scarring.
Given the broad differential diagnosis for his pancytopenia, including infection, GVHD recurrence, drug-induced thrombotic microangiopathy (TMA) from tacrolimus, Myfortic-related myelosuppression, and evolving aplastic anemia he was admitted to the hospital under care of critical care unite (PTD 429) with septic shock for further management and treatment. After resuscitation, initial work up and empirical antibiotic therapy with IV piperacillin-tazobactam and vancomycin, a bone marrow biopsy was performed by hematology team and revealed markedly hypocellular marrow with aplasia and a T-cell–dominant population. Flow cytometry of peripheral blood showed ~88% T cells with dim expression of CD3, CD8, CD52, CD7, and absence of CD4, consistent with a T-cell lymphoproliferative neoplasm.
Blood cultures grew methicillin-sensitive Staphylococcus aureus (MSSA). Therefore, empiric antibiotic therapy transitioned to IV cefazolin. The patient had no indwelling catheters, skin breaks, or prosthetic devices. CT scans of the abdomen/pelvis and femurs showed no abscess or fluid collection, and the WBC scan was negative. Transthoracic echocardiography showed no signs of endocarditis. Transesophageal echocardiography was deferred due to severe thrombocytopenia (platelets 19), with a plan to reassess if bacteremia recurred.
Subsequently, the patient developed recurrent bacteremia with Enterococcus faecium and Enterobacter cloacae complex. He started on vancomycin and meropenem. His condition worsened, with refractory hypotension requiring norepinephrine, respiratory distress needing 4 L/min oxygen by nasal cannula, tachycardia, and fever to 38.2 °C. CT thorax revealed a new large mass-like density (4 × 4 cm) at the right hilum extending into the subcarinal region, encasing the right mainstem bronchus, with additional nodular opacities in the lower lobes and right fissure. Bronchoscopy with BAL identified Aspergillus flavus in fungal cultures. He started on oral voriconazole but switched to IV amphotericin B (5 mg/kg q24h) due to visual hallucinations.
Around the same time, blood cultures grew Pseudomonas aeruginosa, likely due to gut translocation from profound neutropenia. Given resistance to carbapenems, meropenem was replaced with ceftazidime based on susceptibility testing.
He also developed severe Clostridioides difficile colitis and was treated with IV metronidazole TID and high-dose oral vancomycin (500 mg Q6H).
Despite broad-spectrum antimicrobial therapy (ceftazidime, vancomycin, amphotericin B) and treatment for C. difficile infection, his condition progressively declined with persistent distributive shock.
Following a detailed discussion with the patient and his family, he expressed a clear preference not to pursue further life-sustaining interventions. A decision was made to transition to comfort-focused care, and his code status was changed to DNR-Comfort Measures Only. He passed away shortly thereafter, following withdrawal of life-sustaining treatments (PTD 464).
3. Discussion
Although a well-known and common complication of hematopoietic stem cell transplants, graft-versus-host-disease (GVHD) was only first reported in an orthotopic liver transplant recipient by Burdick and colleagues in 1988 [
1].In the solid organ transplant setting, GVHD is thought to occur due to donor-derived immunocompetent lymphocytes within the transplanted organ, which manage to evade the recipient’s immune system and then encounter recipient alloantigens, causing the donor lymphocytes to mount an immune response against the recipient’s tissues [
2,
3]. Thus, the risk of SOT-GVHD is felt to be higher for transplanted organs which contain larger amounts of viable lymphoid tissue such as the small intestine and liver when compared with kidney, heart, and lung [
4]. Pancreas transplant recipients are also at an increased risk of this complication as a small portion of the donor’s duodenum is routinely implanted alongside the donor pancreas [
5] although this risk may be underrecognized by clinicians. Other established risk factors for SOT-GVHD include HLA mismatch, re-transplantation, large graft volume, donor–recipient age and race mismatch, and pre-existing immunodeficiency in the recipient [
4].
SOT-GVHD most commonly occurs in the first 2–12 weeks post-transplant [
6]. Manifestations of SOT-GVHD are varied: cutaneous, gastrointestinal, hepatic, bone marrow, and oropharyngeal mucosal involvement have all been well-described in the literature, and multi-system involvement is common [
4]. Bone marrow involvement is thought to be unique to SOT-GVHD, and hemophagocytic lymphohistiocytosis (HLH) may also be seen [
7]. Fever and other non-specific symptoms such as nausea, vomiting, anorexia, and malaise can also occur [
6]. Cutaneous involvement is reported to be the most common and often earliest presentation [
8], although the rash is often non-specific, with maculopapular, morbilliform, desquamating, and confluent skin eruptions all reported in case series [
9]. Lichenoid eruptions, as was seen with our patient, have been noted to be more common in SOT-GVHD than GVHD following allogeneic stem cell transplant [
10].
The signs and symptoms of SOT-GVHD may be mistaken for more commonly encountered infections or medication side effects, leading to delays in diagnosis [
4]. Biopsies of the affected organs can show characteristic histopathologic findings and remain the gold standard for diagnosis [
3]. Chimerism assays and FISH analysis may confirm the persistence of donor cells and support the diagnosis; these modalities are also being explored for early disease detection and risk stratification [
11].
The optimal management of SOT-GVHD has not yet been determined. Systemic and topical corticosteroids are generally the first line of therapy, with the addition of ATG, alemtuzumab, and ruxolitinib in steroid-refractory cases [
4]. For those with bone marrow involvement not responding to steroids, allogeneic bone marrow transplant may be considered [
4]. Antimicrobial prophylaxis and G-CSF support is recommended for those with bone marrow involvement, noting that mortality in SOT-GVHD is often attributable to secondary infections [
4].
The clinical deterioration of this patient following post-transplant day (PTD) 425 illustrates the devastating and multifactorial nature of graft-versus-host disease (GVHD) following solid organ transplantation (SOT), particularly in the setting of immunosuppression and profound cytopenia.
GVHD is a rare but often fatal complication following SOT, most frequently associated with liver and intestinal transplants, though it has also been documented after kidney and pancreas transplantation [
11,
12,
13]. GVHD results from the activation of donor-derived immunocompetent T cells that recognize recipient antigens as foreign, initiating a cytotoxic immune response [
11]. The patient’s presentation at PTD 429, with systemic symptoms and marrow aplasia, is consistent with both acute and chronic GVHD manifestations reported weeks to months after SOT [
14,
15].
Bone marrow biopsy revealed marked hypocellularity and a T-cell–dominant population with flow cytometry showing ~88% aberrant CD8 + T cells, findings which are characteristic of GVHD-related marrow infiltration [
15]. Cytopenias and marrow suppression have been frequently reported as early manifestations of GVHD after SOT and are often a harbinger of a poor prognosis [
13,
15].
The patient’s subsequent polymicrobial infections—including MSSA, Enterococcus faecium, Enterobacter cloacae, Pseudomonas aeruginosa, Aspergillus flavus, and Clostridioides difficile—reflect the profound immunodeficiency associated with GVHD. Infection remains the leading cause of death in GVHD after SOT, typically due to neutropenia and epithelial barrier breakdown [
11,
14].
Therapeutic approaches in GVHD after SOT remain empiric and challenging. Corticosteroids are the standard first-line therapy, but response rates are low compared to hematopoietic stem cell transplant-associated GVHD [
11]. Other agents such as anti-CD25 monoclonal antibodies (e.g., basiliximab, daclizumab), mesenchymal stromal cells, and extracorporeal photopheresis have shown some success in case reports and small series [
13,
15]. In this case, the patient’s critical illness and multi-organ dysfunction likely precluded the use of these second-line therapies.
This case contributes significantly to the limited literature on GVHD following simultaneous pancreas–kidney transplantation by offering a detailed timeline of multisystem involvement, including skin, liver, and bone marrow biopsy confirmed diagnosis and microchimerism evidence. Unlike many previously reported cases, our patient initially responded to corticosteroids and supportive therapy, but later experienced delayed marrow aplasia and fatal infectious complications, highlighting the unpredictable trajectory of SOT-GVHD and its diagnostic complexity. The use of repeat tissue biopsies and chimerism assays, including fluorescence in situ hybridization (FISH) and microchimerism PCR, was critical for confirming donor T-cell persistence and diagnosing GVHD, as supported by prior literature [
3,
4,
11]. The presentation with a lichenoid skin eruption more commonly observed in SOT-GVHD than in hematopoietic stem cell transplant-associated GVHD also contributes to the evolving recognition of cutaneous phenotypes in this setting [
9,
10]. Additionally, this case reinforces that even in kidney-pancreas transplant recipients, where GVHD is infrequently reported, clinicians must remain vigilant for this life-threatening syndrome, particularly in the setting of donor duodenum implantation and immunosuppression [
4,
5,
12,
15].
To date, approximately 20 cases of GVHD have been reported in pancreas transplant recipients, including both isolated pancreas and simultaneous pancreas–kidney (SPK) transplants [
14,
15]. Compared to these cases, our patient presented within the typical timeframe of 2–12 weeks post-transplant, with initial cutaneous involvement—consistent with the most frequently reported early manifestation [
4,
6,
9]. However, unlike some previously documented cases where gastrointestinal symptoms or pancytopenia were the primary presenting signs, our patient progressed from isolated lichenoid skin lesions to multisystem involvement, including liver injury and ultimately bone marrow aplasia. While many earlier reports relied primarily on histopathology from skin or gastrointestinal tissue, our diagnostic approach incorporated serial organ biopsies and quantitative microchimerism testing, which confirmed donor-origin T-cell engraftment—a method that has been underutilized but is gaining recognition for its diagnostic precision [
3,
4,
11]. In terms of treatment, corticosteroids remain the mainstay across most cases, including ours, but the mortality rate remains high. Notably, second-line agents such as anti-T-cell therapies or bone marrow transplants have been attempted in select reports, but were not feasible in our case due to advanced disease and critical illness [
4,
13]. This comparison underscores the importance of high clinical suspicion, early tissue diagnosis, and emerging diagnostic tools like chimerism assays to improve recognition and outcomes in this rare but lethal transplant complication (
Table 2).
Published outcome data on solid organ transplant-associated graft-versus-host disease (SOT-GVHD) remain limited due to its rarity but consistently point to a poor overall prognosis. Reported mortality rates exceed 80%, particularly in cases involving the bone marrow, where profound cytopenias and susceptibility to opportunistic infections are common [
3,
4,
11,
14]. In a case series by Taylor et al., mortality was primarily attributed to marrow failure and secondary infections [
11]. Similarly, Newell et al. observed high rates of hemophagocytic lymphohistiocytosis and fatal outcomes in patients with SOT-GVHD and marrow involvement [
7]. While corticosteroids remain the mainstay of therapy, treatment response is often limited compared to hematopoietic stem cell transplant-associated GVHD [
4,
13]. A small number of case reports have explored second-line agents such as anti-thymocyte globulin (ATG), alemtuzumab, or ruxolitinib, but standardized treatment guidelines are lacking and outcomes remain variable. Early recognition, tissue diagnosis, and supportive care are therefore critical, but even with these measures, prognosis in multisystem GVHD remains guarded. Lauterio et al [
16] presented a compelling clinical case supporting the potential use of ruxolitinib, a Janus kinase (JAK) 1/2 inhibitor, as a pharmacologic treatment for cutaneous grade 2 GVHD following LT. This case offers valuable clinical insight into a pharmacological strategy that could prove beneficial in other instances of SOT-GVHD.
This case reinforces the need for early suspicion of GVHD in SOT recipients presenting with unexplained fever, rash, cytopenia, and gastrointestinal symptoms. Delays in diagnosis are common due to overlapping presentations with infections and drug toxicities, contributing to high mortality rates—often exceeding 80% in multisystem GVHD [
11,
14]. Given that donor-derived T cells can persist and expand even months post-transplant, clinicians should maintain vigilance well beyond the perioperative period [
12,
15].