Long-Term Outcomes of Kidney Transplant Recipients with Glomerulonephritides by Induction Type and Steroid Avoidance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Outcomes of Interest
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
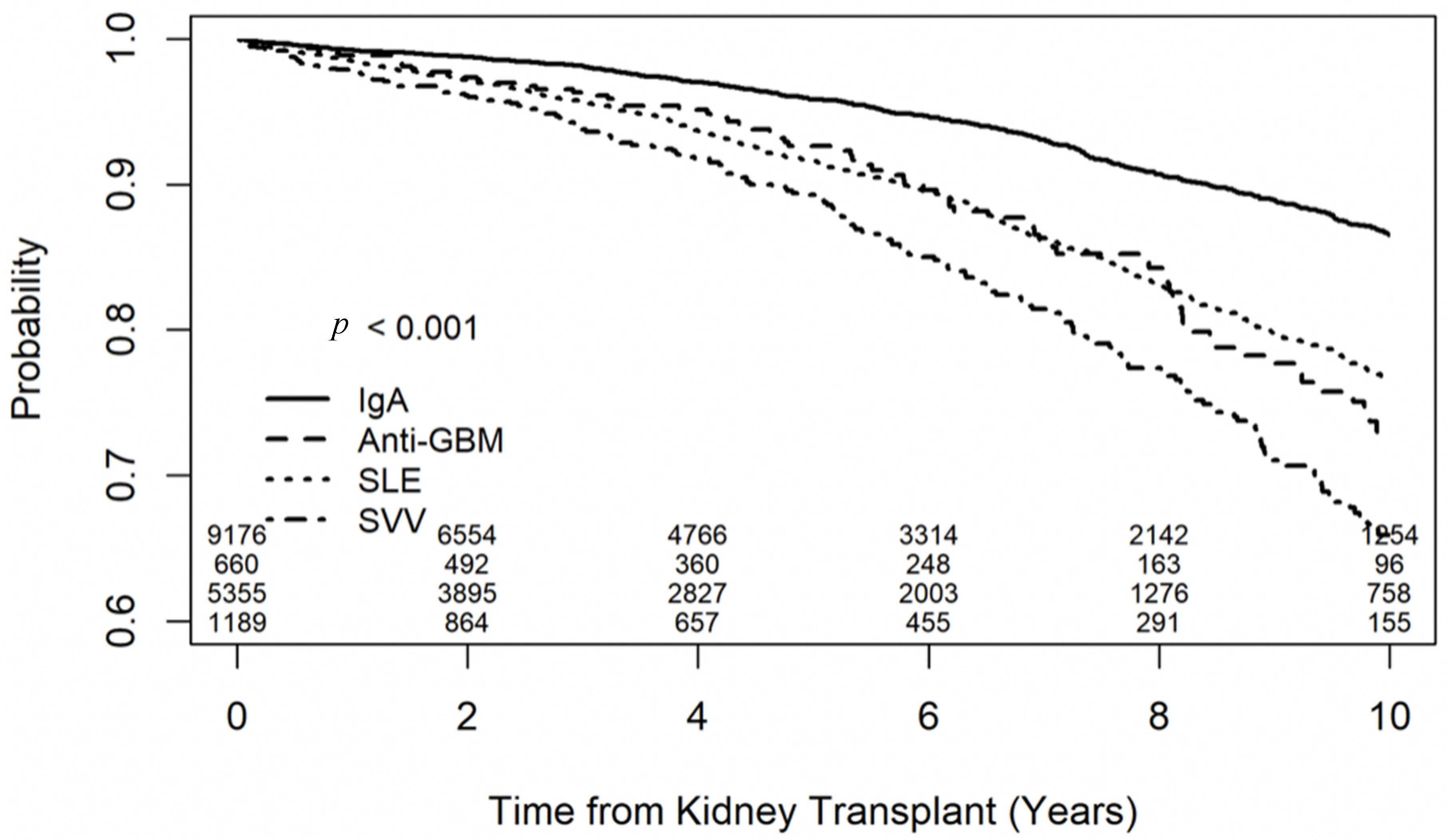
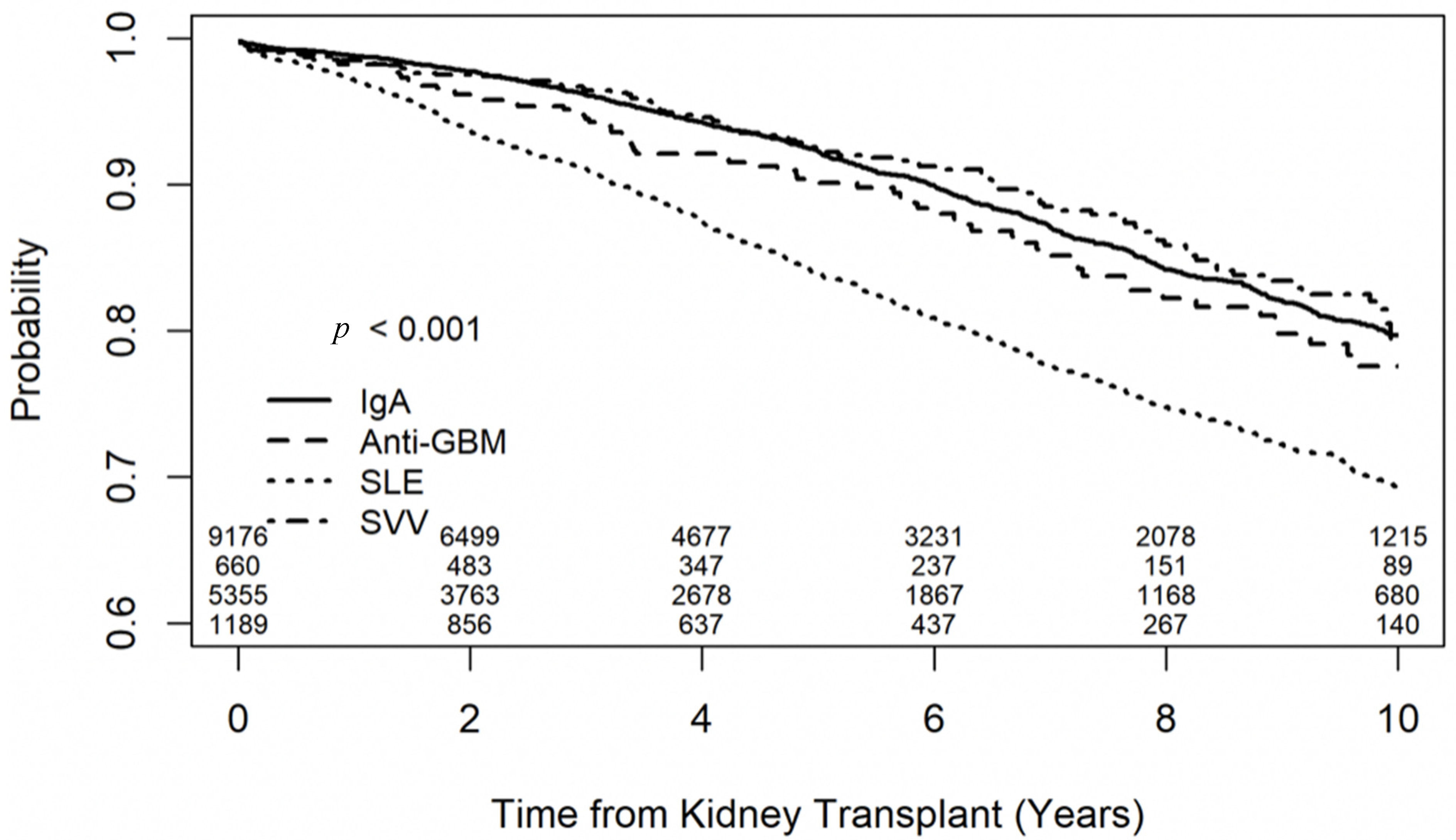
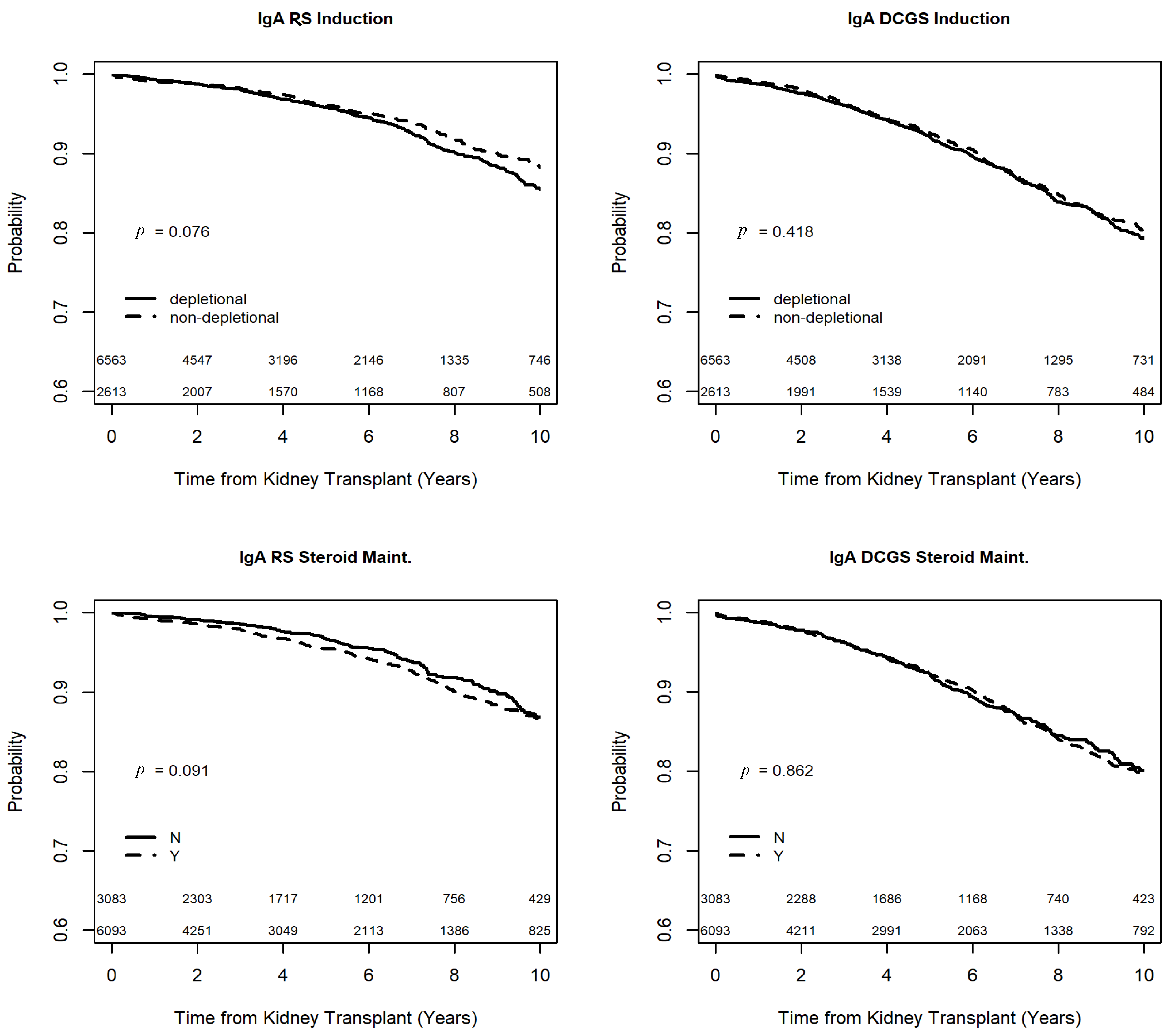
3.2. Outcomes of Recipients with IgA Nephropathy
3.3. Outcomes of Recipients with SLE-Related Glomerulonephritis
3.4. Outcomes of Recipients with Anti-GBM Glomerulonephritis
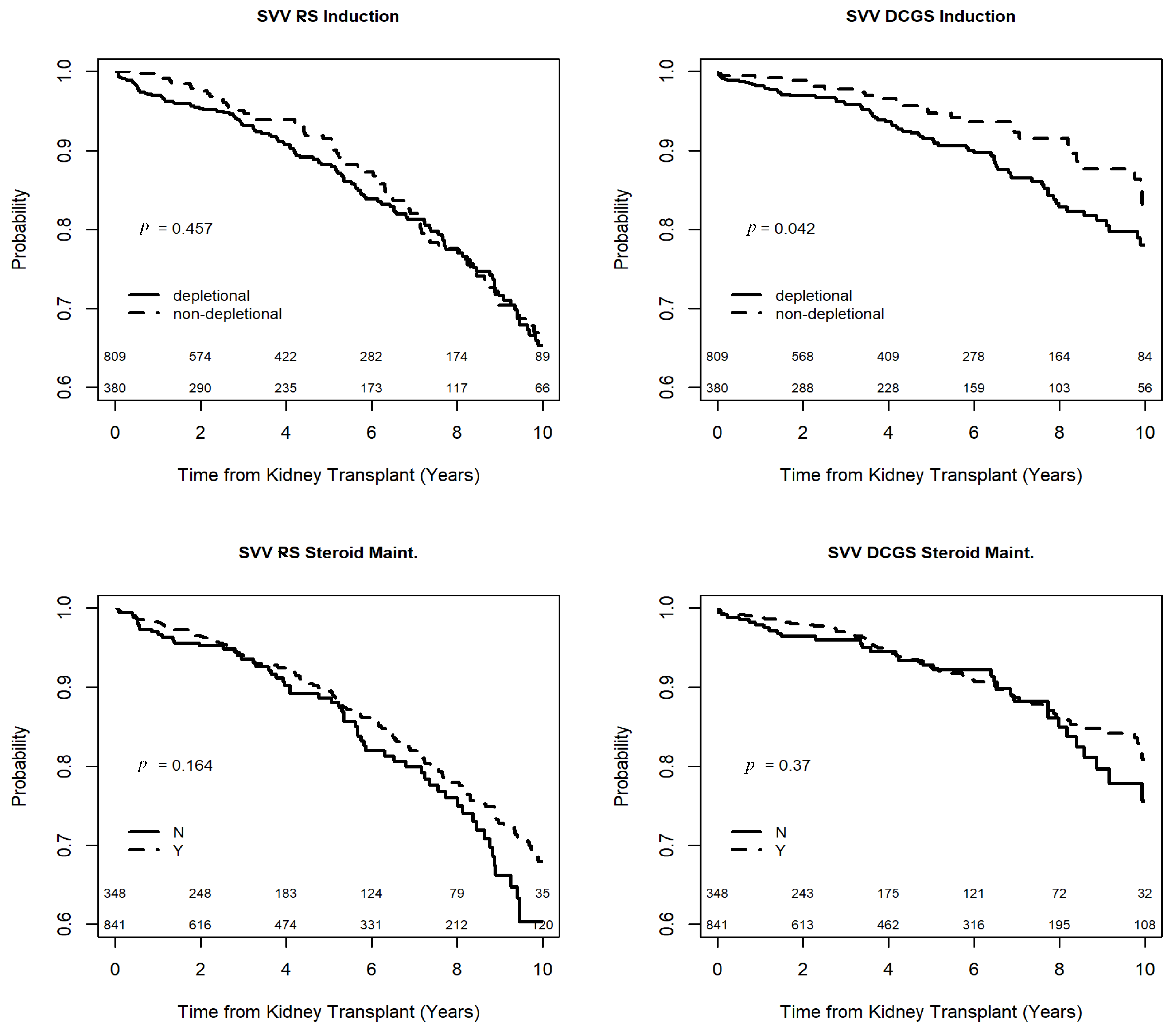
3.5. Outcomes of Recipients with SVV
3.6. Secondary Outcomes
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, W.H.; Shingde, M.; Wong, G. Recurrent and de novo Glomerulonephritis After Kidney Transplantation. Front. Immunol. 2019, 10, 1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, E.F. T-cell exhaustion limits immune reactivity and is associated with good prognosis in autoimmune disease. Nat. Rev. Rheumatol. 2015, 11, 501. [Google Scholar] [CrossRef]
- George, R.P.; Mehta, A.K.; Perez, S.D.; Winterberg, P.; Cheeseman, J.; Johnson, B.; Kwun, J.; Monday, S.; Stempora, L.; Warshaw, B.; et al. Premature T Cell Senescence in Pediatric CKD. J. Am. Soc. Nephrol. 2017, 28, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.J.; Kandaswamy, R.; Humar, A.; Payne, W.D.; Dunn, D.L.; Najarian, J.S.; Gruessner, R.W.G.; Gillingham, K.J.; McHugh, L.E.; Sutherland, D.E.R. Long-term Immunosuppression, Without Maintenance Prednisone, After Kidney Transplantation. Ann. Surg. 2004, 240, 510–517. [Google Scholar] [CrossRef]
- Kukla, A.; Chen, E.; Spong, R.; Weber, M.; El-Shahawi, Y.; Gillingham, K.; Matas, A.J.; Ibrahim, H.N. Recurrent Glomerulonephritis Under Rapid Discontinuation of Steroids. Transplantation 2011, 91, 1386–1391. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.; Rogers, T.; Casingal, V.; Sturdevant, M.; Tan, M.; Humar, A.; Gillingham, K.; Matas, A. Graft Loss from Recurrent Glomerulonephritis Is Not Increased with a Rapid Steroid Discontinuation Protocol. Transplantation 2006, 81, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.C.; Daller, J.A.; Lake, K.D.; Cibrik, D.; Del Castillo, D.; Thymoglobulin Induction Study Group. Rabbit Antithymocyte Globulin versus Basiliximab in Renal Transplantation. N. Engl. J. Med. 2006, 355, 1967–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.T.; Collins, C.D.; Stuckey, L.J.; Luan, F.L.; Englesbe, M.J.; Magee, J.C.; Park, J.M. Clinical and Economic Outcomes of Rabbit Antithymocyte Globulin Induction in Adults Who Received Kidney Transplants from Living Unrelated Donors and Received Cyclosporine-Based Immunosuppression. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 1166–1174. [Google Scholar] [CrossRef] [Green Version]
- Webster, A.C.; Ruster, L.P.; McGee, R.G.; Matheson, S.L.; Higgins, G.Y.; Willis, N.S.; Chapman, J.R.; Craig, J.C. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst. Rev. 2010, 2010, CD003897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riad, S.; Alexy, T.; Jackson, S.; Goswami, U.; Martin, C. Kidney Allograft and Recipient Survival After Heart Transplantation by Induction Type in the United States. Transplantation 2021. [Google Scholar] [CrossRef] [PubMed]
- Riad, S.; Goswami, U.; Jackson, S.; Hertz, M.; Matas, A. Induction type and outcomes for kidney graft and patient survival in recipients with prior lung transplantation in the United States. J. Hear. Lung Transplant. 2019, 39, 157–164. [Google Scholar] [CrossRef]
- Riad, S.M.; Lim, N.; Jackson, S.; Matas, A.J.; Lake, J. Outcomes of Kidney Allograft and Recipient Survival After Liver Transplantation by Induction Type in the United States. Liver Transplant. 2021, 27, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Hurst, F.P.; Belur, P.; Nee, R.; Agodoa, L.Y.; Patel, P.; Abbott, K.C.; Jindal, R.M. Poor Outcomes Associated With Neutropenia After Kidney Transplantation: Analysis of United States Renal Data System. Transplantation 2011, 92, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Henningsen, M.; Pisarski, P.; Walz, G.; Jänigen, B. Impact of G-CSF Therapy on Leukopenia and Acute Rejection Following Kidney Transplantation. Int. J. Organ. Transplant. Med. 2021, 12, 1–8. [Google Scholar] [PubMed]
- Sewgobind, V.D.K.D.; Kho, M.M.L.; van der Laan, L.J.W.; Hendrikx, T.K.; van Dam, T.; Tilanus, H.W.; Ijzermans, J.N.M.; Weimar, W.; Baan, C.C. The effect of rabbit anti-thymocyte globulin induction therapy on regulatory T cells in kidney transplant patients. Nephrol. Dial. Transplant. 2009, 24, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Mendoza Rojas, A.; Dieterich, M.; Roelen, D.L.; Clahsen-van Groningen, M.C.; Wang, L.; Van Gelder, T.; Hesselink, D.A.; van Besouw, N.M.; Baan, C.C. Immunosuppression Has Long-Lasting Effects on Circulating Follicular Regulatory T Cells in Kidney Transplant Recipients. Front. Immunol. 2020, 11, 1972. [Google Scholar] [CrossRef]
- Herrnstadt, G.R.; Steinmetz, O.M. The role of Treg subtypes in glomerulonephritis. Cell Tissue Res. 2020, 385, 293–304. [Google Scholar] [CrossRef]
- Barbour, S.; Djurdjev, O.; Gill, J.S.; Dong, J.J.; Gill, J. A propensity score matched analysis shows no adverse effect of early steroid withdrawal in non-diabetic kidney transplant recipients with and without glomerulonephritis. Kidney Int. 2019, 96, 460–469. [Google Scholar] [CrossRef]
- Luan, F.L.; Steffick, D.E.; Ojo, A.O. Steroid-free maintenance immunosuppression in kidney transplantation: Is it time to consider it as a standard therapy? Kidney Int. 2009, 76, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Augustine, J.J.; Hricik, D.E. Steroid Sparing in Kidney Transplantation: Changing Paradigms, Improving Outcomes, and Remaining Questions. Clin. J. Am. Soc. Nephrol. 2006, 1, 1080–1089. [Google Scholar] [CrossRef]
- Leeaphorn, N.; Garg, N.; Khankin, E.V.; Cardarelli, F.; Pavlakis, M. Recurrence of IgA nephropathy after kidney transplantation in steroid continuation versus early steroid-withdrawal regimens: A retrospective analysis of the UNOS/OPTN database. Transpl. Int. 2018, 31, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosh, S.M.; Knechtle, S.J.; Sollinger, H.W. Campath-1H Use in Pediatric Renal Transplantation. Am. J. Transplant. 2005, 5, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Riad, S.; Jackson, S.; Chinnakotla, S.; Verghese, P. Primary pediatric deceased-donor kidney transplant recipients outcomes by immunosuppression induction received in the United States. Pediatr. Transplant. 2021, 25, e13925. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Zugich, J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- DuBay, D.A.; MacLennan, P.A.; Reed, R.D.; Shelton, B.A.; Redden, D.T.; Fouad, M.; Martin, M.Y.; Gray, S.H.; White, J.A.; Eckhoff, D.E.; et al. Insurance Type and Solid Organ Transplantation Outcomes: A Historical Perspective on How Medicaid Expansion Might Impact Transplantation Outcomes. J. Am. Coll. Surg. 2016, 223, 611–620.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Congress.gov, Library of Congress. H.R.5534-116th Congress (2019–2020): Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2020. Available online: https://www.congress.gov/bill/116th-congress/house-bill/5534 (accessed on 24 December 2020).
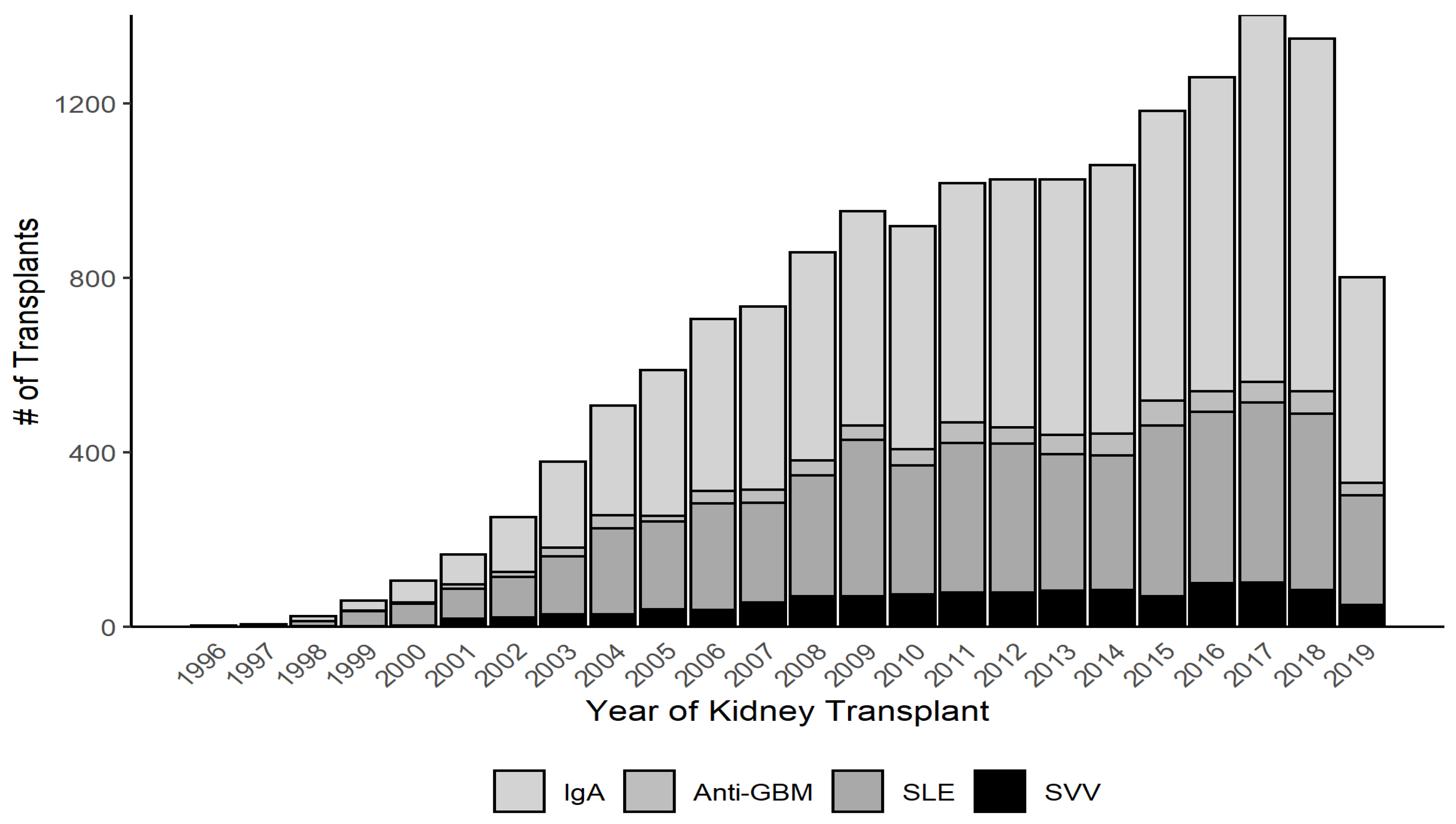


| IgAN n = 9176 | SLE n = 5355 | Anti-GBM n = 660 | SVV n = 1189 | |
|---|---|---|---|---|
| Recipient Age in Years | 44.61 (12.83) | 40.92 (12.23) | 49.05 (16.18) | 52.45 (15.23) |
| Recipient Sex (Male) | 5910 (64.4) | 994 (18.6) | 303 (45.9) | 680 (57.2) |
| Recipient Ethnicity | ||||
| Black | 471 (5.1) | 2283 (42.6) | 62 (9.4) | 91 (7.7) |
| Other | 1994 (21.7) | 521 (9.7) | 24 (3.6) | 44 (3.7) |
| White | 6711 (73.2) | 2551 (47.7) | 574 (87.0) | 1054 (88.6) |
| Diabetes Mellitus | 429 (4.7) | 239 (4.5) | 38 (5.8) | 91 (7.7) |
| Peripheral Vascular Disease | 160 (1.8) | 162 (3.1) | 12 (1.8) | 48 (4.1) |
| Preemptive Transplant | 2368 (25.6) | 697 (12.8) | 41 (6.2) | 195 (16.2) |
| Dialysis Vintage (years) | 2.08 (2.55) | 3.64 (3.37) | 2.87 (2.28) | 2.68 (2.58) |
| Panel Reactive Antibody | 11.87 (23.96) | 27.44 (34.44) | 17.83 (28.94) | 15.07 (27.35) |
| HLA Antigen Mismatches | ||||
| 0 | 716 (7.9) | 436 (8.2) | 73 (11.1) | 94 (7.9) |
| 1–3 | 2948 (32.4) | 1523 (28.6) | 221 (33.8) | 416 (35.1) |
| 4–6 | 5437 (59.7) | 3369 (63.2) | 361 (55.1) | 676 (57.0) |
| Non-Depletional Induction | 2613 (28.5) | 1319 (24.6) | 165 (25.0) | 380 (32.0) |
| Steroid Maintenance Therapy | 6093 (66.4) | 4225 (78.9) | 456 (69.1) | 841 (70.7) |
| Primary Public Payer | 4129 (45.2) | 3601 (67.5) | 429 (65.1) | 723 (61.1) |
| Donor Age in Years | 38.60 (14.41) | 36.57 (14.61) | 40.11 (15.18) | 40.69 (14.09) |
| Donor Sex (Male) | 4601 (50.1) | 2908 (54.3) | 329 (49.8) | 564 (47.4) |
| Donor Race | ||||
| Black | 610 (6.6) | 999 (18.6) | 59 (8.9) | 87 (7.3) |
| Other | 796 (8.7) | 287 (5.4) | 12 (1.8) | 38 (3.2) |
| White | 7770 (84.7) | 4069 (76.0) | 589 (89.3) | 1064 (89.5) |
| Donation after Cardiocirculatory Death | 792 (8.6) | 489 (9.1) | 64 (9.7) | 111 (9.3) |
| Donation after Brain Death | 3526 (38.4) | 2843 (53.1) | 276 (41.8) | 515 (43.3) |
| Living Donor | 4857 (52.9) | 2022 (37.8) | 320 (48.5) | 563 (47.4) |
| Cold Ischemia Time 1 | 17.14 (8.66) | 17.24 (8.53) | 18.28 (7.95) | 17.74 (8.88) |
| Variable | Recipient Survival | Graft Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | p-Value | HR | 95% Confidence Interval | p-Value | |
| Non-Depletional Induction | 0.83 | (0.67, 1.03) | 0.09 | 0.98 | (0.83, 1.15) | 0.78 |
| Steroid Maintenance | 1.21 | (0.98, 1.51) | 0.08 | 0.97 | (0.83, 1.13) | 0.66 |
| HLA Antigen Mismatches | ||||||
| 0 | Ref | Ref | ||||
| 1–3 | 1.41 | (0.94, 2.11) | 0.10 | 1.47 | (1.08, 1.98) | 0.01 |
| 4–6 | 1.26 | (0.85, 1.87) | 0.24 | 1.48 | (1.11, 1.99) | 0.01 |
| Recipient Age (Year) | 1.06 | (1.05, 1.07) | <0.001 | 0.97 | (0.96, 0.97) | <0.001 |
| Recipient Sex (Male) | 1.33 | (1.07, 1.64) | 0.01 | 0.85 | (0.74, 0.99) | 0.03 |
| Recipient Race | ||||||
| Black | Ref | Ref | ||||
| Other | 0.61 | (0.38, 0.98) | 0.04 | 0.37 | (0.28, 0.50) | <0.001 |
| White | 1.04 | (0.69, 1.57) | 0.85 | 0.58 | (0.45, 0.75) | <0.001 |
| Diabetes Mellitus | 1.65 | (1.22, 2.22) | 0.001 | 1.60 | (1.15, 2.23) | 0.005 |
| Pre-Transplant Dialysis | 1.71 | (1.30, 2.27) | <0.001 | 1.44 | (1.17, 1.78) | 0.001 |
| Dialysis Vintage (per year) | 1.06 | (1.02, 1.10) | 0.004 | 1.02 | (0.98, 1.06) | 0.28 |
| Deceased Donor Kidney | 1.27 | (1.01, 1.61) | 0.04 | 1.69 | (1.42, 2.02) | <0.001 |
| Peripheral Vascular Disease | 2.05 | (1.24, 3.39) | 0.005 | 1.75 | (1.03, 2.98) | 0.04 |
| Donor Age (Year) | 1.00 | (0.99, 1.01) | 0.43 | 1.01 | (1.01, 1.02) | <0.001 |
| Donor Sex (Male) | 0.83 | (0.69, 1.00) | 0.053 | 0.69 | (0.60, 0.80) | <0.001 |
| Donor Race | ||||||
| Black | Ref | Ref | ||||
| Other | 0.67 | (0.37, 1.21) | 0.18 | 0.98 | (0.65, 1.47) | 0.92 |
| White | 0.84 | (0.60, 1.18) | 0.32 | 1.02 | (0.78, 1.34) | 0.89 |
| Public Primary Payor | 1.39 | (1.13, 1.72) | 0.002 | 1.17 | (0.99, 1.38) | 0.06 |
| Variable | Recipient Survival | Graft Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | p-Value | HR | 95% Confidence Interval | p-Value | |
| Non-Depletional Induction | 1.04 | (0.86, 1.26) | 0.69 | 0.90 | (0.76, 1.06) | 0.21 |
| Steroid Maintenance | 1.24 | (0.99, 1.57) | 0.07 | 1.01 | (0.85, 1.21) | 0.91 |
| HLA Antigen Mismatches | ||||||
| 0 | Ref | Ref | ||||
| 1–3 | 0.71 | (0.52, 0.98) | 0.04 | 1.13 | (0.85, 1.51) | 0.39 |
| 4–6 | 0.80 | (0.60, 1.06) | 0.12 | 1.11 | (0.84, 1.46) | 0.46 |
| Recipient Age (Year) | 1.03 | (1.02, 1.04) | <0.001 | 0.95 | (0.95, 0.96) | <0.001 |
| Recipient Sex (Male) | 1.39 | (1.13, 1.70) | 0.002 | 1.30 | (1.11, 1.54) | 0.002 |
| Recipient Race | ||||||
| Black | Ref | Ref | ||||
| Other | 0.61 | (0.41, 0.92) | 0.02 | 0.54 | (0.40, 0.73) | <0.001 |
| White | 0.98 | (0.81, 1.19) | 0.82 | 0.63 | (0.53, 0.74) | <0.001 |
| Diabetes Mellitus | 1.51 | (1.1, 2.09) | 0.01 | 1.08 | (0.74, 1.59) | 0.68 |
| Pre-transplant Dialysis | 1.54 | (1.1, 2.14) | 0.01 | 1.41 | (1.07, 1.86) | 0.02 |
| Dialysis Vintage (per year) | 1.01 | (0.99, 1.04) | 0.34 | 0.99 | (0.97, 1.02) | 0.55 |
| Deceased Donor Kidney | 1.42 | (1.13, 1.78) | 0.003 | 1.62 | (1.34, 1.95) | <0.001 |
| Peripheral Vascular Disease | 1.54 | (1.03, 2.30) | 0.04 | 1.11 | (0.72, 1.72) | 0.64 |
| Donor Age (Year) | 1.01 | (1.01, 1.02) | <0.001 | 1.02 | (1.01, 1.02) | <0.001 |
| Donor Sex (Male) | 0.93 | (0.78, 1.10) | 0.37 | 0.95 | (0.83, 1.10) | 0.50 |
| Donor Race | ||||||
| Black | Ref | Ref | ||||
| Other | 0.65 | (0.37, 1.14) | 0.14 | 0.89 | (0.60, 1.32) | 0.55 |
| White | 0.87 | (0.69, 1.10) | 0.25 | 0.90 | (0.74, 1.08) | 0.24 |
| Public Primary Payor | 1.31 | (1.06, 1.61) | 0.01 | 1.07 | (0.91, 1.26) | 0.43 |
| Variable | Recipient Survival | Graft Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | p-Value | HR | 95% Confidence Interval | p-Value | |
| Non-Depletional Induction | 1.11 | (0.62, 1.98) | 0.73 | 0.73 | (0.40, 1.31) | 0.29 |
| Steroid Maintenance | 1.19 | (0.66, 2.16) | 0.56 | 1.03 | (0.60, 1.77) | 0.91 |
| HLA Antigen Mismatches | ||||||
| 0 | Ref | Ref | ||||
| 1–3 | 1.70 | (0.63, 4.61) | 0.30 | 1.41 | (0.58, 3.42) | 0.45 |
| 4–6 | 2.77 | (1.11, 6.91) | 0.03 | 1.37 | (0.60, 3.15) | 0.46 |
| Recipient Age (Year) | 1.04 | (1.02, 1.06) | <0.001 | 0.96 | (0.95, 0.98) | <0.001 |
| Recipient Sex (Male) | 1.29 | (0.77, 2.18) | 0.33 | 0.98 | (0.61, 1.59) | 0.95 |
| Recipient Race | ||||||
| Black | Ref | Ref | ||||
| Other | 0.37 | (0.04, 3.95) | 0.41 | 0.23 | (0.03, 1.90) | 0.17 |
| White | 2.70 | (0.78, 9.34) | 0.12 | 0.68 | (0.31, 1.50) | 0.34 |
| Diabetes Mellitus | 1.32 | (0.57, 3.07) | 0.51 | 0.38 | (0.05, 2.77) | 0.34 |
| Pre-Transplant Dialysis | 0.88 | (0.29, 2.66) | 0.82 | 0.78 | (0.31, 1.97) | 0.60 |
| Dialysis Vintage (per year) | 1.05 | (0.93, 1.18) | 0.42 | 1.02 | (0.91, 1.15) | 0.70 |
| Deceased Donor Kidney | 1.67 | (0.90, 3.08) | 0.10 | 1.86 | (1.04, 3.31) | 0.04 |
| Peripheral Vascular Disease | 0.77 | (0.10, 6.07) | 0.80 | Data Missing | ||
| Donor Age (Year) | 1.00 | (0.99, 1.02) | 0.78 | 1.02 | (1.01, 1.04) | 0.01 |
| Donor Sex (Male) | 0.96 | (0.57, 1.63) | 0.89 | 0.66 | (0.40, 1.09) | 0.10 |
| Donor Race | ||||||
| Black | Ref | Ref | ||||
| Other | 0.76 | (0.08, 7.07) | 0.81 | Data Missing | ||
| White | 1.29 | (0.51, 3.27) | 0.60 | Data Missing | ||
| Public Primary Payor | 1.31 | (1.06, 1.61) | 0.01 | 1.88 | (1.04, 3.41) | 0.04 |
| Variable | Recipient Survival | Graft Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | p-Value | HR | 95% Confidence Interval | p-Value | |
| Non-Depletional Induction | 0.96 | (0.70, 1.32) | 0.80 | 0.64 | (0.40, 1.04) | 0.07 |
| Steroid Maintenance | 0.91 | (0.66, 1.25) | 0.56 | 0.88 | (0.56, 1.38) | 0.57 |
| HLA Antigen Mismatches | ||||||
| 0 | Ref | Ref | ||||
| 1–3 | 1.01 | (0.54, 1.90) | 0.97 | 5.29 | (1.24, 22.47) | 0.02 |
| 4–6 | 1.24 | (0.69, 2.25) | 0.47 | 6.13 | (1.48, 25.45) | 0.01 |
| Recipient Age (Year) | 1.04 | (1.03, 1.05) | <0.001 | 0.98 | (0.96, 0.99) | 0.001 |
| Recipient Sex (Male) | 1.04 | (0.77, 1.41) | 0.81 | 0.80 | (0.53, 1.21) | 0.29 |
| Recipient Race | ||||||
| Black | Ref | Ref | ||||
| Other | 0.40 | (0.11, 1.48) | 0.17 | 1.03 | (0.30, 3.58) | 0.97 |
| White | 0.81 | (0.44, 1.49) | 0.49 | 0.96 | (0.43, 2.17) | 0.92 |
| Diabetes Mellitus | 1.52 | (0.98, 2.34) | 0.06 | 1.73 | (0.90, 3.32) | 0.10 |
| Pre-Transplant Dialysis | 1.60 | (0.89, 2.87) | 0.12 | 1.65 | (0.80, 3.38) | 0.17 |
| Dialysis Vintage (per year) | 1.06 | (0.99, 1.13) | 0.10 | 0.98 | (0.88, 1.09) | 0.65 |
| Deceased Donor Kidney | 1.02 | (0.71, 1.47) | 0.93 | 1.37 | (0.81, 2.32) | 0.24 |
| Peripheral Vascular Disease | 1.08 | (0.49, 2.40) | 0.84 | 0.73 | (0.17, 3.05) | 0.66 |
| Donor Age (Year) | 1.005 | (0.995, 1.02) | 0.30 | 1.02 | (1.00, 1.03) | 0.04 |
| Donor Sex (Male) | 0.85 | (0.63, 1.16) | 0.31 | 1.26 | (0.83, 1.92) | 0.28 |
| Donor Race | ||||||
| Black | Ref | Ref | ||||
| Other | 1.03 | (0.36, 2.92) | 0.96 | 1.69 | (0.43, 6.60) | 0.45 |
| White | 0.76 | (0.46, 1.26) | 0.29 | 1.20 | (0.51, 2.82) | 0.67 |
| Public Primary Payor | 1.26 | (0.88, 1.82) | 0.21 | 0.97 | (0.61, 1.53) | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravipati, P.; Jackson, S.; Tumer, G.; Nachman, P.H.; Riad, S.M. Long-Term Outcomes of Kidney Transplant Recipients with Glomerulonephritides by Induction Type and Steroid Avoidance. Transplantology 2022, 3, 68-82. https://doi.org/10.3390/transplantology3010007
Ravipati P, Jackson S, Tumer G, Nachman PH, Riad SM. Long-Term Outcomes of Kidney Transplant Recipients with Glomerulonephritides by Induction Type and Steroid Avoidance. Transplantology. 2022; 3(1):68-82. https://doi.org/10.3390/transplantology3010007
Chicago/Turabian StyleRavipati, Prasanth, Scott Jackson, Gizem Tumer, Patrick H. Nachman, and Samy M. Riad. 2022. "Long-Term Outcomes of Kidney Transplant Recipients with Glomerulonephritides by Induction Type and Steroid Avoidance" Transplantology 3, no. 1: 68-82. https://doi.org/10.3390/transplantology3010007
APA StyleRavipati, P., Jackson, S., Tumer, G., Nachman, P. H., & Riad, S. M. (2022). Long-Term Outcomes of Kidney Transplant Recipients with Glomerulonephritides by Induction Type and Steroid Avoidance. Transplantology, 3(1), 68-82. https://doi.org/10.3390/transplantology3010007







