Strategies to Improve Immune Suppression Post-Liver Transplantation: A Review
Abstract
1. Introduction
2. Immunosuppression Protocols: The Past and the Present
3. Effects of Immune-Suppressive Agents on Recipients’ Health
3.1. Mechanisms of Metabolic Derangements Induced by Commonly Used Immunosuppressive Agents
3.1.1. Calcineurin Inhibitors
3.1.2. Mammalian Target of Rapamycin Inhibitors
3.1.3. Steroids
3.2. Approach to the Management of Common Post-Transplant Metabolic Derangements
- (i)
- Post-transplant hypertension is the most commonly reported metabolic derangement; it is estimated that ~66% of liver transplant recipients develop hypertension shortly after transplantation [30]. Calcium channel blockers (CCBs), due to their vasodilator properties, are an effective treatment, especially in CNI-induced hypertension. Some calcium channel blockers, such as Nifedipine, inhibit intestinal cytochrome P450, thus increasing the level of CNIs. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are effective and potent treatments, but they require close monitoring, as they can induce hyperkalemia. Non-selective beta-blockers are also effective, but are not as potent as CCBs and ACE inhibitors [31].
- (ii)
- For the management of PTDM, insulin therapy, especially early after an operation, represents the cornerstone. Metformin showed potential in animal studies; however, it cannot be used in patients with renal impairments. Other antidiabetic agents, such as incretins, insulin sensitizers, and insulin secretagogues, are safe in LT recipients who have stable cardiac, renal, and hepatic functions [32].
- (iii)
- Dyslipidemia management should be primarily through weight reduction. However, hyperlipidemia with MTORis may necessitate termination of its use. The true clinical dilemma in using hypolipidemic drugs, especially statins, is their interaction with cytochrome P450, which metabolizes CNIs. The use of statins that do not interfere with cytochrome P450 (e.g., pravastatin and rosuvastatin) or the use of fish oil and fibrates can be an alternative. Fibrates can be nephrotoxic and they may lower the level of CsA, but they are used as first-line agents in MTORi-induced hypertriglyceridemia. Ezetimibe can be used as a monotherapy to decrease LDL levels without affecting immunosuppression levels [33].
- (iv)
- Post-LT obesity leads to accelerated graft steatosis, exacerbation of hypertension, and deterioration of renal function, especially in patients who received transplants due to NASH [34]. Dietary interventions, pharmacologic treatment, and post-transplant bariatric surgery are the available treatment options. For the medical treatment of obesity, lorcaserin, naltrexone, and bupropion have important drug–drug interactions with CNIs. For bariatric surgery, its impact is variable between individuals, as the absorption of immunosuppressive drugs is highly affected by the type of bariatric surgery. For example, sleeve gastrectomy leads to a 40% decrease in MMF absorption because it is absorbed in the stomach. On the other hand, the absorption of TAC and MTORi decreases by 50% in patients who undergo gastric bypass operations, as their absorption is affected by intestinal cytochrome P450 [35].
3.3. Approach to the Management of Common Non-Metabolic Adverse Effects
4. Effect of Immunosuppression on Health-Related Quality of Life
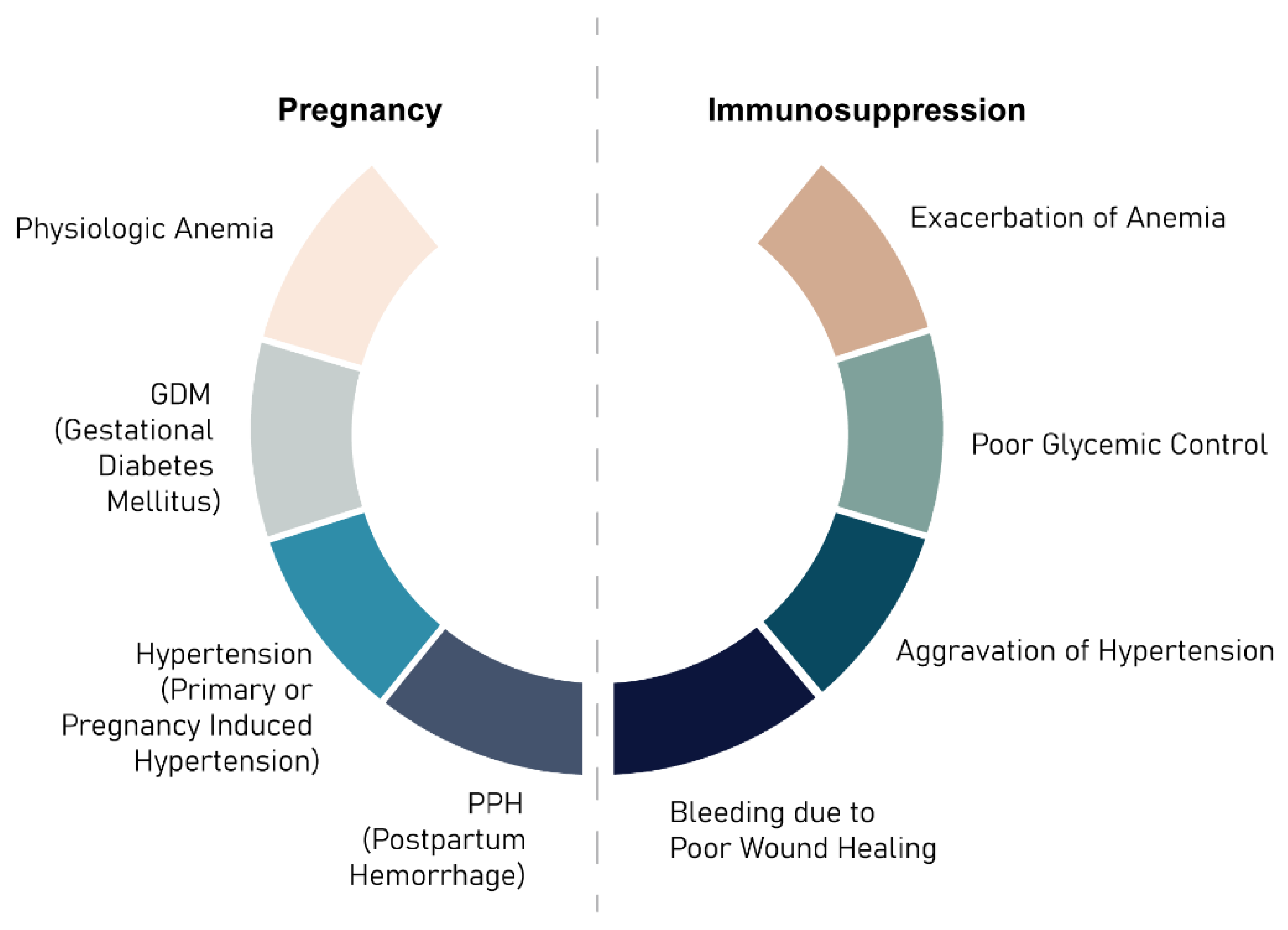
5. Pregnancy and Lactation in Immunosuppressed Patients
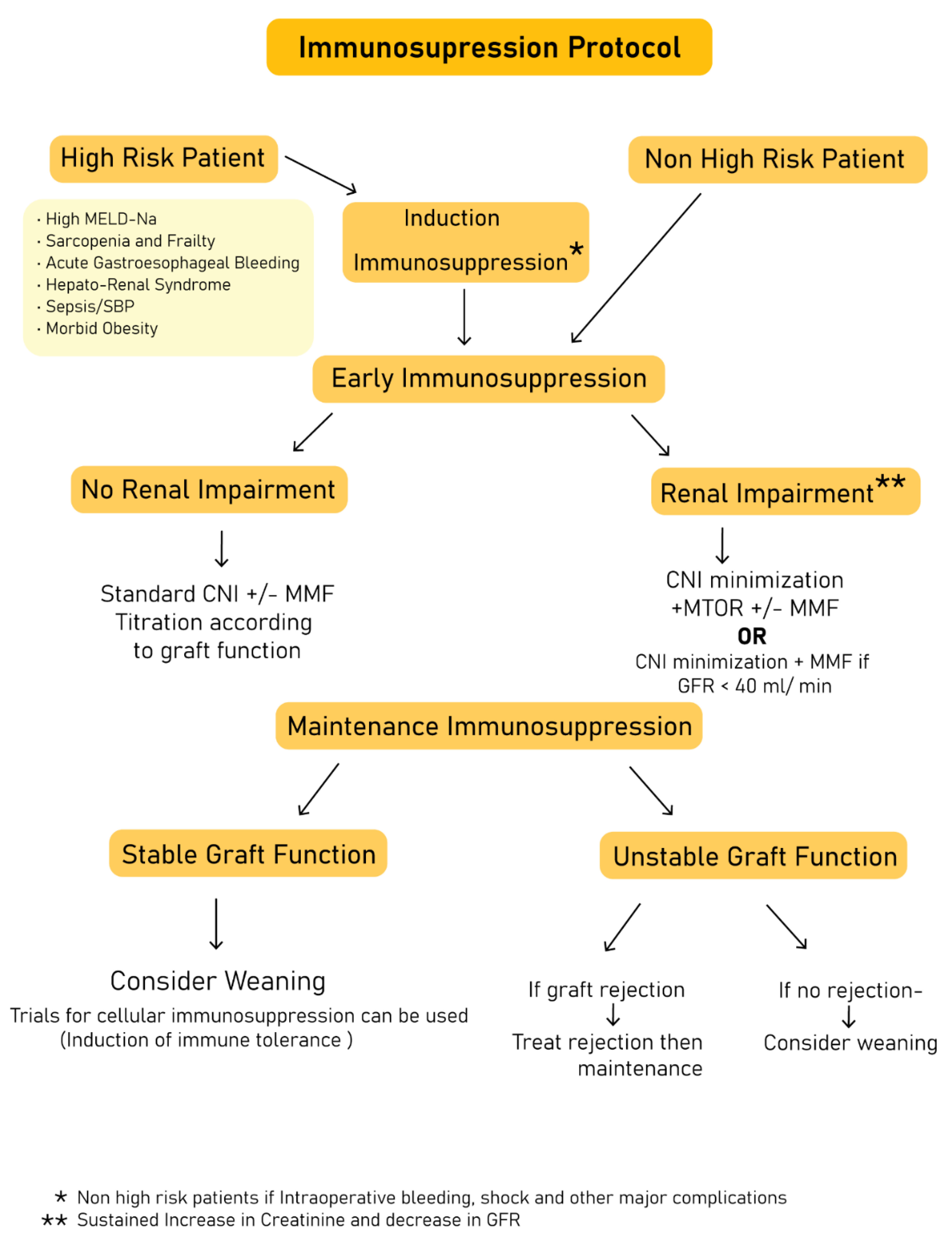
6. Strategies for Immune Suppression Withdrawal/Minimization
7. Immune Tolerance and Its Application in the Liver Transplant Setting: From Bench to Bedside
8. Deliberation on Strategies to Improve Immune Suppression Practice
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LT | Liver transplantation |
| CNIs | Calcineurin inhibitors |
| CsA | Cyclosporine A |
| TAC | Tacrolimus |
| rTAC | Reduced Tacrolimus |
| MTORi | Mammalian target of Rapamycin inhibitors |
| MMF | Mycophenolic acid |
| ATG | Antithymocyte globulin |
| IS | Immunosuppressive |
| Tregs | Regulatory T cells |
| CVD | Cardiovascular diseases; |
| PTDM | Post-transplant diabetes mellitus |
| NASH | Non-alcoholic fatty liver disease |
| HCC | Hepatocellular carcinoma |
| HRQL | Health-related quality of life |
| AMR | Antibody-mediated rejection |
| HCV | Hepatitis C virus |
| HBV | Hepatitis B virus |
References
- Neuberger, J. Follow-up of liver transplant recipients. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101682. [Google Scholar] [CrossRef]
- Scientific Registry of Transplant Recipients. Available online: https://srtr.transplant.hrsa.gov/annual_reports/2016/Economics.aspx#Econ_3_LI_tx_medicare_cov_1_b64 (accessed on 20 August 2021).
- Serper, M.; Reese, P.P.; Patzer, R.R.; Levitsky, J.; Wolf, M.S. The prevalence, risk factors, and outcomes of medication trade-offs in kidney and liver transplant recipients: A pilot study. Transpl. Int. 2018, 31, 870–879. [Google Scholar] [CrossRef]
- Leighton, J.; Wilson, C. Modern immunosuppression. Surgery 2020, 38, 368–374. [Google Scholar] [CrossRef]
- Tasdogan, B.E.; Ma, M.; Simsek, C.; Saberi, B.; Gurakar, A. Update on immunosuppression in liver transplantation. Euroasian J. Hepato Gastroenterol. 2019, 9, 96–101. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Busuttil, R.W. Liver transplantation: Past, present and future. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 434–440. [Google Scholar] [CrossRef]
- Geissler, E.K.; Schlitt, H.J. Immunosuppression for liver transplantation. Gut 2009, 58, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef]
- Liu, C.L.; Fan, S.T.; Lo, C.M.; Chan, S.C.; Ng, I.O.; Lai, C.L.; Wong, J. Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: A protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transplant. 2004, 10, 728–733. [Google Scholar] [CrossRef]
- Magliocca, J.F.; Knechtle, S.J. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl. Int. 2006, 19, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Mendez, R.; Pescovitz, M.; Rajagopalan, P.R.; Wilkinson, A.H.; Butt, K.; Laskow, D.; Slakey, D.P.; Lorber, M.I.; Garg, J.P.; et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am. J. Transplant. 2007, 7, 1770–1777. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Brem-Exner, B.G.; Riquelme, P.; Roelen, D.; Schulze, M.; Ivens, K.; Grabensee, B.; Witzke, O.; Philipp, T.; Renders, L.; et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl. Int. 2008, 21, 742–754. [Google Scholar] [CrossRef]
- Tanimine, N.; Ohira, M.; Tahara, H.; Ide, K.; Tanaka, Y.; Onoe, T.; Ohdan, H. Strategies for deliberate induction of immune tolerance in liver transplantation: From preclinical models to clinical application. Front. Immunol. 2020, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- De Simone, P.; Carrai, P.; Coletti, L.; Ghinolfi, D.; Petruccelli, S.; Filipponi, F. Modification of immunosuppressive therapy as risk factor for complications after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 199–209. [Google Scholar] [CrossRef]
- Cillo, U.; De Carlis, L.; Del Gaudio, M.; De Simone, P.; Fagiuoli, S.; Lupo, F.; Tisone, G.; Volpes, R.; Avolio, A.; Bitetto, D.; et al. Correction to: Immunosuppressive regimens for adult liver transplant recipients in real-life practice: Consensus recommendations from an Italian Working Group. Hepatol. Int. 2021, 14, 930–943. [Google Scholar] [CrossRef]
- Berglund, D.; Bengtsson, M.; Biglarnia, A.; Berglund, E.; Yamamoto, S.; Von Zur-Mühlen, B.; Lorant, T.; Tufveson, G. Screening of mortality in transplant patients using an assay for immune function. Transpl. Immunol. 2011, 24, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Spiritos, Z.; Abdelmalek, M.F. Metabolic syndrome following liver transplantation in nonalcoholic steatohepatitis. Transl. Gastroenterol. Hepatol. 2021, 6, 13. [Google Scholar] [CrossRef]
- Cotter, T.G.; Charlton, M. Nonalcoholic Steatohepatitis after Liver Transplantation. Liver Transplant. 2020, 26, 141–159. [Google Scholar] [CrossRef]
- Anastácio, L.R.; Lima, A.S.; Correia, M.I.T.D. Metabolic syndrome and its components after liver transplantation: Incidence, prevalence, risk factors, and implications. Clin. Nutr. 2010, 29, 175–179. [Google Scholar] [CrossRef]
- Pagadala, M.; Dasarathy, S.; Eghtesad, B.; McCullough, A.J. Posttransplant metabolic syndrome: An epidemic waiting to happen. Liver Transplant. 2009, 15, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Hecking, M.; de Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef]
- Yang, J.; Hutchinson, I.I.; Shah, T.; Min, D.I. Genetic and clinical risk factors of new-onset diabetes after transplantation in hispanic kidney transplant recipients. Transplantation 2011, 91, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Desideri, F.; Geerts, A.; Van Vlierberghe, H.; Berrevoet, F.; Rogiers, X.; Troisi, R.; De Hemptinne, B.; Vanholder, R.; Colle, I. Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transplant. 2010, 16, 1278–1287. [Google Scholar] [CrossRef]
- Lane, J.T.; Dagogo-Jack, S. Approach to the patient with new-onset diabetes after transplant (NODAT). J. Clin. Endocrinol. Metab. 2011, 96, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Hryniewiecka, E.; Żegarska, J.; Paczek, L. Arterial hypertension in liver transplant recipients. Transplant. Proc. 2011, 43, 3029–3034. [Google Scholar] [CrossRef]
- Shivaswamy, V.; Boerner, B.; Larsen, J. Post-transplant diabetes mellitus: Causes, treatment, and impact on outcomes. Endocr. Rev. 2016, 37, 37–61. [Google Scholar] [CrossRef]
- Hakeam, H.A.; Al-Jedai, A.H.; Raza, S.M.; Hamawi, K. Sirolimus induced dyslipidemia in tacrolimus based vs. tacrolimus free immunosuppressive regimens in renal transplant recipients. Ann. Transplant. 2008, 13, 46–53. [Google Scholar] [PubMed]
- Khullar, V.; Dolganiuc, A.; Firpi, R.J. Pre-and-post transplant considerations in patients with nonalcoholic fatty liver disease. World J. Transplant. 2014, 4, 81–92. [Google Scholar] [CrossRef]
- Stone, N.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Neal, D.A.J.; Brown, M.J.; Wilkinson, I.B.; Alexander, G.J.M. Mechanisms of hypertension after liver transplantation. Transplantation 2005, 79, 935–940. [Google Scholar] [CrossRef]
- Pirsch, J.D.; Henning, A.K.; First, M.R.; Fitzsimmons, W.; Gaber, A.O.; Reisfield, R.; Shihab, F.; Woodle, E.S. New-onset diabetes after transplantation: Results from a double-blind early corticosteroid withdrawal trial. Am. J. Transplant. 2015, 15, 1982–1990. [Google Scholar] [CrossRef]
- Aroda, V.R.; Ratner, R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: A review. Diabetes Metab. Res. Rev. 2011, 27, 528–542. [Google Scholar] [CrossRef]
- Laryea, M.; Watt, K.D.; Molinari, M.; Walsh, M.J.; McAlister, V.C.; Marotta, P.J.; Nashan, B.; Peltekian, K.M. Metabolic syndrome in liver transplant recipients: Prevalence and association with major vascular events. Liver Transplant. 2007, 13, 1109–1114. [Google Scholar] [CrossRef]
- Houlihan, D.D.; Armstrong, M.J.; Davidov, Y.; Hodson, J.; Nightingale, P.; Rowe, I.A.; Paris, S.; Gunson, B.K.; Bramhall, S.B.; Mutimer, D.J.; et al. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: Time to reconsider immunosuppression regimens? Liver Transplant. 2011, 17, 1292–1298. [Google Scholar] [CrossRef]
- McGuire, B.M.; Rosenthal, P.; Brown, C.C.; Busch, A.M.H.; Calcatera, S.M.; Claria, R.S.; Hunt, N.K.; Korenblat, K.M.; Mazariegos, G.V.; Moonka, D.; et al. Long-term management of the liver transplant patient: Recommendations for the primary care doctor. Am. J. Transplant. 2009, 9, 1988–2003. [Google Scholar] [CrossRef]
- Vinaixa, C.; Rubín, A.; Aguilera, V.; Berenguer, M. Recurrence of hepatitis C after liver transplantation. Ann. Gastroenterol. 2013, 26, 304–313. [Google Scholar]
- Albekairy, A.M.; Abdel-Razaq, W.S.; Alkatheri, A.M.; Al Debasi, T.M.; Alotaibi, N.; Qandil, A.M. The impact of immunosuppressant therapy on the recurrence of hepatitis C post-liver transplantation. Int. J. Health Sci. 2008, 12, 78–87. [Google Scholar]
- Saliba, F.; Nevens, F. Progression of liver fibrosis in HCV-positive liver transplant recipients randomized to everolimus with reduced calcineurin inhibitor (CNI) therapy or a standard CNI regimen. Transpl. Int. 2015, 28, 373–374. [Google Scholar] [CrossRef]
- John, S.; Andersson, K.; Kotton, C.; Hertl, M.; Markmann, J.F.; Cosimi, A.B.; Chung, R.T. Prophylaxis of hepatitis B infection in solid organ transplant recipients. Ther. Adv. Gastroenterol. 2013, 6, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.; Wu, G.Y. Prevention of HBV recurrence after liver transplant: A review. J. Clin. Transl. Hepatol. 2020, 8, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, S.E.; Heaton, N. Optimization of immunosuppressive medication upon liver transplantation against HCC recurrence. Transl. Gastroenterol. Hepatol. 2016, 1, 25. [Google Scholar] [CrossRef]
- Sposito, C.; Mariani, L.; Germini, A.; Reyes, M.F.; Bongini, M.; Grossi, G.; Bhoori, S.; Mazzaferro, V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: A case-control study. J. Hepatol. 2013, 59, 59–66. [Google Scholar] [CrossRef]
- Finn, R.S.; Poon, R.T.; Yau, T.; Klümpen, H.-J.; Chen, L.-T.; Kang, Y.-K.; Kim, T.-Y.; Gomez-Martin, C.; Rodriguez-Lope, C.; Kunz, T.; et al. Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma. J. Hepatol. 2013, 59, 1271–1277. [Google Scholar] [CrossRef]
- Cholongitas, E.; Antoniadis, N.; Goulis, I.; Theocharidou, E.; Ιmvrios, G.; Giouleme, O.; Filis, D.; Mouloudi, E.; Akriviadis, E.; Fouzas, I. Trough levels of everolimus are associated with recurrence rates of hepatocellular carcinoma after liver transplantation. Transplant. Proc. 2019, 51, 450–453. [Google Scholar] [CrossRef]
- Cholongitas, E.; Mamou, C.; Rodríguez-Castro, K.I.; Burra, P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: A systematic review. Transpl. Int. 2014, 27, 1039–1049. [Google Scholar] [CrossRef]
- Åberg, F. From prolonging life to prolonging working life: Tackling unemployment among liver-transplant recipients. World J. Gastroenterol. 2016, 22, 3701–3711. [Google Scholar] [CrossRef] [PubMed]
- Scientific Registry of Transplant Recipients. Available online: https://srtr.transplant.hrsa.gov/annual_reports/2019/Economics.aspx#ECON_tx_hosp_li_rec_oneyear_outcome_b64 (accessed on 20 August 2021).
- Rahim, M.N.; Long, L.; Penna, L.; Williamson, C.; Kametas, N.A.; Nicolaides, K.H.; Heneghan, M.A. Pregnancy in liver transplantation. Liver Transplant. 2020, 26, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Rouas-Freiss, N.; Naji, A.; Durrbach, A.; Carosella, E.D. Tolerogenic functions of human leukocyte antigen G: From pregnancy to organ and cell transplantation. Transplantation 2007, 84, S21–S25. [Google Scholar] [CrossRef]
- Kim, M.; Rostas, S.; Gabardi, S. Mycophenolate fetal toxicity and risk evaluation and mitigation strategies. Am. J. Transplant. 2013, 13, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Valentin, N.; Guerrido, I.; Rozenshteyn, F.; Pinotti, R.; Wu, Y.C.; Collins, K.; Shah, M.; Hershman, M.; Weisberg, I. Pregnancy outcomes after liver transplantation: A systematic review and meta-analysis. Am. J. Gastroenterol. 2021, 116, 491–504. [Google Scholar] [CrossRef]
- Ghazali, S.; Czuzoj-Shulman, N.; Spence, A.R.; Mishkin, D.S.; Abenhaim, H.A. Pregnancy outcomes in liver transplant patients, a population-based study. J. Matern. Fetal Neonatal Med. 2017, 30, 261–266. [Google Scholar] [CrossRef]
- Lim, T.Y.; Gonsalkorala, E.; Cannon, M.D.; Gabeta, S.; Penna, L.; Heaton, N.D.; Heneghan, M.A. Successful pregnancy outcomes following liver transplantation is predicted by renal function. Liver Transplant. 2018, 24, 606–615. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Arakali, S.R.; Mealey, K.J.; Cardonick, E.; Gaughan, W.J.; Davison, J.M.; Moritz, M.J.; Armenti, V.T. Safety considerations: Breastfeeding after transplant. Prog. Transplant. 2013, 23, 137–146. [Google Scholar] [CrossRef]
- Kwon, H.; Jeon, J.; Kim, D.; Jang, H.; Sung, H.; Han, D.; Park, J.; Lee, J.; Huh, W.; Kim, S.; et al. Clinical impact of a protocolized kidney donor follow-up system. Transplant. Proc. 2019, 51, 692–700. [Google Scholar] [CrossRef]
- Shaked, A.; DesMarais, M.R.; Kopetskie, H.; Feng, S.; Punch, J.D.; Levitsky, J.; Reyes, J.; Klintmalm, G.B.; Demetris, A.J.; Burrell, B.E.; et al. Outcomes of immunosuppression minimization and withdrawal early after liver transplantation. Am. J. Transplant. 2019, 19, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Bucuvalas, J.C.; Mazariegos, G.V.; Magee, J.C.; Sanchez-Fueyo, A.; Spain, K.M.; Lesniak, A.; Kanaparthi, S.; Perito, E.; Venkat, V.L.; et al. Efficacy and safety of immunosuppression withdrawal in pediatric liver transplant recipients: Moving toward personalized management. Hepatology 2021, 73, 1985–2004. [Google Scholar] [CrossRef]
- Levitsky, J.; Burrell, B.E.; Kanaparthi, S.; Turka, L.A.; Kurian, S.; Sanchez-Fueyo, A.; Lozano, J.J.; Demetris, A.; Lesniak, A.; Kirk, A.D.; et al. Immunosuppression withdrawal in liver transplant recipients on sirolimus. Hepatology 2020, 72, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Benítez, C.; Londoño, M.-C.; Miquel, R.; Manzia, T.M.; Gonzalez-Abraldes, J.; Lozano, J.J.; Martínez-Llordella, M.; López, M.; Angelico, R.; Bohne, F.; et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013, 58, 1824–1835. [Google Scholar] [CrossRef]
- Pons, J.A.; Revilla-Nuin, B.; Baroja-Mazo, A.; Ramírez, P.; Martínez-Alarcón, L.; Sánchez-Bueno, F.; Robles, R.; Rios, A.; Aparicio, P.; Parrilla, P. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplanation 2008, 86, 1370–1378. [Google Scholar] [CrossRef]
- Neuberger, J.M.; Mamelok, R.D.; Neuhaus, P.; Pirenne, J.; Samuel, D.; Isoniemi, H.; Rostaing, L.; Rimola, A.; Marshall, S.; Mayer, A.D.; et al. delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: The ‘ReSpECT’ study. Am. J. Transplant. 2009, 9, 327–336. [Google Scholar] [CrossRef]
- De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Saliba, F.; Jonas, S.; Sudan, D.; Fung, J.; Fischer, L.; et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am. J. Transplant. 2012, 12, 3008–3020. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F.; De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Jonas, S.; Sudan, D.; Fischer, L.; Duvoux, C.; et al. Renal function at two years in liver transplant patients receiving everolimus: Results of a randomized, multicenter study. Am. J. Transplant. 2013, 13, 1734–1745. [Google Scholar] [CrossRef]
- Fischer, L.; Saliba, F.; Kaiser, G.M.; De Carlis, L.; Metselaar, H.J.; De Simone, P.; Duvoux, C.; Nevens, F.; Fung, J.J.; Dong, G.; et al. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: Follow-up results from a randomized, multicenter study. Transplantation 2015, 99, 1455–1462. [Google Scholar] [CrossRef]
- De Simone, P.; Saliba, F.; Dong, G.; Escrig, C.; Fischer, L. Do patient characteristics influence efficacy and renal outcomes in liver transplant patients receiving everolimus? Clin. Transplant. 2015, 30, 279–288. [Google Scholar] [CrossRef]
- De Simone, P.; Fagiuoli, S.; Cescon, M.; De Carlis, L.; Tisone, G.; Volpes, R.; Cillo, U. Use of everolimus in liver transplantation. Transplantation 2017, 101, 239–251. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Guerrero-Misas, M.; Thorburn, U.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Maintenance immunosuppression for adults undergoing liver transplantation: A network meta-analysis. Cochrane Database Syst. Rev. 2017, 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fueyo, A.; Strom, T.B. Immunological tolerance and liver transplantation. J. Hepatol. 2004, 41, 698–705. [Google Scholar] [CrossRef]
- Sykes, M. Mixed Chimerism and Transplant Tolerance. Immunity 2001, 14, 417–424. [Google Scholar] [CrossRef][Green Version]
- Lu, L.; A Rudert, W.; Qian, S.; McCaslin, D.; Fu, F.; Rao, A.S.; Trucco, M.; Fung, J.; E Starzl, T.; Thomson, A.W. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1995, 182, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Yoshida, O.; Ono, Y.; Geller, D.A.; Thomson, A.W. Liver transplantation in the mouse: Insights into liver immunobiology, tissue injury, and allograft tolerance. Liver Transplant. 2015, 22, 536–546. [Google Scholar] [CrossRef]
- Akkaya, B.; Oya, Y.; Akkaya, M.; Al Souz, J.; Holstein, A.H.; Kamenyeva, O.; Kabat, J.; Matsumura, R.; Dorward, D.W.; Glass, D.D.; et al. Regulatory T cells mediate specific suppression by depleting peptide–MHC class II from dendritic cells. Nat. Immunol. 2019, 20, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Onoe, T.; Ohdan, H. The role of liver sinusoidal endothelial cells in induction of carbohydrate reactive B cells tolerance through the programmed death 1/programmed death ligand 1 pathway. Transplantation 2015, 99, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Kuo, L.-M.; Chang, Y.; Wu, W.; Goldbach, C.; Ross, M.A.; Stolz, D.B.; Chen, L.; Fung, J.; Lu, L.; et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology 2006, 44, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Gratz, I.K.; Truong, H.-A.; Yang, S.H.-Y.; Maurano, M.M.; Lee, K.; Abbas, A.K.; Rosenblum, M.D. Cutting edge: Memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 2013, 190, 4483–4487. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Z.; Li, C.; Wei, Q.; Zheng, S.; Saeb-Parsy, K.; Xu, X. Regulatory T cell therapy following liver transplantation. Liver Transplant. 2021, 27, 264–280. [Google Scholar] [CrossRef]
- Todo, S.; Yamashita, K.; Goto, R.; Zaitsu, M.; Nagatsu, A.; Oura, T.; Watanabe, M.; Aoyagi, T.; Suzuki, T.; Shimamura, T.; et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016, 64, 632–643. [Google Scholar] [CrossRef]
- Tang, Q.; Vincenti, F. Transplant trials with tregs: Perils and promises. J. Clin. Investig. 2017, 127, 2505–2512. [Google Scholar] [CrossRef]

| Year of Discovery/FDA Approval | Indication | Immunosuppressive Agent | Mode of Action | Main Adverse Effect |
|---|---|---|---|---|
| 1972/1983 TAC in 1994 | Maintenance | CNI | Blocks protein transcription in response to Il-2 and prevents cellular proliferation | Neurotoxicity Nephrotoxicity Diabetogenic |
| 1893/1995 | Maintenance | MMF | Interferes with DNA synthesis | Pancytopenia Abdominal discomfort |
| 1997 | AMR | Rituximab | Anti-CD 20 | Reactivation of TB |
| 2007 | Maintenance | MTORi | Inhibits cytokine receptor signal transduction | Hyperlipidemia Pedal edema Oral ulcers HAT |
| 1998/2008 | Induction | Basiliximab | IL2 receptor blocker | Hypersensitivity |
| 2011 | Induction or maintenance | Balatacept | Blocks CD80 co-stimulation ligand | Post-TX lymphoproliferative disorder |
| Induction, Maintenance, and Rejection | Corticosteroids | Affects the production of several inflammatory mediators and multiple cytokines, including IL-1, IL-2, IL-3, IL-6, TNF-a, IFN-Y, leukotrienes, and prostaglandins | Diabetes Hypertension Osteoporosis Hirsutism Weight gain | |
| 2014 | Chronic rejection | Alemtuzumab (Campath 1H) | Anti-CD52 on nucleated cells of BM | Hirsutism Post-TX Lymphoproliferative disorder |
| 2017 | Induction or rejection | ATG | Anti-CD3 on lymphocytes | Anaphylaxis, post-TX lymphoproliferative disorders |
| Drug Class | Effect of Maternal Health |
|---|---|
| Corticosteroids | Hypertension, diabetes mellitus, weight gain, delayed wound healing |
| Calcineurin inhibitors | Hypertension, diabetes mellitus, renal impairment, pregnancy-induced hypertension |
| MMF | Increased infection, not recommended during pregnancy due to teratogenicity |
| MTORi | Increased infection, GI side effects |
| Study | Population | Type | Duration of Withdrawal | Total Subjects | Successful Withdrawal Arm | Follow Up |
|---|---|---|---|---|---|---|
| Feng/iWITH Study [59] | Pediatrics | Multicenter | 36–48 weeks | 2909 | 37.5%% of 88 subjects | 1, 2, 3, 4 years |
| Levitsky [60] | Adults | Single center | >3 years post-LTWithdrawal over 6 months | 1255 | >50% of 15 subjects | 1 year |
| Benitez [61] | Adults | Multicenter | >3 years post-LTWithdrawal over 6–9 months | 500 | 40% of 98 subjects | Up to 3 years |
| Pons [62] | Adults | Single center | >2 years post-LT | 490 | 42% of 12 subjects | 2 years |
| Trial Name | Induction Therapy | Immunosuppressive Regimen | Timing of Start of Tregs | Status |
|---|---|---|---|---|
| ARTEMIS NCT02474199 | Yes (ATG) | CNI based | 2–6 years post-transplant | Completed |
| ThRIL NCT02166177 | Yes (ATG) | CNI and MTORi | 2 months | Completed |
| ITN073ST NCT03577431 | Yes (cyclocphosphamide) | EVR and TAC | 2–6 months | Recruiting |
| DAIT RTB-002 NCT02188719 | Yes (ATG) | TAC+ MMF EVR+ rTAC | Early post-LT | Terminated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, I.B.; Aloor, F.Z.; Jalal, P.K. Strategies to Improve Immune Suppression Post-Liver Transplantation: A Review. Transplantology 2021, 2, 441-454. https://doi.org/10.3390/transplantology2040042
Mohamed IB, Aloor FZ, Jalal PK. Strategies to Improve Immune Suppression Post-Liver Transplantation: A Review. Transplantology. 2021; 2(4):441-454. https://doi.org/10.3390/transplantology2040042
Chicago/Turabian StyleMohamed, Islam B, Fuad Z Aloor, and Prasun K Jalal. 2021. "Strategies to Improve Immune Suppression Post-Liver Transplantation: A Review" Transplantology 2, no. 4: 441-454. https://doi.org/10.3390/transplantology2040042
APA StyleMohamed, I. B., Aloor, F. Z., & Jalal, P. K. (2021). Strategies to Improve Immune Suppression Post-Liver Transplantation: A Review. Transplantology, 2(4), 441-454. https://doi.org/10.3390/transplantology2040042






