Epitope-Level Matching—A Review of the Novel Concept of Eplets in Transplant Histocompatibility
Abstract
:1. Introduction
2. The Effects of Poor HLA Matching
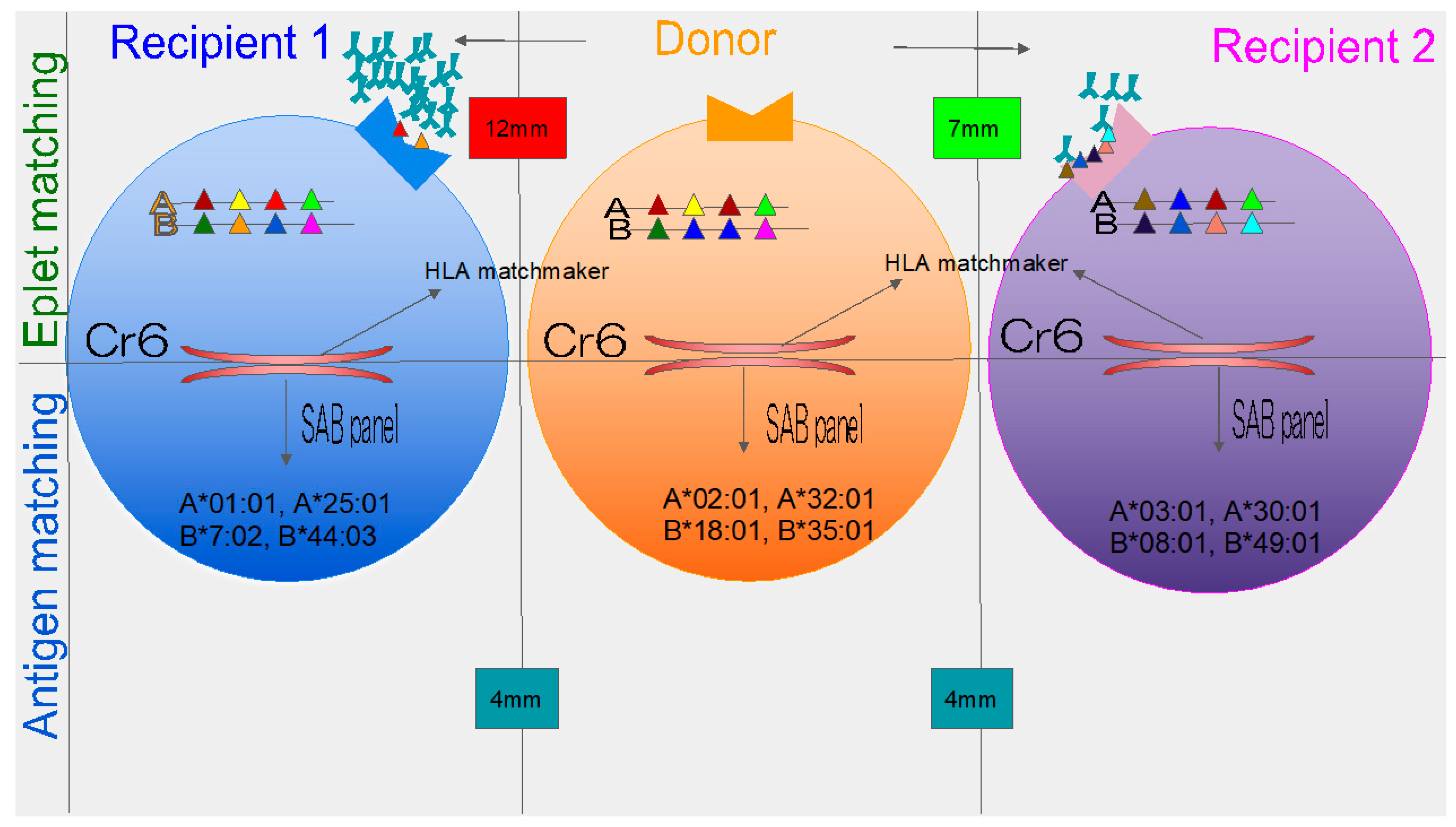
3. New Concept of Eplets
4. Organs and Eplet
4.1. Mechanism for How dnDSA Are Developed
4.2. Renal
4.3. Lung
4.4. Cardiac
4.5. Liver
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiebe, C.; Gibson, I.W.; Blydt-Hansen, T.D.; Karpinski, M.; Ho, J.; Storsley, L.J.; Goldberg, A.; Birk, P.E.; Rush, D.N.; Nickerson, P.W. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am. J. Transplant. 2012, 12, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Larkins, N.G.; Wong, G.; Taverniti, A.; Lim, W.H. Epitope matching in kidney transplantation: Recent advances and current limitations. Curr. Opin. Organ. Transplant. 2019, 24, 370–377. [Google Scholar] [CrossRef]
- Taherkhani, N.; Sepehri, M.M.; Shafaghi, S.; Khatibi, T. Identification and weighting of kidney allocation criteria: A novel multi-expert fuzzy method. BMC Med. Inform. Decis. Mak. 2019, 19, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.B.; Bennett, L.E.; Cecka, J.M. Effect of HLA matching on the relative risk of mortality for kidney recipients: A comparison of the mortality risk after transplant to the mortality risk of remaining on the waiting list. Transplantation 1997, 64, 1274–1277. [Google Scholar] [CrossRef]
- Nissel, R.; Brázda, I.; Feneberg, R.; Wigger, M.; Greiner, C.; Querfeld, U.; Haffner, D. Effect of renal transplantation in childhood on longitudinal growth and adult height. Kidney Int. 2004, 66, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Foster, B.J.; Dahhou, M.; Zhang, X.; Platt, R.W.; Smith, J.M.; Hanley, J.A. Impact of HLA mismatch at first kidney transplant on lifetime with graft function in young recipients. Am. J. Transplant. 2014, 14, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Crafter, S.R.; Bell, L.; Foster, B.J. Balancing organ quality, HLA-matching, and waiting times: Impact of a pediatric priority allocation policy for deceased donor kidneys in Quebec. Transplantation 2007, 83, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Doxiadis, I.I.N.; De Fijter, J.W.; Mallat, M.J.K.; Haasnoot, G.W.; Ringers, J.; Persijn, G.G.; Claas, F.H.J. Simpler and equitable allocation of kidneys from postmortem donors primarily based on full HLA-DR compatibility. Transplantation 2007, 83, 1207–1213. [Google Scholar] [CrossRef]
- Williams, R.C.; Opelz, G.; McGarvey, C.J.; Weil, E.J.; Chakkera, H.A. The risk of transplant failure with hla mismatch in first adult kidney allografts from deceased donors. Transplantation 2016, 100, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Sellarés, J.; De Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, C.; Pochinco, D.; Blydt-Hansen, T.D.; Ho, J.; Birk, P.E.; Karpinski, M.; Goldberg, A.; Storsley, L.J.; Gibson, I.W.; Rush, D.N.; et al. Class II HLA epitope matching—A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am. J. Transplant. 2013, 13, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Banner, N.R.; Hamour, I.M.; Ozawa, M.; Goh, A.; Robinson, D.; Terasaki, P.I.; Rose, M.L. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am. J. Transplant. 2011, 11, 312–319. [Google Scholar] [CrossRef]
- Duquesnoy, R.J. Epitope-based human leukocyte antigen matching for transplantation: A personal perspective of its future. Curr. Opin. Organ. Transplant. 2018, 23, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Hönger, G.; Niemann, M.; Schawalder, L.; Jones, J.; van Heck, M.R.; van de Pasch, L.A.L.; Vendelbosch, S.; Rozemuller, E.H.; Hösli, I.; Blümel, S.; et al. Toward defining the immunogenicity of HLA epitopes: Impact of HLA class I eplets on antibody formation during pregnancy. HLA 2020, 96, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Schawalder, L.; Hönger, G.; Kleiser, M.; van Heck, M.R.; van de Pasch, L.A.L.; Vendelbosch, S.; Rozemuller, E.H.; Schaub, S. Development of an immunogenicity score for HLA-DQ eplets: A conceptual study. HLA 2021, 97, 30–43. [Google Scholar] [CrossRef]
- Sypek, M.; Kausman, J.; Holt, S.; Hughes, P. HLA Epitope Matching in Kidney Transplantation: An Overview for the General Nephrologist. Am. J. Kidney Dis. 2018, 71, 720–731. [Google Scholar] [CrossRef]
- Duquesnoy, R.J.; Askar, M. HLAMatchmaker: A Molecularly Based Algorithm for Histocompatibility Determination. V. Eplet Matching for HLA-DR, HLA-DQ and HLA-DP. Hum. Immunol. 2007, 68, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.M.; Warner, P.; Kemna, M.S.; Albers, E.L.; Law, S.P.; Weiss, N.S.; Law, Y.M. HLA molecular epitope mismatching and long-term graft loss in pediatric heart transplant recipients. J. Heart Lung Transplant. 2015, 34, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Do Nguyen, H.T.; Wong, G.; Chapman, J.R.; McDonald, S.P.; Coates, P.T.; Watson, N.; Russ, G.R.; D’Orsogna, L.; Lim, W.H. The Association between Broad Antigen HLA Mismatches, Eplet HLA Mismatches and Acute Rejection after Kidney Transplantation. Transplant. Direct. 2016, 2, e120. [Google Scholar] [CrossRef]
- Bryan, C.F.; Chadha, V.; Warady, B.A. Donor selection in pediatric kidney transplantation using DR and DQ eplet mismatching: A new histocompatibility paradigm. Pediatr. Transplant. 2016, 20, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Engen, R.M.; Jedraszko, A.M.; Conciatori, M.A.; Tambur, A.R. Substituting imputation of HLA antigens for high-resolution HLA typing: Evaluation of a multiethnic population and implications for clinical decision making in transplantation. Am. J. Transplant. 2021, 21, 344–352. [Google Scholar] [CrossRef]
- Kausman, J.Y.; Walker, A.M.; Cantwell, L.S.; Quinlan, C.; Sypek, M.P.; Ierino, F.L. Application of an epitope-based allocation system in pediatric kidney transplantation. Pediatr. Transplant. 2016, 20, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, C.; Nevins, T.E.; Robiner, W.N.; Thomas, W.; Matas, A.J.; Nickerson, P.W. The Synergistic Effect of Class II HLA Epitope-Mismatch and Nonadherence on Acute Rejection and Graft Survival. Am. J. Transplant. 2015, 15, 2197–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobashevsky, A.; Goggins, W.; Rosner, K.; Taber, T. Immunogenicity of Class I HLA but not preformed low MFI donor specific antibodies correlates with outcomes after first renal transplantation. Transpl. Immunol. 2017, 43, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tafulo, S.; Malheiro, J.; Santos, S.; Dias, L.; Almeida, M.; Martins, L.S.; Pedroso, S.; Mendes, C.; Lobato, L.; Castro-Henriques, A. HLA class II eplet mismatch load improves prediction of dnDSA development after living donor kidney transplantation. Int. J. Immunogenet. 2021, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tafulo, S.; Malheiro, J.; Santos, S.; Dias, L.; Almeida, M.; Martins, L.S.; Pedroso, S.; Mendes, C.; Lobato, L.; Castro-Henriques, A. Degree of HLA class II eplet mismatch load improves prediction of antibody-mediated rejection in living donor kidney transplantation. Hum. Immunol. 2019, 80, 966–975. [Google Scholar] [CrossRef]
- Hayes, D.; Black, S.M.; Tobias, J.D.; Kopp, B.T.; Kirkby, S.E.; Mansour, H.M.; Whitson, B.A. Influence of human leukocyte antigen mismatching on bronchiolitis obliterans syndrome in lung transplantation. J. Heart Lung Transplant. 2016, 35, 186–194. [Google Scholar] [CrossRef] [Green Version]
- McCaughan, J.A.; Battle, R.K.; Singh, S.K.S.; Tikkanen, J.M.; Moayedi, Y.; Ross, H.J.; Singer, L.G.; Keshavjee, S.; Tinckam, K.J. Identification of risk epitope mismatches associated with de novo donor-specific HLA antibody development in cardiothoracic transplantation. Am. J. Transplant. 2018, 18, 2924–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio-Jaramillo, E.; Haasnoot, G.W.; Kaider, A.; Schaefer, A.K.; Haberl, T.; Goekler, J.; Angleitner, P.; Moayedifar, R.; Zuckermann, A.; Fischer, G.F.; et al. Molecular-level HLA mismatch is associated with rejection and worsened graft survival in heart transplant recipients—A retrospective study. Transpl. Int. 2020, 33, 1078–1088. [Google Scholar] [CrossRef]
- Nilsson, J.; Ansari, D.; Ohlsson, M.; Höglund, P.; Liedberg, A.S.; Smith, J.G.; Nugues, P.; Andersson, B. Human Leukocyte Antigen-Based Risk Stratification in Heart Transplant Recipients—Implications for Targeted Surveillance. J. Am. Heart Assoc. 2019, 8, e11124. [Google Scholar] [CrossRef] [Green Version]
- Guiral, S.; Segundo, D.S.; Irure, J.; Casafont, F.; Fortea, J.I.; Puente, Á.; López-Hoyos, M.; Crespo, J.; Fabrega, E. Number of antibody-verified eplet in HLA-C locus as an independent factor of t-cell-mediated rejection after liver transplantation. Transplantation 2020, 104, 562–567. [Google Scholar] [CrossRef]
- Forner, D.; Liwski, R.; Alwayn, I. Human leukocyte antigen, allele, and eplet mismatches in liver transplantation; observations from a small, single center cohort. Hum. Immunol. 2018, 79, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ekong, U.D.; Antala, S.; Bow, L.; Sese, D.; Morotti, R.; Rodriguez-Davalos, M.; Gan, G.; Deng, Y.; Emre, S.H. HLA, Non-HLA Antibodies, and eplet mismatches in pediatric liver transplantation: Observations from a small, single-center cohort. Exp. Clin. Transplant. 2019, 17, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Kubal, C.A.; Mangus, R.; Ekser, B.; Mihaylov, P.; Ceballos, B.; Higgins, N.; Chalasani, N.; Ghabril, M.; Nephew, L.; Lobashevsky, A. Class II Human Leukocyte Antigen Epitope Mismatch Predicts De Novo Donor-Specific Antibody Formation After Liver Transplantation. Liver Transplant. 2018, 24, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Kosmoliaptsis, V.; Mallon, D.H.; Chen, Y.; Bolton, E.M.; Bradley, J.A.; Taylor, C.J. Alloantibody Responses After Renal Transplant Failure Can Be Better Predicted by Donor–Recipient HLA Amino Acid Sequence and Physicochemical Disparities Than Conventional HLA Matching. Am. J. Transplant. 2016, 16, 2139–2147. [Google Scholar] [CrossRef] [Green Version]
- Süsal, C.; Opelz, G. Good kidney transplant outcome in recipients with presensitization against HLA class II but not HLA class I. Hum. Immunol. 2004, 65, 810–816. [Google Scholar] [CrossRef]
- Lim, W.H.; Chadban, S.J.; Clayton, P.; Budgeon, C.A.; Murray, K.; Campbell, S.B.; Cohney, S.; Russ, G.R.; Mcdonald, S.P. Human leukocyte antigen mismatches associated with increased risk of rejection, graft failure, and death independent of initial immunosuppression in renal transplant recipients. Clin. Transplant. 2012, 26, E428–E437. [Google Scholar] [CrossRef]
- Heidt, S.; Haasnoot, G.W.; van Rood, J.J.; Witvliet, M.D.; Claas, F.H.J. Kidney allocation based on proven acceptable antigens results in superior graft survival in highly sensitized patients. Kidney Int. 2018, 93, 491–500. [Google Scholar] [CrossRef]
- Bestard, O.; Meneghini, M.; Crespo, E.; Bemelman, F.; Koch, M.; Volk, H.D.; Viklicky, O.; Giral, M.; Banas, B.; Ruiz, J.C.; et al. Preformed T cell alloimmunity and HLA eplet mismatch to guide immunosuppression minimization with tacrolimus monotherapy in kidney transplantation: Results of the CELLIMIN trial. Am. J. Transplant. 2021, 21, 2833–2845. [Google Scholar] [CrossRef]
- Heidt, S.; Claas, F.H.J. Not all HLA epitope mismatches are equal. Kidney Int. 2020, 97, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.C.; Hiho, S.J.; Cantwell, L.S.; Diviney, M.B.; Wright, S.T.; Snell, G.I.; Paraskeva, M.A.; Westall, G.P. HLA Matching at the Eplet Level Protects Against Chronic Lung Allograft Dysfunction. Am. J. Transplant. 2016, 16, 2695–2703. [Google Scholar] [CrossRef] [Green Version]
- Woodle, E.S.; Shields, A.R.; Ejaz, N.S.; Sadaka, B.; Girnita, A.; Walsh, R.C.; Alloway, R.R.; Brailey, P.; Cardi, M.A.; Abu Jawdeh, B.G.; et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am. J. Transplant. 2015, 15, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, J.M.; Singer, L.G.; Kim, S.J.; Li, Y.; Binnie, M.; Chaparro, C.; Chow, C.W.; Martinu, T.; Azad, S.; Keshavjee, S.; et al. De novo DQ donor-specific antibodies are associated with chronic lung allograft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2016, 194, 596–606. [Google Scholar] [CrossRef]
- Sato, M. Chronic lung allograft dysfunction after lung transplantation: The moving target. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.; Robinson, D.R.; Soresi, S.; Carby, M.; Smith, J.D. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J. Heart Lung Transplant. 2014, 33, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.R.; Pilewski, J.M.; Gries, C.J.; Pipeling, M.R.; Crespo, M.M.; Ensor, C.R.; Yousem, S.A.; D’Cunha, J.; Shigemura, N.; Bermudez, C.A.; et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J. Heart Lung Transplant. 2014, 33, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Everly, M.J.; Terasaki, P.I. Monitoring and treating posttransplant human leukocyte antigen antibodies. Hum. Immunol. 2009, 70, 655–659. [Google Scholar] [CrossRef]
- Claas, F.H.J.; Dankers, M.K.; Oudshoorn, M.; van Rood, J.J.; Mulder, A.; Roelen, D.L.; Duquesnoy, R.J.; Doxiadis, I.I.N. Differential immunogenicity of HLA mismatches in clinical transplantation. Transpl. Immunol. 2005, 14, 187–191. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Demetris, A.J.; Friedman, L.S.; Gebel, H.M.; Halloran, P.F.; Kirk, A.D.; Knechtle, S.J.; McDiarmid, S.V.; Shaked, A.; Terasaki, P.I.; et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am. J. Transplant. 2014, 14, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Starzl, T.E. The “privileged” liver and hepatic tolerogenicity. Liver Transplant. 2001, 7, 918–920. [Google Scholar] [CrossRef]
| References | Year, Author | Study | Organ | HLA Loci | Observations | Clinical Correlate | Eplet |
|---|---|---|---|---|---|---|---|
| [11] | 2013, Wiebe | p-cohort, n = 286 | Renal | DR, DQ | HLA-DR epitope MM load OR = 1.06 * (1.03–1.10) a HLA-DQ epitope MM load OR = 1.04 * (1.0–1.02) a | dnDSA | - |
| [19] | 2016, Do Nguyen | R-cohort, n = 3499 | Renal | All | 0–2 HLA MMs + >20 eplet MMs, HR = 1.85 (1.11–3.08) a | Risk of rejection | - |
| [20] | 2016, Bryan | R-cochort, n = 16 | Renal (peds) | DR, DQ | HLA-DR > 10 MMs, HLA-DQ > 17 MMs | dnDSA | - |
| [22] | 2016, Kaussman | p-cohort, n = 19 | Renal (peds) | All | Class I < 10 MMs, class II < 30 MMs | AMR | - |
| [23] | 2015, Wiebe | R-cohort, n = 195 | Renal | DR, DQ | HLA-DR > 10 MMs, HLA-DQ > 17 MMs | Synergistic with Tx nonadherence | - |
| [24] | 2017, Lobashevsky | R-cohort, n = 141 | Renal | Class I | Threshold > 12 MMs, OR = 9 (1.0–81.2) a | AMR, TG, dnDSA, existing DSA | 127K |
| [25] | 2021, Tafulo | R-cohort, n = 96 | Renal | Class II | Class II eplet MMs, HR = 1.105 (1.011–1.208) a | dnDSA | - |
| [26] | 2019, Tafulo | R-cohort, n = 151 | Renal | Class II | Class II eplet MMs, HR = 14.839 (1.846–119.282) a | AMR | - |
| [27] | 2016, Walton | R-cohort, n = 175 | Lung | All | Threshold > 60 eplets MMs | CLAD, ROS | - |
| [28] | 2019, McCaughan | R-cohort, n = 433 | Lung | DQ | OR = 4.9 | dnDSA | 45EV, 45GE3 |
| [28] | 2019, McCaughan | R-cohort, n = 265 | Cardiac | DQ | OR = 4.2 | dnDSA | 45EV, 45GE3 |
| [29] | 2020, Osorio-Jaramillo | R-cohort, n = 1167 | Cardiac | DR, AB | HLA-DR eplet MMs had inferior 1 year graft survival, HR = 1.14 (1.01–1.28) a Risk of rejection: HLA-AB MM load HR = 1.70 (1.29–2.24) a and HLA-DR HR = 1.32 (1.09–1.61) a | Graft survival and rejection | - |
| [30] | 2019, Nilsson | R-cohort, n = 34,681 | Cardiac | DR, DQ | HLA-DR/DQ > 40 eplets MMS HR = 1.11 (1.03–1.21) a | Graft loss | - |
| [18] | 2015, Sullivan | R-cohort n = 4851 | Cardiac(peds) | All | Class I > 10 MMs | Graft loss | - |
| [31] | 2019, Guiral | R-cohort, n = 43 | Liver | C | OR = 3.8 (1.59–8.93) a | TCMR | - |
| [32] | 2018, Forner | R-cohort, n = 67 | Liver | A | Decreased graft survival, not significant | - | - |
| [33] | 2019, Ekong | R-cohort, n = 42 | Liver | DQ | HLA-DQ > 5 MMs | ACR, portal fibrosis score | 4Q, 45GE, 52PQ, 52PL |
| [34] | 2018, Kubal | p-cohort, n = 80 | Liver | DRB1,DQA1/B1 | Class II MM eplets was associated with dnDSA OR = 1.2 | dnDSA | - |
| HLA Antigen Mismatch | Eplet Mismatch | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renaldo, A.; Roa-Bautista, A.; González-López, E.; López-Hoyos, M.; San Segundo, D. Epitope-Level Matching—A Review of the Novel Concept of Eplets in Transplant Histocompatibility. Transplantology 2021, 2, 336-347. https://doi.org/10.3390/transplantology2030033
Renaldo A, Roa-Bautista A, González-López E, López-Hoyos M, San Segundo D. Epitope-Level Matching—A Review of the Novel Concept of Eplets in Transplant Histocompatibility. Transplantology. 2021; 2(3):336-347. https://doi.org/10.3390/transplantology2030033
Chicago/Turabian StyleRenaldo, André, Adriel Roa-Bautista, Elena González-López, Marcos López-Hoyos, and David San Segundo. 2021. "Epitope-Level Matching—A Review of the Novel Concept of Eplets in Transplant Histocompatibility" Transplantology 2, no. 3: 336-347. https://doi.org/10.3390/transplantology2030033
APA StyleRenaldo, A., Roa-Bautista, A., González-López, E., López-Hoyos, M., & San Segundo, D. (2021). Epitope-Level Matching—A Review of the Novel Concept of Eplets in Transplant Histocompatibility. Transplantology, 2(3), 336-347. https://doi.org/10.3390/transplantology2030033








