1. Introduction
Thrombophilias, whether inherited or acquired, constitute a heterogeneous group of disorders characterized by increased coagulability and a predisposition to thrombotic events, which may compromise uteroplacental circulation and adversely affect pregnancy outcomes. These conditions are frequently associated with obstetric complications such as preeclampsia, intrauterine growth restriction (IUGR), abruptio placentae, and recurrent pregnancy loss, particularly in the second and third trimesters [
1,
2,
3].
The clinical relevance of thrombophilia is further supported by its notable prevalence among women of reproductive age. Inherited thrombophilias are estimated to affect approximately 8–15% of pregnant women in European and North American populations, although prevalence varies depending on ethnicity, geographic region, and screening protocols [
1,
4,
5]. Factor V Leiden remains the most common mutation, with heterozygous prevalence rates ranging from 3 to 8% among Caucasian pregnant women [
1,
3]. The Prothrombin G20210A mutation is found in 1–3% of women, while deficiencies of natural anticoagulants such as protein C, protein S, or antithrombin are less common (0.03–0.5%) but associated with a significantly increased thrombotic risk during pregnancy [
4,
6,
7]. Despite this, many cases remain undiagnosed, as universal screening is not routinely recommended in the absence of clinical or obstetric risk factors [
7,
8]. This diagnostic gap highlights the need for adjunctive or indirect markers—such as placental histopathological changes—to retrospectively raise suspicion and guide postpartum management or future pregnancy care.
The placenta serves as a key interface between the maternal and fetal circulations, and its structural integrity is essential for a successful pregnancy. In thrombophilic pregnancies, impaired perfusion and microthrombotic events can lead to histological changes, including infarctions, intervillous thrombi, and delayed villous maturation. These lesions often remain subclinical and may be overlooked during routine gross examination, highlighting the value of systematic histopathological assessment in uncovering the underlying pathophysiological processes [
9,
10].
Among the most frequently reported lesions in thrombophilic pregnancies are intervillous thrombosis, placental infarctions, villous agglutination, villous stasis and immaturity, and degenerative vascular changes. These findings suggest a state of chronic placental dysfunction, although their diagnostic relevance remains insufficiently evaluated in standardized studies [
11,
12,
13].
While individual placental lesions associated with thrombophilia are well described, their diagnostic significance remains poorly defined, and no standardized scoring system has been widely adopted. Most existing approaches rely on qualitative assessment and lack reproducible metrics to stratify thrombophilic risk. Therefore, a composite histopathological score could provide a more objective framework for assessing cumulative lesion burden, improving communication between pathologists and clinicians, and guiding further thrombophilia testing.
While several histopathological studies have described individual placental lesions associated with thrombophilia, standardized composite scoring systems are scarce. To date, most diagnostic approaches rely on qualitative assessments, without a unified tool to stratify risk based on lesion burden [
14]. Our proposed histological score provides a simplified, additive model incorporating the most frequent lesions associated with thrombophilic pregnancies. By quantifying pathology in a reproducible format, it may enhance retrospective diagnosis and clinical decision-making.
The objectives of this study are to compare macroscopic and histopathological placental parameters between pregnancies with thrombophilia and those without complications; to identify histopathological features predictive of thrombophilia; to explore the potential of these features to form a clinically meaningful placental histological score. This study complements our previous work [
15], which analyzed clinical and systemic parameters in the same patient population. The current analysis focuses specifically on placental histopathology and proposes a practical composite score for assessing thrombophilia-related lesions.
2. Materials and Methods
2.1. Study Design and Setting
This was a retrospective observational study conducted on two groups of placental samples collected from pregnancies delivered in a tertiary care maternity hospital. The study was approved by the local institutional ethics committee, and all procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki. The study was carried out at “Emergency County Hospital Bihor” between September 2020 and September 2024. Ethical approval was granted by the Institutional Review Board, and all participants provided written informed consent before enrollment. The study protocol (approval no. 14146/15.06.2018) was reviewed and approved by the Institutional Ethics Committee of the University of Oradea. All participants provided written informed consent for the use of anonymized clinical and histopathologic data for research purposes, in accordance with institutional and national regulations and the Declaration of Helsinki.
2.2. Study Population
The study included two distinct cohorts:
- 1.
Thrombophilia group (TG)—placentas obtained from pregnancies diagnosed with hereditary thrombophilia with or without obstetrical complications (80 persons, 72.1%).
- 2.
Control group (CG)—placentas from pregnancies without known maternal pathology, delivering healthy newborns at term or late preterm (31 cases, 27.9%).
Inclusion criteria for both groups were the availability of complete placental examination (both macroscopic and histologic) and adequate clinical records. Exclusion criteria included multiple pregnancies, major fetal anomalies, intrauterine infections, or incomplete pathology data.
Sample size justification was based on preliminary power calculations estimating that a minimum of 30–34 subjects per group would allow detection of moderate effect sizes (Cohen’s d ≈ 0.7–0.8) with 80% power at α = 0.05. The final cohort exceeded this threshold for the thrombophilia group. Selection bias was minimized by applying uniform inclusion/exclusion criteria and blinding pathologists to clinical diagnosis during histopathologic evaluation.
2.3. Placental Examination
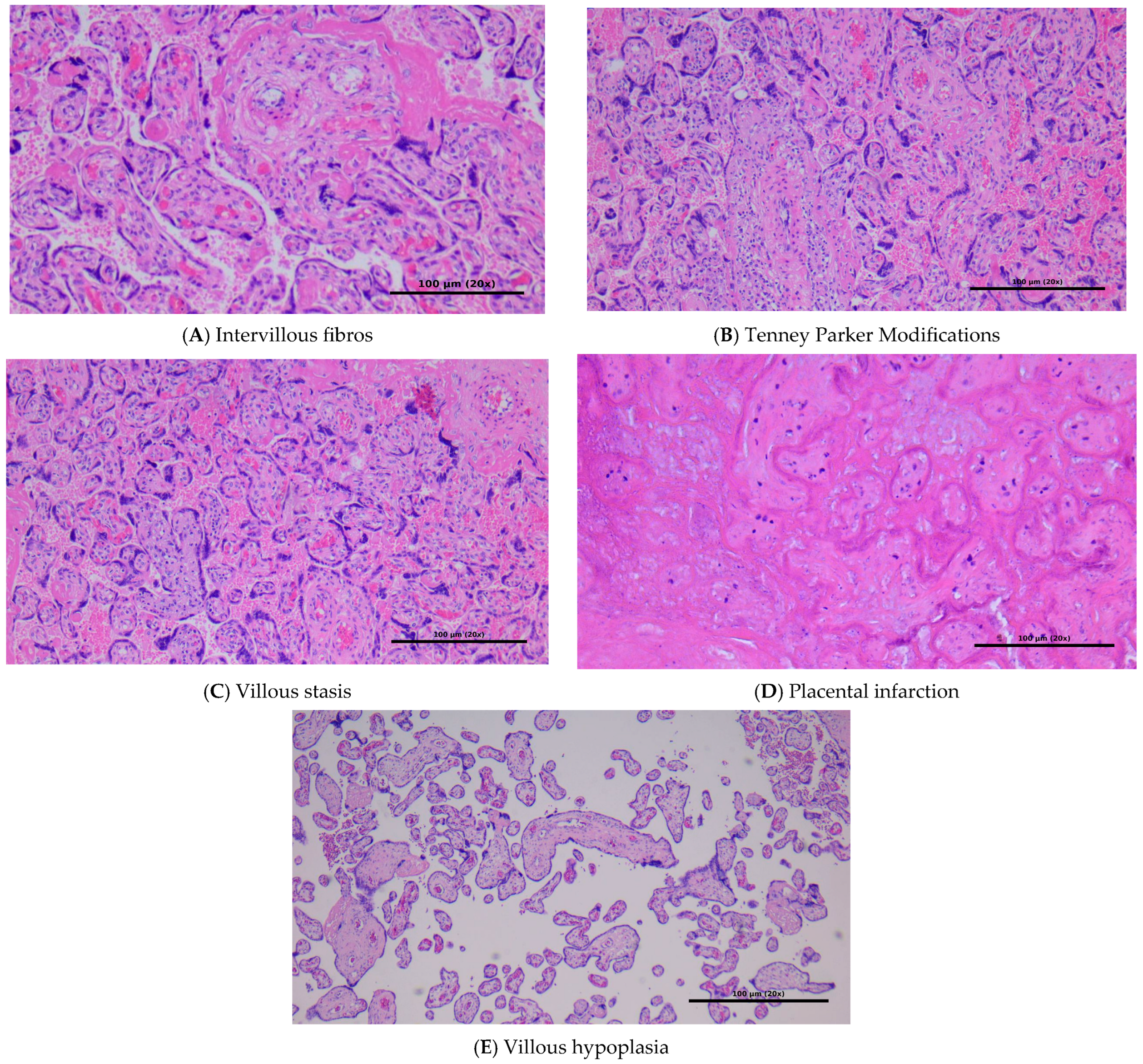
All placentas underwent standardized examination by the same team of pathologists. The pathologists performing the histopathological evaluations were blinded to the clinical diagnosis (thrombophilia vs. control group) to reduce potential bias in lesion identification and score assignment. Representative microphotographs (
Figure 1) were taken directly from the analyzed cases and illustrate the main placental lesions assessed according to standardized histopathological criteria.
Macroscopic parameters included:
Placental weight (g),
Maximum diameter (cm),
Thickness (cm),
Umbilical cord length and insertion type.
Histopathologic features were evaluated according to standard criteria and included the presence or absence of the following lesions:
Intervillous thrombosis,
Villous agglutination,
Placental infarction,
Acute atherosis,
Stromal fibrosis,
Villous stasis,
Immature villi (villous maturation delay).
Histopathologic lesions were assessed according to standardized criteria. Although this study initially used descriptive terms, we have now aligned terminology with the Amsterdam Placental Workshop Group Consensus Statement (2016), categorizing findings as consistent with maternal vascular malperfusion (MVM) or fetal vascular malperfusion (FVM) where appropriate [
16]. Lesions such as placental infarctions, decidual arteriopathy (acute atherosis), and accelerated villous maturation were classified under the category of MVM. Although features like avascular villi or thrombosed fetal vessels would indicate FVM, such findings were not identified in our cohort and thus were not included in
Table 1.
2.4. Histologic Score Development
A synthetic histologic score (range 0–5) was developed by assigning one point for each of the following lesions when present:
Villous stasis
Stromal fibrosis
Intervillous thrombosis
Placental infarction
Acute atherosis
This score was calculated for each placenta, and its distribution was compared between the two groups. A cutoff value of ≥3 was tested for association with the thrombophilia group.
Although individual histopathologic variables did not reach conventional statistical significance, all demonstrated consistent directional trends toward higher prevalence in the thrombophilia group. Given the exploratory nature of this study and the interrelated pathophysiology of ischemic placental lesions, a simple unweighted additive model was used to capture the cumulative burden of pathology rather than the significance of single features. This approach is consistent with early validation stages of composite histologic scores.
2.5. Placental Histological Score for Suspected Thrombophilia
2.5.1. Concept
Based on the data obtained, we propose a simple additive score in which each histopathological lesion suggestive of thrombophilia is assigned a value of 1 point. The higher the score, the greater the probability that the placenta has been affected by ischemic or thrombotic changes characteristic of thrombophilia.
2.5.2. Parameters Included in the Score
Based on their increased frequency in the thrombophilia group and
p-values approaching statistical significance, the following parameters are included in
Table 2.
2.6. Statistical Analysis
All statistical analyses were performed using Python (v3.11; Python Software Foundation, Wilmington, DE, USA), SciPy (v1.12.0), and statsmodels (v0.14.1). Continuous variables were tested for normality and compared using the Student’s t-test or Mann–Whitney U test as appropriate. Categorical variables were analyzed with the Chi-square test or Fisher’s exact test. The multivariate analysis was performed using several tests (Pillai’s Trace, Wilks’ Lambda, Hotelling’s Trace, Roy’s Largest Root).
Correlations between the histologic score and continuous variables (placental weight, diameter, thickness, fetal weight, gestational age) were assessed using Pearson and Spearman coefficients. A linear regression model was built to assess predictors of the histologic score. A p-value < 0.05 was considered statistically significant.
For analyses involving multiple pairwise group comparisons, Bonferroni correction was applied to control for type I error inflation. When assumptions of normality were violated, non-parametric tests (Mann–Whitney U or Fisher’s exact) were used. All statistical analyses were two-tailed with α = 0.05 after adjustment.
3. Results
3.1. Study Cohort Characteristics
A total of 111 placentas were included in the final analysis. Of these, 80 were obtained from pregnancies complicated by confirmed maternal thrombophilia (study group), while 31 represented normal pregnancies without any documented maternal comorbidity (control group). All included cases had completed clinical records and placental pathology reports, with no exclusions for missing data or inadequate sampling.
The thrombophilia group included only hereditary forms. The control group was composed of singleton pregnancies that progressed uneventfully and resulted in healthy term or late-preterm neonates. The thrombophilia group included both term and late-preterm deliveries (34–36.9 weeks). Approximately 68% of cases in this group delivered before 37 weeks, often due to obstetric complications such as preeclampsia or fetal growth restriction.
The mean maternal age in the thrombophilia group was 35.8 ± 4.7 years, compared to 31.2 ± 3.9 years in the control group. Most participants were multiparous (68%) and of Caucasian ethnicity. The thrombophilia group included patients with confirmed hereditary conditions: Factor V Leiden mutation (42.5%), Prothrombin G20210A mutation (21.3%), MTHFR C677T mutation (18.8%), Protein S deficiency (10.0%), and Antithrombin deficiency (7.5%). Severity stratification (homozygous/heterozygous) was inconsistently reported in historical records and is listed as a study limitation.
The maternal age ranged between 21 and 46 years (mean 36.03 ± 2.8), and all women in the thrombophilia group had confirmed hereditary thrombophilia, though the specific genotypes were not stratified. The control group consisted of healthy, normotensive women with no personal or family history of thrombotic events or pregnancy complications. All neonates had 5 min Apgar scores ≥ 7, and no cases of fetal distress were recorded in the control group.
Multivariate analysis (
Table 2) was performed using several tests (Pillai’s Trace, Wilks’ Lambda, Hotelling’s Trace, Roy’s Largest Root) to assess the combined effect of placental weight and newborn weight on the dependent variables.
The intercept was highly significant in all tests (p < 0.001), indicating that the overall model explains a substantial portion of the variance.
For placental weight, none of the multivariate tests were significant (p = 0.551), suggesting that it does not have a significant multivariate effect on the outcomes.
For newborn weight, all tests were highly significant (p < 0.01), with large effect sizes (e.g., Partial Eta Squared = 0.567 to 0.740), indicating a strong multivariate association with the dependent variables.
Table 3 was included to explore whether placental and neonatal morphometric parameters could explain the variability of histologic findings. Although placental weight did not contribute significantly, newborn weight showed a strong multivariate association with dependent outcomes, supporting the independence of histologic pathology from macroscopic metrics.
3.2. Sample Size Considerations
The size of the study groups was determined retrospectively, based on the availability of complete placental histopathological assessments during the study period. For exploratory analysis with continuous outcomes (e.g., histologic score), an a priori estimation suggested that a minimum of 30–34 cases per group would allow detection of moderate effect sizes (Cohen’s d ≈ 0.7–0.8) with a power of 80% and α = 0.05. The final cohort exceeded this requirement in the thrombophilia group, ensuring adequate statistical power for group comparisons.
Descriptive statistics for maternal and placental baseline parameters are summarized in
Table 4.
3.3. Histopathologic Findings
The comparative histopathologic analysis between the thrombophilia group and the control group revealed a higher frequency of placental lesions among patients with thrombophilia. The most prevalent finding in this group was villous stasis, identified in 59.4% of cases, compared to 27.3% in the control group (
p = 0.053), suggesting a near-significant difference (
Figure 2).
Similarly, stromal fibrosis was more frequently observed in placentas from thrombophilic pregnancies (39.7% vs. 18.2%), with a p-value of 0.076. Although these two parameters did not reach conventional statistical significance (p < 0.05), their consistent association with the thrombophilia group indicates a relevant trend worth further investigation.
Massive perivillous fibrin deposition (MPVFD), a known feature associated with thrombophilic and immune-mediated placental injury, was not consistently reported in our cohort and was therefore not included in the current scoring model. We acknowledge this as a limitation of the retrospective design and recommend systematic assessment of fibrin deposition in future studies.
Other lesions, such as intervillous thrombosis (37.5% vs. 36.4%), placental infarctions (42.2% vs. 22.7%), and acute atherosis (42.2% vs. 13.6%) were also more frequent in the thrombophilia group, but did not demonstrate statistical significance in this cohort (all p > 0.05). Likewise, villous agglutination, vascular thrombi, and delayed villous maturation were variably distributed but showed no significant group differences.
These findings point toward a distinct histopathologic profile in thrombophilia, marked by a higher prevalence of ischemic and congestive lesions. Despite the lack of statistical significance in some cases, possibly due to sample size limitations, the consistent pattern observed may reflect an underlying placental dysfunction associated with prothrombotic states (
Table 5). Terms such as ‘villous stasis’ and ‘boiled meat appearance’ were previously used descriptively by the examining pathologists. For clarity, we now interpret villous stasis as delayed villous maturation, and ‘boiled meat’ as a gross descriptor of homogenous parenchymal discoloration, often associated with infarction or perivillous fibrin deposition. These have been re-categorized according to consensus criteria.
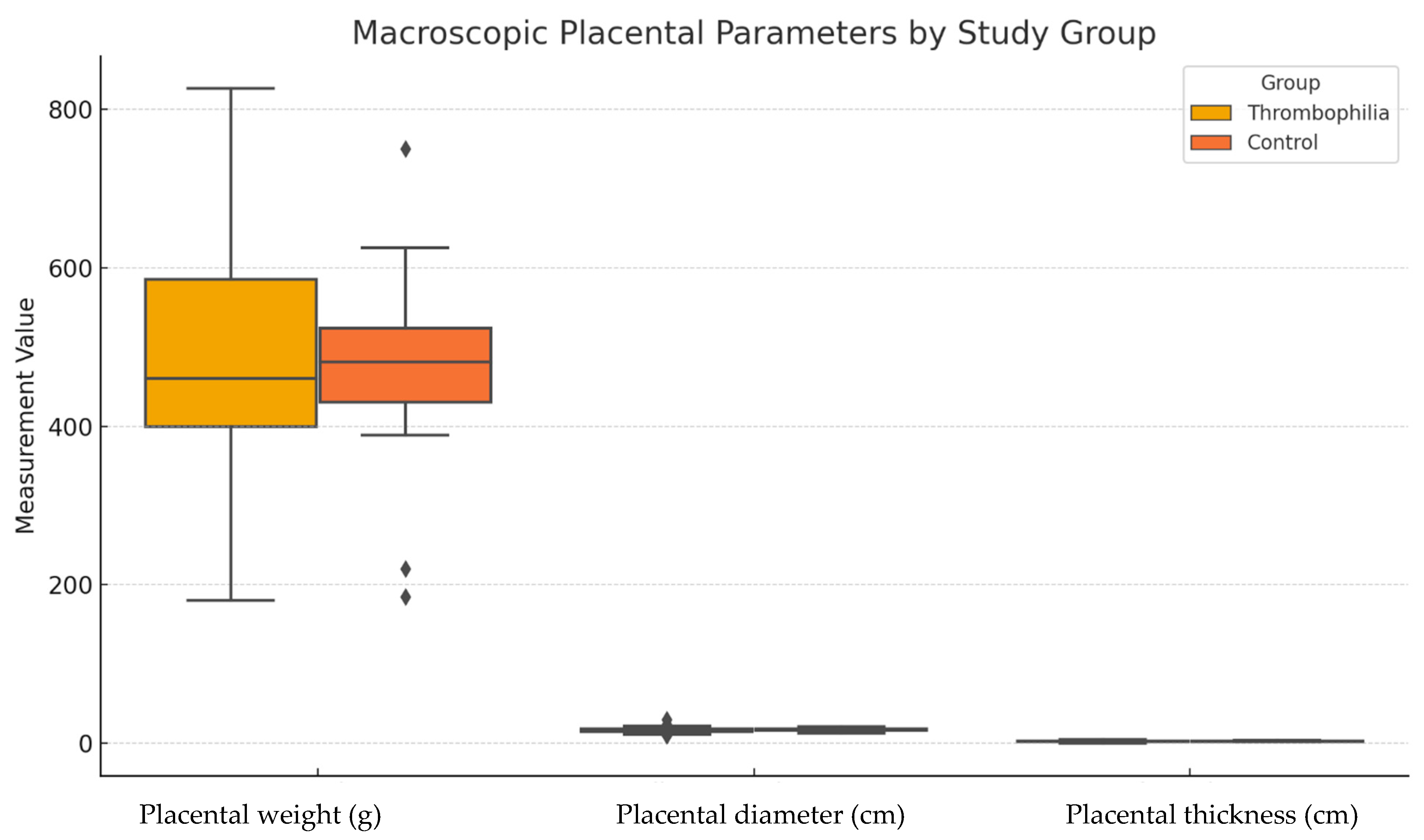
3.4. Macroscopic Placental Parameters
Macroscopic analysis of placental dimensions revealed no statistically significant differences between the thrombophilia and control groups. The mean placental weight was 476.4 ± 135.5 g in the thrombophilia group and 470.2 ± 120.9 g in the control group (p = 0.8403). Similarly, the mean placental diameter did not differ significantly between the groups (16.88 ± 3.47 cm vs. 17.14 ± 1.96 cm, p = 0.6657). The mean placental thickness was also comparable (2.44 ± 0.99 cm vs. 2.27 ± 0.61 cm, p = 0.3678).
These findings suggest that, despite the higher frequency of histopathologic abnormalities in the thrombophilia group, macroscopic placental features alone are insufficient to distinguish between pathologic and non-pathologic placentas in this context. This reinforces the importance of histologic examination, particularly in cases with obstetrical complications but unremarkable gross findings (
Table 6 and
Figure 3).
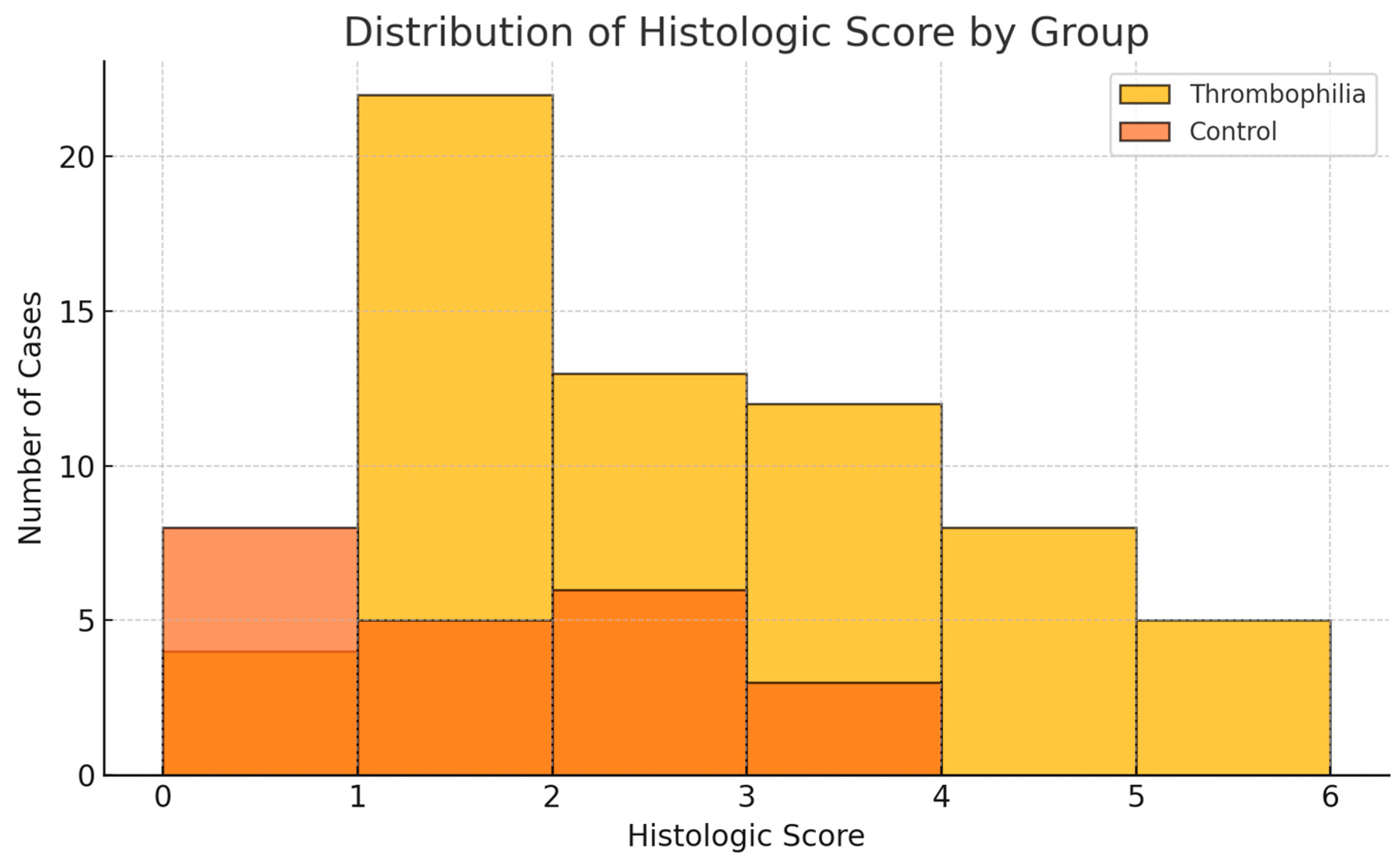
3.5. Histologic Score Analysis
Based on the five most prevalent histopathologic lesions observed in the study cohort, a composite histologic score (range 0–5) was developed. Each of the following lesions contributed one point to the score when present: villous stasis, stromal fibrosis, intervillous thrombosis, placental infarction, and acute atherosis.
The threshold of ≥3 was selected post hoc based on its ability to discriminate between groups. In our cohort, this threshold yielded a sensitivity of 42.2% and specificity of 86.4% for identifying thrombophilic pregnancies. While not a definitive diagnostic cutoff, it may serve as a screening trigger for further clinical evaluation in the postpartum period.
The mean histologic score was significantly higher in the thrombophilia group compared to the control group (2.20 ± 1.4 vs. 1.18 ± 1.1, p = 0.0011), indicating a greater burden of histopathologic abnormalities in thrombophilic pregnancies.
Furthermore, a cutoff of ≥3 points was used to identify placentas with a high density of pathologic changes. This threshold was exceeded in 39.1% of placentas in the thrombophilia group, compared to only 13.6% in the control group (p = 0.015, Chi-square test), reinforcing the potential of this score as a screening tool for thrombophilia-related placental pathology. The threshold of ≥3 was chosen based on its ability to capture a cluster of multiple co-existing placental lesions. This value marked a significant inflection point in group differences and corresponds to the upper tertile of the score distribution. It also offered a pragmatic balance between sensitivity and specificity, identifying a subgroup with substantial histologic burden.
The cutoff of ≥3 was empirically determined as the upper tertile of the score distribution. Although sensitivity (42.2%) was modest, the specificity (86.4%) suggests good discriminative capacity for identifying placentas with a high burden of ischemic lesions. Future validation in larger cohorts will refine the optimal threshold.
Table 7 presents the comparative distribution of the histologic score, and
Figure 4 illustrates its group-wise distribution.
3.6. Risk Threshold and Clinical Interpretation
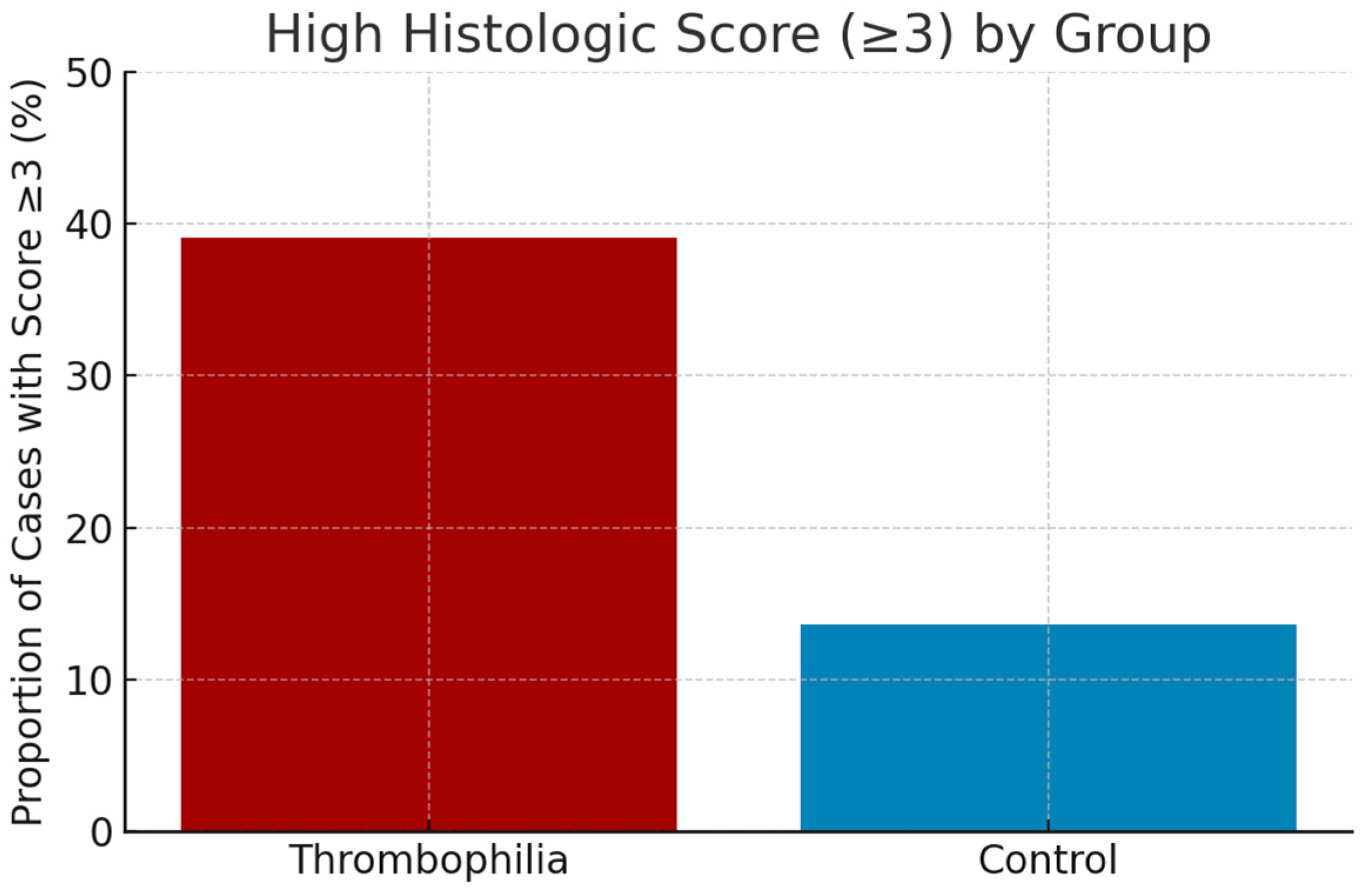
To evaluate the practical utility of the histologic score, a threshold of ≥3 points was proposed as an indicator of increased risk for thrombophilia. This cutoff was chosen based on the observation that scores ≥ 3 occurred significantly more frequently in the thrombophilia group compared to the control group.
Application of this threshold to the study population yielded the following results:
Percentage of cases with histologic score ≥ 3:
Thrombophilia group: 42.5% (34 out of 80)
Control group: 9.6% (3 out of 31)
This marked difference supports the validity of the proposed threshold and suggests that a histologic score of ≥3 may serve as an indirect marker of placental pathology suggestive of underlying maternal thrombophilia, with potential relevance for retrospective screening and clinical evaluation (
Figure 5).
3.7. Correlations Between Placental Histologic Score and Clinico-Morphological Parameters
To assess the potential impact of histopathological lesions on placental and fetal characteristics, correlations were analyzed between the placental histologic score and the following indicators: fetal weight, placental weight, placental diameter and thickness, and gestational age at birth.
The results of the Pearson correlation analysis in the thrombophilia group are presented in
Table 8.
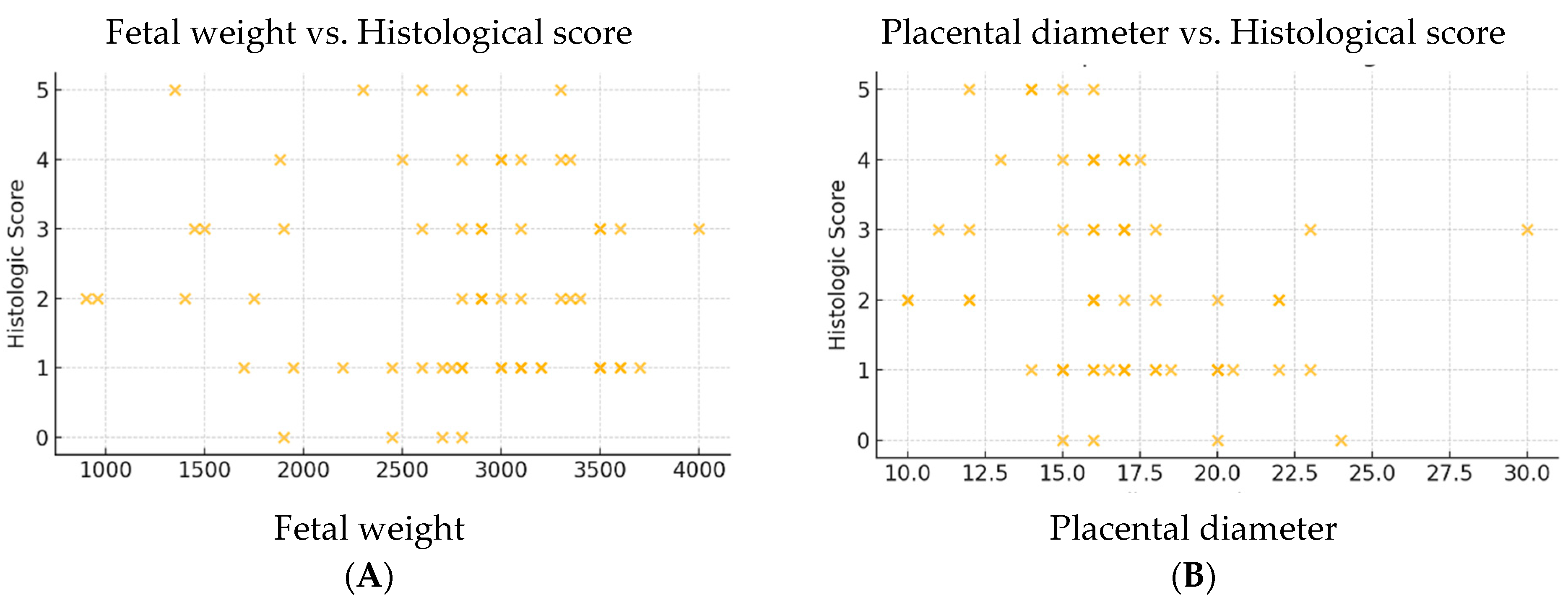
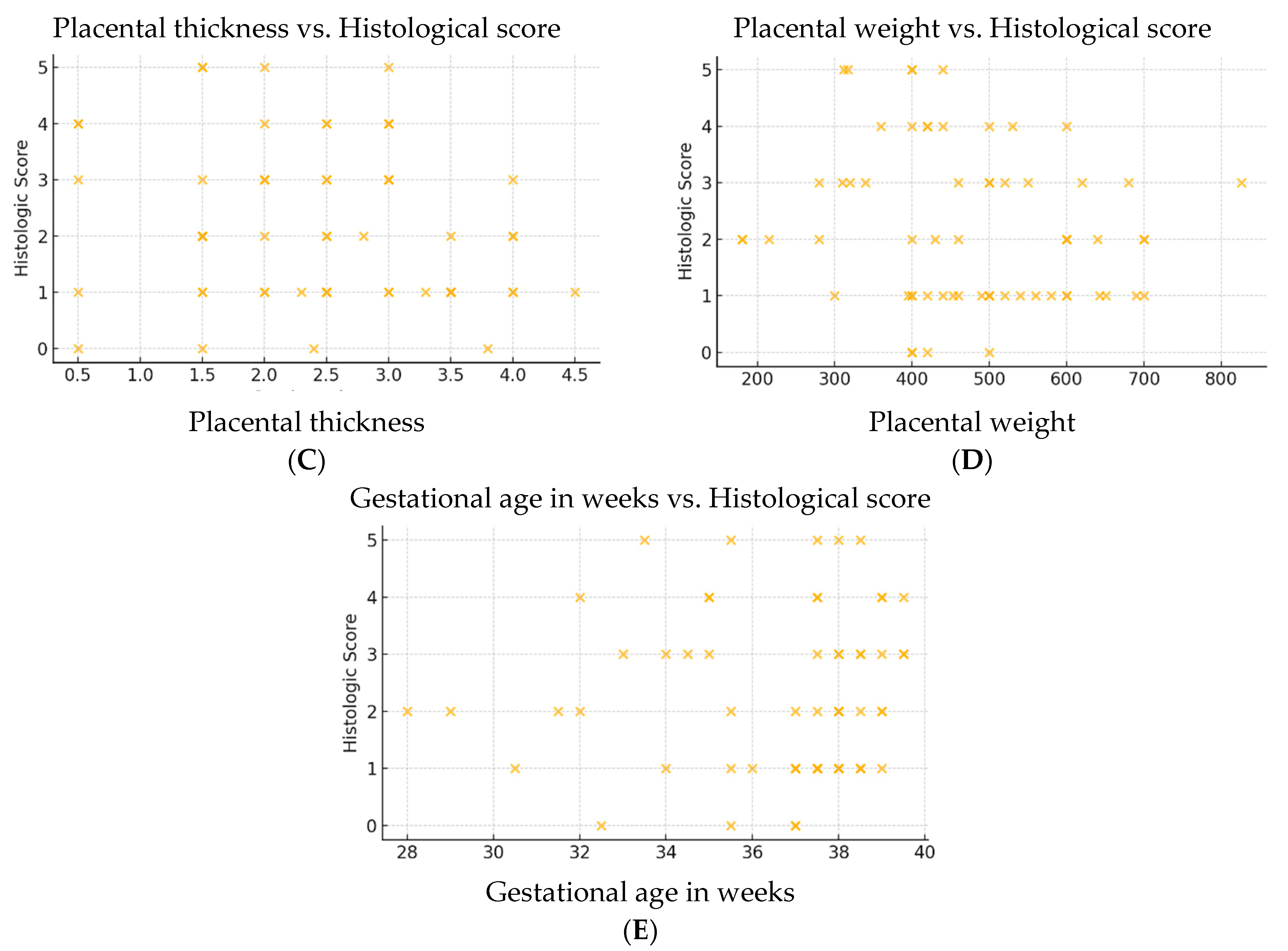
Of all the variables analyzed, only placental diameter showed a statistically significant, albeit weak and negative, correlation (r = −0.270, p = 0.0311), suggesting that a reduced placental diameter is associated with a higher histologic score and, implicitly, with a greater presence of histopathological lesions.
The remaining parameters, including fetal weight and gestational age, did not demonstrate significant correlations with the histologic score. This may indicate that such microscopic tissue changes are not always reflected in macroscopic placental measurements or in fetal growth parameters. These findings emphasize the subclinical and potentially insidious nature of placental lesions in thrombophilia, and support the importance of histopathological examination—even in the presence of apparently normal gross features.
3.8. Linear Regression
The multiple linear regression model failed to identify a combination of clinico-morphological variables that significantly predicted the histologic placental score. This result supports the conclusions drawn from the correlation analysis, indicating that histopathologic alterations are not directly mirrored by the clinical or macroscopic variables assessed. These findings reinforce the notion that histologic evaluation is essential for the detection of placental pathology in thrombophilic pregnancies, particularly when macroscopic findings are unremarkable (
Table 9).
The only predictor that reached statistical significance (p < 0.05) was placental diameter, which showed a negative coefficient, further supporting the association between smaller placentas and the severity of histopathological lesions.
To explore potential associations between histologic placental changes and clinical or morphological parameters, Pearson correlation coefficients were calculated between the histologic score and the following continuous variables: fetal weight, placental weight, placental diameter, placental thickness, and gestational age (
Table 6).
The analysis revealed that only placental diameter was significantly correlated with the histologic score (r = −0.270, p = 0.0311). All other variables showed weak or non-significant correlations, suggesting that macroscopic and clinical parameters do not reliably predict the extent of microscopic placental injury in thrombophilic pregnancies.
To better visualize these relationships, scatter plots were generated for each variable (
Figure 6A–E). The inverse correlation between placental diameter and histologic score was the only one to show a consistent trend, supporting its potential use as an indirect marker of underlying placental pathology.
4. Discussion
In this study, we identified a distinct histopathological profile in placentas from pregnancies complicated by thrombophilia, including a higher prevalence of lesions suggestive of chronic ischemic and thrombotic injury. Although macroscopic placental parameters did not differ significantly between groups, histologic examination revealed more pronounced abnormalities in the thrombophilia group, which were captured effectively by the proposed histologic score [
17,
18,
19,
20].
Among the parameters evaluated, placental diameter emerged as the only variable significantly associated with the histologic score. Both the multiple linear regression model and Pearson correlation analysis indicated a statistically significant, though weak, negative relationship between diameter and histologic score (r = −0.270,
p = 0.0311). This suggests that a smaller placental diameter may serve as an indirect marker of underlying histological damage. In contrast, fetal weight, placental weight, gestational age, and placental thickness showed no significant association with the severity of microscopic lesions [
21,
22,
23,
24,
25].
Few standardized histologic scores have been proposed for thrombophilia-related placental pathology. Existing systems, such as those used for maternal vascular malperfusion (MVM) classification, focus on individual lesion severity rather than composite burden. Our score complements these by integrating five frequent lesions into a simple numeric framework, similar in concept to semi-quantitative indices in placental insufficiency studies [
14,
16,
26].
Previous studies have described placental lesions such as infarctions, intervillous thrombi, and decidual vasculopathy in association with thrombophilia [
12,
17,
27,
28,
29]. Redline et al. and others have reported a higher prevalence of these features in women with adverse pregnancy outcomes and hypercoagulable states. However, most studies have focused on individual lesions or lacked a unified scoring system. Our work extends these findings by integrating common histopathologic alterations into a simplified scoring model, potentially enhancing diagnostic yield and facilitating communication between obstetricians and pathologists. To enhance the clinical relevance of our findings, we retrospectively classified the histopathologic lesions based on the Amsterdam Consensus, which provides a robust framework for categorizing placental abnormalities by etiology. Most lesions identified in the thrombophilia group are consistent with maternal vascular malperfusion (MVM), further supporting the underlying prothrombotic state as a contributor to impaired uteroplacental circulation.
Previous studies have identified increased perivillous fibrin, particularly MPVFD, as a key marker of placental insufficiency in thrombophilia. While not incorporated in our current score due to reporting limitations, its role warrants further investigation and possible inclusion in revised scoring frameworks. Although our analysis was retrospective and did not include longitudinal follow-up, all patients diagnosed with hereditary thrombophilia received individualized counseling and hematologic evaluation. In most cases, postpartum thromboprophylaxis with low-molecular-weight heparin (LMWH) was recommended, and preventive anticoagulant therapy was planned for subsequent pregnancies based on risk stratification and obstetric history. Future prospective studies will aim to correlate histologic scores with therapeutic outcomes.
These findings reinforce the notion that macroscopic or clinical characteristics alone are insufficient to detect placental damage related to thrombophilia. The subclinical and often insidious nature of such changes highlights the importance of systematic histopathological evaluation [
30], especially in pregnancies with adverse outcomes but unremarkable placental morphology [
12,
28]. The proposed score was not intended for diagnostic use but as an exploratory framework summarizing lesion co-occurrence. Further refinement and weighting based on multivariate regression or ROC analysis will be necessary for external validation.
The implementation of a standardized placental histologic score could influence clinical care by prompting retrospective thrombophilia screening in cases with high scores (≥3), even when not previously suspected during pregnancy. Identifying subclinical thrombophilia postpartum may allow for targeted thromboprophylaxis in subsequent pregnancies or non-obstetric settings. Additionally, in high-risk women, antenatal surveillance protocols could be intensified in future pregnancies, including earlier Doppler assessment, maternal coagulation profiling, and potential prophylactic anticoagulation based on interdisciplinary risk stratification [
31,
32,
33]. The implementation of a simple additive histologic score, based on key lesions such as villous stasis, fibrosis, and infarction, allowed for a semi-quantitative assessment of placental damage. A threshold score of ≥3 points proved useful in differentiating between thrombophilic and physiological pregnancies, with potential applicability in retrospective diagnostic screening or postpartum risk stratification [
34,
35].
While placental diameter emerged as the only significantly correlated macroscopic feature with the histologic score, this was unexpected given the broader range of parameters typically associated with placental insufficiency (e.g., weight, fetal–placental ratio, cord abnormalities) [
36]. The lack of statistical associations in other parameters may reflect the study’s limited sample size and the heterogeneity of thrombophilic disorders. This further emphasizes the potential value of microscopic over macroscopic examination in detecting subclinical placental pathology.
This study has several limitations that should be acknowledged. First, the sample size—particularly in the control group—was relatively small, which may have reduced the statistical power to detect more subtle differences. This imbalance resulted from the retrospective nature of the study and the requirement for comprehensive placental histopathological analysis, which is rarely performed in uncomplicated pregnancies). Furthermore, we applied appropriate statistical methods, including non-parametric and exact tests, to account for unequal group sizes. Second, the diagnosis of thrombophilia was not stratified by subtype (e.g., inherited vs. acquired, homozygous vs. heterozygous mutations), which may have introduced heterogeneity within the study group and diluted potential associations. Third, the retrospective design precluded the evaluation of dynamic or longitudinal changes during pregnancy and relied on available histological records and standardized reporting. Future studies should incorporate blinded assessments to strengthen objectivity. Additionally, the proposed histologic score was internally developed and applied to a single-center population, without external validation. As such, further studies on its generalizability to larger or more diverse cohorts are warranted [
6,
29,
37].
This study has several limitations. First, as a single-center retrospective analysis, unmeasured confounding variables—such as maternal comorbidities, treatment regimens, or genetic heterogeneity—may have influenced histologic findings. Second, the unequal group sizes and predominance of hereditary thrombophilia limit generalizability to acquired forms and other populations. Third, absence of longitudinal follow-up restricts interpretation of causal relationships. Future multicenter prospective studies with larger and ethnically diverse cohorts are planned to externally validate the proposed histologic score and assess its predictive value for pregnancy outcomes.
Future prospective studies involving larger, well-characterized, and more diverse populations, including external validation cohorts, are needed to confirm the diagnostic and predictive value of the histologic score, to refine its clinical applicability, and to explore its potential role in ongoing pregnancies—particularly in conjunction with biomarkers and imaging-based assessments. From a clinical perspective, the histologic score offers a potential adjunct for postpartum risk stratification in pregnancies with adverse outcomes. By identifying women with placental signs suggestive of undiagnosed thrombophilia, this tool could support the initiation of thrombophilia workup, guide decisions regarding future pregnancy management, and reduce the recurrence of thrombo-embolic obstetric events. Its ease of application and reliance on standard histological assessments make it suitable for widespread use, pending validation.
5. Conclusions
This study highlights the diagnostic value of placental histopathological examination in pregnancies complicated by thrombophilia. Although macroscopic placental features did not differ significantly between groups, histological evaluation revealed a consistent pattern of lesions suggestive of ischemic and thrombotic injury. The development and application of a simple histologic score allowed for effective differentiation between thrombophilic and physiological pregnancies.
A score ≥ 3 was significantly more frequent in the thrombophilia group and may serve as an indirect marker for underlying maternal thrombotic pathology. While placental diameter showed a weak yet statistically significant inverse correlation with histologic severity, most clinical and morphological parameters failed to predict the extent of microscopic damage.
The findings underscore the subclinical nature of placental lesions in thrombophilia and support the integration of standardized histological scoring in routine obstetric and pathological practice. Future validation in larger, prospective cohorts is essential to confirm its utility and explore its potential role in postpartum screening, risk stratification, and management of thrombophilic patients.