1. Introduction
The presence of type 1 diabetes mellitus (T1DM) during pregnancy significantly increases the risk of both maternal and perinatal complications. Maternal risks include miscarriage, preterm birth, caesarean delivery, preeclampsia, and hypertension, while perinatal risks involve macrosomia, congenital anomalies, neonatal hypoglycaemia, hyperbilirubinemia, and neonatal death [
1,
2]. Maternal hyperglycaemia, particularly during the second and third trimesters, has been associated with a higher incidence of preeclampsia, preterm delivery, large-for-gestational-age (LGA) and small-for-gestational-age (SGA) neonates, and neonatal hypoglycaemia [
2]. Optimal glycaemic control plays a critical role throughout pregnancy. Achieving tight metabolic control in the first trimester reduces the risk of congenital malformations and perinatal mortality, while maintaining normoglycaemia in the second and third trimesters lowers the risk of hypertensive disorders, LGA neonates, premature delivery, and the need for neonatal intensive care unit (NICU) [
3]. However, stringent glycaemic targets are accompanied by an increased risk of maternal hypoglycaemia, particularly in early gestation [
1]. Recent qualitative studies demonstrate that pregnant women using closed-loop systems report significantly improved sleep, confidence, and reduced anxiety around hypoglycaemia [
4,
5]. Furthermore, large, mixed-methods RCTs (randomised controlled trials) comparing advanced hybrid closed-loop therapy with sensor-augmented pump therapy confirm enhanced quality of life and decreased diabetes distress [
6].
Murphy et al. (2021) conducted a comprehensive 5-year national population-based cohort study in the UK, which included 4089 pregnancies in women with T1DM and 1768 with type 2 diabetes mellitus (T2DM). The study revealed significant differences in baseline characteristics and pregnancy outcomes between the two groups. Women with T2DM were generally older, more frequently obese (66.9% vs. 27.7%), and had a higher prevalence of chronic hypertension (23.1% vs. 12.3%) than women with T1DM. Nevertheless, perinatal outcomes were consistently worse in the T1DM group. The incidence of congenital anomalies (4.3% vs. 2.1%), preterm birth before 37 weeks (37.3% vs. 21.7%), macrosomia (27.9% vs. 21.8%), and NICU admission (27.2% vs. 17.6%) was significantly higher among women with T1DM. The rate of stillbirth was also slightly higher in the T1DM group (0.8% vs. 0.5%). The study further highlighted the underutilization of preconception counselling, with only 41.3% of women with T1DM and 14.3% with T2DM receiving structured advice before conception. Moreover, fewer than 20% of women with T1DM achieved the recommended pre-pregnancy HbA1c target of <6.5%, compared to 38.3% of women with T2DM. Glycaemic control remained suboptimal throughout pregnancy, especially among women with T1DM [
7].
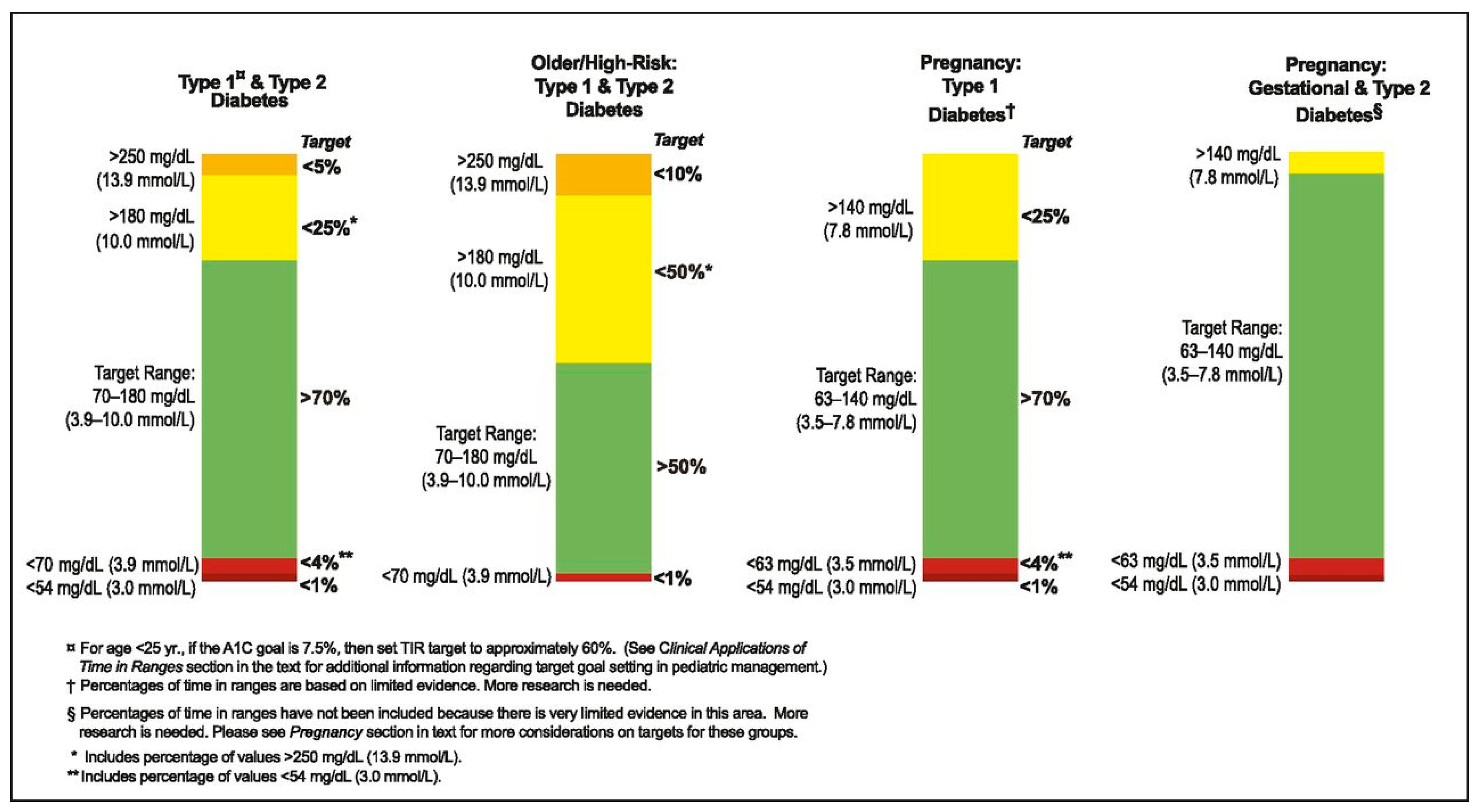
To improve pregnancy outcomes, the current guidelines recommend a target HbA1c of <6.5% and a pregnancy-specific time in range (TIR) of ≥70%, defined as glucose values between 3.5 and 7.8 mmol/L [
1]. Achieving these targets requires intensive insulin therapy, either via multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII), combined with frequent glucose monitoring using self-monitoring of blood glucose (SMBG) or continuous glucose monitoring (CGM) [
2].
Sensor-augmented pump (SAP) therapy, which integrates real-time CGM with insulin pumps, offers enhanced glycaemic control by facilitating more precise insulin adjustments. More recently, advanced hybrid closed loop (AHCL) systems have emerged as a promising innovation. These systems automate basal insulin delivery based on CGM data, while still requiring user-initiated bolus administration for meals. In non-pregnant individuals with T1DM, AHCL systems have been shown to improve TIR, reduce glycaemic variability, and decrease the risk of hypoglycaemia. Despite these advantages, maintaining optimal glycaemic control during pregnancy remains complex. Hormonal fluctuations lead to progressive changes in insulin sensitivity, particularly insulin resistance in late gestation, and insulin absorption becomes increasingly variable. These factors complicate insulin dose titration and can impair glycaemic stability [
3]. While AHCL technology offers the potential to support optimal glycaemic control before and during pregnancy, regulatory approvals are still limited, and not all AHCL systems are currently approved for use in pregnancy [
1].
CamAPS FX is the first AID system formally approved for pregnancy. The AiDAPT RCT demonstrated a 10.5% absolute increase in pregnancy-specific TIR compared with SAP therapy [
4,
8,
9]. MiniMed 780G, although not pregnancy-approved, has been evaluated in RCTs such as CRISTAL, showing safety and improved overnight TIR [
1,
10]. Tandem Control-IQ, which operates with a higher default target band, has also been trialled in pregnancy with promising results [
6,
11]. NICE explicitly recommends AHCL use for all pregnant women with T1DM, regardless of baseline HbA1c [
12].
The aim of this narrative review is to critically evaluate the role of AHCL systems in the management of T1DM during pregnancy. Specifically, it synthesises current evidence from clinical trials and observational studies, compares AHCL performance with conventional therapies, and assesses maternal, neonatal, and psychosocial outcomes. By doing so, this article intends to highlight both the potential benefits and limitations of AHCL technology, and to outline directions for future research.
2. Materials and Methods
This work is structured as a narrative review. A comprehensive literature search was conducted to identify studies evaluating AHCL systems and related diabetes technologies in women with T1DM during pregnancy. The search was performed in PubMed, Scopus, and Web of Science databases, supplemented by manual screening of reference lists in key articles and clinical guidelines. The time frame was set from January 2010 to June 2025 to cover the evolution of diabetes technology in the past decade and a half. Keywords included combinations of “type 1 diabetes,” “pregnancy,” “hybrid closed loop,” “automated insulin delivery,” “insulin pump,” and “continuous glucose monitoring.”
Eligible publications comprised randomised controlled trials, observational cohort studies, case series, qualitative investigations, and relevant consensus guidelines. Although no formal quality scoring system was applied—consistent with the narrative review approach—preference was given to studies with larger sample sizes, robust design, or significant clinical impact.
The evidence was synthesised thematically, focusing on maternal glycaemic control, perinatal outcomes, safety considerations, and psychosocial aspects. Key results from individual studies are summarised in comparative tables where appropriate to facilitate interpretation.
3. Insulin Pumps with Automated Insulin Delivery
Automated insulin delivery (AID) systems combine CGM, an insulin pump, and a predictive algorithm to mimic the physiological function of the pancreas. Their use is increasingly integrated into the routine management of T1DM in both adults and children [
13]. AID systems operate as closed-loop platforms and consist of three main components: [
1] a CGM device that continuously measures interstitial glucose levels, [
2] an insulin pump for subcutaneous insulin delivery, and [
3] a computerised control algorithm that adjusts basal insulin delivery in real time based on glucose trends [
8]. AHCL systems represent the most sophisticated form of AID technology currently available. In AHCL systems, basal insulin delivery is automated based on sensor glucose data, while users are still required to manually administer prandial boluses using an integrated bolus calculator. These systems have demonstrated the ability to safely improve glycaemic control, increase TIR, and reduce glycaemic variability in non-pregnant individuals with T1DM, including paediatric populations [
1,
8].
In addition to improving metabolic outcomes in the general T1DM population, AHCL systems have shown potential to optimise glycaemic control in women planning pregnancy. However, despite these promising results, the safety and efficacy of AHCL technology during pregnancy remain under investigation. Given the dynamic physiological changes in gestation and the stringent glycaemic targets required to minimise perinatal complications, further evidence is needed to confirm whether AHCL systems can be reliably and safely implemented throughout pregnancy [
1].
Key differences between systems are clinically relevant in pregnancy: CamAPS FX uses an adaptive Model Predictive Control (MPC) algorithm with lower set targets (4.4 mmol/L), aligning well with pregnancy goals. MiniMed 780G employs a Proportional–Integral–Derivative controller (PID) algorithm with target options of 5.5–6.7 mmol/L. Control-IQ uses a fixed band (6.2–8.9 mmol/L) but includes sleep and exercise modes for narrower ranges [
4,
9,
11]. Earlier systems, such as Medtronic 670G, with a fixed 6.7 mmol/L target, were unsuitable for pregnancy [
11].
5. Review of Studies on Insulin Pump Use During Pregnancy
Stewart et al. (2016) conducted a randomised crossover trial to evaluate the efficacy and safety of an AHCL system in pregnant women with T1DM. Sixteen participants completed two 4-week intervention phases: one with conventional CSII combined with CGM, and the other with an AHCL system that autonomously adjusted basal insulin delivery based on sensor glucose levels. Use of the closed-loop system significantly increased time in the pregnancy-specific glycaemic target range (3.5–7.8 mmol/L), particularly during the night (75% vs. 59%;
p < 0.001), without increasing the incidence of hypoglycaemia. Overall TIR improved from 61% with standard CSII to 69% with AHCL (
p = 0.002), accompanied by a reduction in time spent in hyperglycaemia. No episodes of severe hypoglycaemia or diabetic ketoacidosis were reported. Participants subjectively reported improved sleep quality and reduced anxiety during the closed-loop treatment phase. The study concluded that AHCL insulin delivery is safe and enhances glycaemic control during pregnancy, particularly in the overnight period, underscoring its potential role in the management of T1DM in pregnant women [
15].
The CONCEPTT trial (Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy), conducted by Feig et al. was a pivotal multicentre, international, randomised controlled trial investigating the impact of real-time CGM on glycaemic control and pregnancy outcomes in women with T1DM. The study enrolled 325 women, including 215 who were already pregnant at the time of randomization. Pregnant participants were assigned to either real-time CGM or conventional SMBG, while continuing intensive insulin therapy via MDI or insulin pumps. The primary endpoint was the change in HbA1c from baseline to 34 weeks’ gestation. Although the CGM group demonstrated only a modest reduction in HbA1c compared to the SMBG group (mean difference −0.2%, p = 0.02), CGM use resulted in a significantly greater percentage of TIR spent within the pregnancy-specific target glycaemic range (3.5–7.8 mmol/L), reduced time in hyperglycaemia, and no increase in hypoglycaemia.
Importantly, CGM was associated with improved neonatal outcomes. The incidence of LGA neonates was significantly lower in the CGM group compared to SMBG (53% vs. 69%,
p = 0.02), as were the rates of neonatal hypoglycaemia and neonatal NICU admissions exceeding 24 h (27% vs. 43%,
p = 0.01). The authors concluded that CGM use in pregnant women with T1DM improves neonatal outcomes and confers modest benefits in maternal glycaemic control, supporting its integration into routine antenatal diabetes management [
3].
A retrospective observational study assessing the impact of combining CSII with CGM on glycemic control and pregnancy outcomes in women with pregestational T1DM was conducted by Lason et al., (2021). The analysis included 81 singleton pregnancies from 109 women treated between 2016 and 2017, stratified into three groups: CSII + CGM, CSII alone, and MDI. Women using CSII with CGM achieved significantly better glycaemic control throughout pregnancy and postpartum compared to the other groups. In this group, mean HbA1c remained consistently low: 5.3% in the first and second trimesters, 5.2% in the third, and 5.5% postpartum. Despite superior metabolic control, no significant differences were found between groups in obstetric outcomes, including gestational age at delivery, rates of preterm birth, or neonatal birth weight. Macrosomia remained prevalent (~20%) even among women with optimal glycaemic control. The study concluded that CGM combined with CSII, particularly with advanced safety algorithms (AID), significantly improves maternal glycaemic outcomes. However, the persistent incidence of macrosomia suggests that factors beyond maternal glycaemia may contribute to foetal overgrowth in T1DM pregnancies [
16].
A retrospective observational study evaluating the effectiveness of SAP therapy in pregnant women with T1DM was conducted by Imafuku et al. (2023). The study compared maternal metabolic control and neonatal outcomes between women treated with SAP and those using MDI in combination with CGM. A total of 41 pregnant women with T1DM were included: 21 in the SAP group and 20 in the MDI + CGM group. Assessed outcomes included mean glucose levels, TIR (3.5–7.8 mmol/L), incidence of LGA neonates, neonatal birth weight, and frequency of maternal hypoglycaemia. The SAP group demonstrated superior glycaemic control, with significantly lower mean glucose levels and a higher proportion of TIR during the second and third trimesters. Moreover, SAP therapy was associated with a significantly lower incidence of LGA infants and fewer hypoglycaemic episodes compared to the MDI + CGM group. These findings support the clinical benefit of SAP therapy in optimising metabolic control and reducing the risk of adverse neonatal outcomes in pregnant women with T1DM [
2].
The AiDAPT study (Lee TTM et al., 2023) was a multicentre, randomised controlled trial designed to assess the efficacy and safety of AID systems in pregnant women with T1DM. A total of 124 women were enrolled early in pregnancy, at a mean gestational age of 11 weeks, and randomly assigned to receive either an AID system (CamAPS FX closed-loop technology) or conventional SAP therapy without automation. The primary outcome was the percentage of time spent within the pregnancy-specific glycaemic target range (3.5–7.8 mmol/L) from 16 to 36 weeks of gestation. Women using the AID system achieved significantly better glycaemic control, spending on average 14 percentage points more TIR compared to the SAP group. The AID group also exhibited lower mean glucose concentrations and reduced time in hyperglycaemia, with no significant increase in time spent in hypoglycaemia. Although the trial was not powered to detect differences in maternal or neonatal clinical outcomes, there were no significant differences between the groups in the incidence of serious adverse events. The findings demonstrate that AID systems are both effective and safe for use in pregnancy, offering substantial improvements in glycaemic control. The authors concluded that AID should be considered a preferred therapeutic option for pregnant women with T1DM to optimise maternal glucose levels and potentially improve pregnancy outcomes [
12,
17].
Rankin et al. (2023) examined healthcare professionals’ perspectives on implementing AHCL systems in pregnant women with T1DM. As AID technologies become more widely available, understanding the practical and systemic barriers to their integration into antenatal care is essential. The study involved semi-structured interviews with 29 clinicians—including diabetologists, obstetricians, diabetes specialist nurses, and midwives—across multiple NHS centres in the UK. Thematic analysis revealed overall support for AHCL use in pregnancy, citing benefits such as improved glycaemic control, reduced hypoglycaemia risk, and decreased psychological burden. However, several concerns were raised, including the complexity of the technology, increased workload for clinical staff, the need for specialised training, and disparities in access between centres. Participants highlighted the importance of multidisciplinary education, clear care pathways, and robust patient support systems. System-level factors, including funding, device availability, and workflow integration, were identified as critical to successful implementation. The authors concluded that while there is strong clinical enthusiasm for AHCL use in pregnancy, effective rollout requires coordinated planning, resource allocation, and efforts to ensure equitable access across healthcare settings [
18].
As part of a qualitative sub-study embedded in the AiDAPT randomised controlled trial, Lawton et al. (2023) explored the experiences of pregnant women with T1DM using AHCL systems. Semi-structured interviews were conducted with 26 women who used the CamAPS FX system during pregnancy. Participants reported a marked reduction in the mental and emotional burden of diabetes management. Automated insulin adjustments provided a sense of safety—particularly overnight—and contributed to improved sleep, greater confidence, and a feeling of normalcy. Women also noted enhanced glycaemic control and increased day-to-day flexibility. Despite these benefits, some challenges were identified, including device wearability issues, occasional technical difficulties, and the continued need for user input for meal bolusing. The study concluded that AHCL systems offer significant psychological and practical benefits for pregnant women with T1DM. The authors emphasised the importance of incorporating patient experiences into clinical care models and service planning to support equitable and patient-centred implementation of this technology in routine antenatal diabetes management [
5].
According to NICE (2023), AHCL therapy should be offered to adults with T1DM who have an HbA1c level of 7.5% (58 mmol/mol or higher) despite optimised use of CSII or CGM, or to those experiencing disabling hypoglycaemia. In contrast, children, adolescents, and women who are pregnant or planning pregnancy are eligible for HCL therapy regardless of their current HbA1c level. These recommendations are supported by clinical trial data and real-world evidence demonstrating improvements in glycaemic control, including reductions in HbA1c and TAR, as well as increased time in the target glucose range (3.5–7.8 mmol/L). Additionally, patients report reduced psychological distress and fear of hypoglycaemia when using these systems. HCL systems are classified by NICE as a technology class rather than by brand; however, commercially available systems such as the Medtronic MiniMed 780G, Tandem t: slim X2 with Control-IQ, and CamAPS FX fall within the scope of the recommendation [
12].
The CRISTAL study (Benhalima et al., 2024) was a multicentre, open label, randomised controlled trial evaluating the efficacy and safety of AHCL insulin therapy in pregnant women with T1DM. A total of 124 women (≥18 years, ≤13 + 6 weeks’ gestation, HbA1c ≤ 9%) were randomised 1:1 to receive either AHCL therapy with the MiniMed 780G system or standard insulin therapy (MDI or CSII, with or without CGM) across nine Belgian centres. Participants were followed until delivery. The primary endpoint—TIR (3.5–7.8 mmol/L) from 14 to 34 weeks’ gestation—was significantly higher in the AHCL group (68.4%) compared to standard care (55.6%), with an adjusted mean difference of 12.8 percentage points (95% CI: 7.8–17.8;
p < 0.0001). AHCL users also had lower mean glucose levels, reduced time in hyperglycaemia, and significantly lower HbA1c at 34 weeks (6.2% vs. 6.6%), with no increase in hypoglycaemia. Neonatal outcomes favoured the AHCL group, with lower rates of LGA infants (31% vs. 41%) and neonatal hypoglycaemia (33% vs. 50%), though differences were not statistically significant. No cases of severe hypoglycaemia or diabetic ketoacidosis were reported. The study concluded that AHCL therapy during pregnancy significantly improves glycaemic control without increasing adverse events, supporting its use as a preferred treatment strategy in pregnant women with T1DM [
1].
The CRISTAL study was the first randomised trial to evaluate off-label use of the MiniMed 780G system in automated mode during pregnancy. While no significant improvement in daytime TIR was observed, the AHCL system significantly increased overnight TIR and reduced the risk of hypoglycaemia, which was associated with improved glycaemic variability, reduced hypoglycaemia unawareness, and greater maternal satisfaction. Given that hypoglycaemia is a major barrier to tight glycaemic control during pregnancy, this finding is clinically important. The study also confirmed the safety of AHCL use during pregnancy. In comparison, the AiDAPT study demonstrated a significantly greater overall TIR (+10.5%) with the CamAPS FX system versus standard care. However, baseline characteristics differed: women in AiDAPT had higher initial HbA1c (7.7% vs. 6.5%), lower baseline TIR and TBR, and most control participants were on BBT, unlike CRISTAL, where 77.5% used insulin pumps in manual mode. Furthermore, the CamAPS system targeted a lower glucose threshold for automated delivery (4.4 mmol/L) compared to MiniMed 780G (5.5 mmol/L), which may explain the greater glycaemic improvements observed in AiDAPT [
1].
The CopenFast study (Nørgaard et al., 2023) was a single-centre, open-label, randomised controlled trial comparing faster-acting insulin aspart with conventional insulin aspart in pregnant women with pregestational diabetes (type 1 or type 2). A total of 208 women (133 with T1DM, 75 with T2DM) were enrolled before 14 weeks’ gestation and randomised 1:1 to receive either insulin as part of a basal-bolus regimen throughout pregnancy and 6 weeks postpartum. The primary outcome was 1 h postprandial glucose, which was significantly lower with faster aspart (mean difference −0.37 mmol/L;
p = 0.015). HbA1c, TIR, insulin requirements, and maternal weight gain were similar between groups. Neonatal outcomes, including rates of LGA infants (30% in both groups) and neonatal hypoglycaemia, did not differ significantly. These findings support its use as a safe and effective prandial insulin option during pregnancy in women with diabetes [
19].
A multicentre observational study (2023) examined the feasibility and safety of a pregnancy-specific zone-MPC AHCL system for women with T1DM during pregnancy. Conducted as a single-arm, at-home trial across several sites, the study evaluated the system’s performance outside of the hospital setting, focusing on its capacity to manage the complex glycaemic fluctuations characteristic of pregnancy. The results showed that the AHCL algorithm achieved high levels of TIR, with glucose maintained effectively within pregnancy-specific targets, while reducing exposure to both hyperglycaemia and hypoglycaemia. Importantly, the system was well tolerated by participants, with no major safety concerns reported. These findings support the practicality of at-home use of pregnancy-tailored AHCL technology and underscore its potential to enhance glycaemic control in T1DM pregnancies beyond conventional pump and sensor therapy [
20].
6. Additional RCTs Confirm Postpartum AHCL Safety
Recent evidence has increasingly underscored the clinical value of AHCL systems in the management of T1DM during pregnancy, labour, and the postpartum period. A secondary analysis of two randomised crossover trials demonstrated that the Cambridge AHCL system effectively maintained glucose levels within the target range during labour, delivery, and the immediate postpartum phase, despite rapid changes in insulin requirements. Importantly, this stability was achieved without an increased risk of hypoglycaemia, highlighting the robustness of AHCL in adapting to the unpredictable metabolic shifts surrounding childbirth [
21]. These findings align with results from a subsequent randomised clinical trial conducted within six months postpartum, where women allocated to CamAPS FX achieved significantly greater TIR (3.9–10.0 mmol/L) compared with standard insulin pump therapy combined with CGM. This improvement was accompanied by reductions in hyperglycaemia and no increase in hypoglycaemia risk, with efficacy sustained even under the added complexity of breastfeeding and hormonal variability [
10]. The consistency of these results is further supported by real-world data. A case series published in Diabetes Technology and Obesity Medicine described three women using AHCL during the intrapartum and immediate postpartum period, phases characterised by sudden shifts in insulin requirements. In all three cases, the closed-loop approach successfully stabilised glucose profiles and adapted rapidly to the marked decline in insulin needs following delivery, again without compromising safety [
22]. Taking together, these studies demonstrate that AHCL systems not only deliver superior glycaemic outcomes in controlled trial settings but also perform effectively in highly variable real-world contexts.
Synthesising this body of evidence, a 2025 systematic review and meta-analysis of randomised controlled trials confirmed that AHCL therapy consistently improves maternal glycaemic outcomes compared with standard therapy. Across multiple stages of pregnancy, women using AHCL achieved greater TIR and reduced hyperglycaemia, without a significant increase in hypoglycaemia, thereby reinforcing the robustness of this technology across diverse physiological states [
11]. The robustness observed clinically resonates with qualitative findings, which explored the psychosocial burden of diabetes in pregnancy. Women described persistent anxiety over glucose fluctuations and adverse outcomes but reported that closed-loop systems and digital support tools alleviated some of these pressures by reducing uncertainty and enabling greater confidence in self-management. At the same time, the study emphasised that technology alone cannot replace the need for sustained psychosocial support [
23]. This convergence of clinical trial data, real-world evidence, and qualitative perspectives is mirrored by further insights from the CRISTAL randomised controlled trial. A secondary analysis focusing on the intrapartum and early postpartum periods confirmed that AHCL provided more stable glycaemic profiles and enhanced safety compared with standard therapy, reducing the risk of sudden hyperglycaemic or hypoglycaemic events during physiologically vulnerable transitions [
24]. Collectively, these findings indicate that AHCL is adaptable across multiple peripartum phases, while also addressing broader patient-reported concerns around the psychological burden of diabetes care.
An emerging direction in diabetes technology research has explored the potential of artificial intelligence (AI) to personalise insulin therapy through reinforcement learning. A recent in silico study evaluated such an approach for people with diabetes on intensive insulin treatment. The investigators developed and tested a reinforcement learning–based algorithm capable of adapting insulin adjustments to individual patient profiles by continuously “learning” from glucose and insulin data. Using validated computer simulation models, the system was benchmarked against conventional strategies for insulin dosing. Results demonstrated that the reinforcement learning framework was able to achieve comparable or superior glycaemic outcomes, improving TIR and reducing variability, while maintaining safety with respect to hypoglycaemia risk. The in silico validation highlighted the potential of reinforcement learning to deliver fully personalised and dynamic insulin management, representing a significant step toward the integration of advanced AI in future AHCL systems [
25].
The clinical evidence regarding diabetes technologies in pregnancy is summarised in
Table 1. These trials consistently demonstrate that AHCL systems improve maternal glycaemic outcomes, particularly TIR, without increasing hypoglycaemia. Moreover, studies such as CONCEPTT, AiDAPT, and CRISTAL suggest a potential reduction in neonatal complications, including LGA infants, neonatal hypoglycaemia, and NICU admissions, although statistical significance varies across trials.
7. Conclusions
The management of T1DM in pregnancy remains a major clinical challenge, with women continuing to face elevated risks of maternal and perinatal complications despite advances in insulin analogues, glucose monitoring, and structured preconception care. Achieving stringent glycaemic targets is essential to reduce adverse outcomes, yet these targets are rarely met in real-world settings due to physiological changes in gestation, fluctuating insulin requirements, and the increased risk of hypoglycaemia.
Evidence from recent trials demonstrates that AHCL systems provide significant improvements in maternal glycaemic outcomes compared with standard therapy. Randomised studies, including AiDAPT and CRISTAL, confirm that AHCL systems increase TIR, lower mean glucose levels, and reduce exposure to hyperglycaemia without increasing the risk of severe hypoglycaemia or diabetic ketoacidosis. Findings from CONCEPTT highlight that better glycaemic control translates into improved neonatal outcomes such as lower rates of macrosomia, neonatal hypoglycaemia, and NICU admissions, although some of these benefits have not reached statistical significance across all trials.
In addition to metabolic effects, qualitative studies consistently report that women using AHCL systems experience substantial psychosocial benefits, including improved sleep, reduced anxiety about hypoglycaemia, and a greater sense of safety and normalcy during pregnancy. These patient-reported outcomes are of particular importance, as they directly impact adherence, quality of life, and maternal well-being. Clinician perspectives also emphasise enthusiasm for AHCL adoption, while noting barriers related to training, access, and health system capacity, which must be addressed to ensure equitable implementation.
Nevertheless, several limitations remain. Most available trials are relatively small and often conducted in highly specialised centres, which may limit generalisability. Some systems, such as MiniMed 780G, have been studied in pregnancy only in an off-label context, underscoring the need for further regulatory clarity. Long-term outcomes for mothers and offspring, particularly beyond delivery and postpartum, are not yet fully understood. Moreover, persistent rates of macrosomia even in women with excellent glycaemic control suggest that additional mechanisms beyond maternal glucose may contribute to adverse outcomes, warranting further investigation.
Looking ahead, larger multicentre trials and real-world implementation studies are essential to confirm the safety and efficacy of AHCL systems across diverse populations. Integration of AI and reinforcement learning holds promise for more personalised insulin delivery, potentially refining glycaemic targets specific to pregnancy. Broader adoption will also depend on addressing cost, accessibility, and training to ensure that these technologies are available to all women who may benefit.
In summary, AHCL systems represent one of the most promising developments in the management of T1DM in pregnancy. They offer the potential not only to improve maternal glycaemic outcomes but also to reduce perinatal complications and alleviate the psychosocial burden of diabetes management. With further evidence and equitable implementation, AHCL technologies could become the standard of care for pregnant women with T1DM.