Abstract
Background/Objectives: In the past 50 years, human reproductive capacity has steadily declined with elusive and idiopathic origins. Amongst theorized causes, oxidative stress has been proposed to directly contribute to male infertility. The glutathione (GSH) and glutathione disulfide (GSSG) molecular couple reflect cellular redox environments and are thus reflective of oxidative stress in most cells. Shifting GSH/GSSG redox states to abnormal, more oxidizing conditions can disrupt normal cellular activities. This study explores the correlation between the GSH/GSSG redox system and factors involved in male infertility, including sperm quality, specifically sperm motility and total count. Methods: Semen samples from 98 patients underwent high-performance liquid chromatography (HPLC) for GSH/GSSG analysis. A protein assay determined the protein concentration for normalization, and GSH/GSSG redox potentials (Eh) were calculated using the Nernst equation. Results: A significant inverse correlation between GSH/GSSG Eh and sperm count was identified (p = 0.0046 and R2 = 0.071). Analysis also found that cellular GSH concentrations (p < 0.001 and R2 = 0.11) and total GSH (GSH + (GSSG × 2); p = 0.0039 and R2 = 0.074) were significantly and positively correlated with total sperm count, whereas GSSG concentrations were not. The correlation between redox potential and motility was not significantly different (p = 0.11 and R2 = 0.02). Conclusions: This study shows that total sperm count decreases with increasing redox potential, indicating that more oxidized systems, such as the GSH/GSSG system, are associated with lower sperm counts in ejaculated sperm samples. These findings support a potential link between oxidative stress and sperm parameters. As understanding of the relationship between GSH/GSSG Eh and sperm quality improves, this may inform future potential therapies and approaches aimed at supporting male reproductive health.
1. Introduction
Oxidative stress is a physiological phenomenon that occurs when there is a dramatic increase in the amount of cellular oxidizing equivalents and a concomitant decrease in cellular reducing equivalents. While oxidative stress has many origins, a common mechanism by which it occurs is through the generation/overproduction of reactive oxygen species (ROSs), including oxygen free radicals [1,2]. Common ROSs include hydrogen peroxide, singlet oxygen, superoxide anions, and hydroxyl radicals [3]. Although ROSs produced from O2 are needed for signaling reactions within the body, when cellular radicals accumulate, toxicity and inflammation can occur [1]. ROS production and oxidative stress are known to increase with many environmental/chemical exposures, including pollutants, such as traffic exhaust emission, tobacco smoke, alcohol, or high levels of radiation [3,4]. Interestingly, multiple diseases, such as Alzheimer’s and Parkinson’s Disease, diabetes, and autoimmune disorders have also been linked to oxidative stress [5,6]. Under oxidative stress conditions, cellular function is often affected. As such, our study initiative was to determine whether sperm cell function and/or parameters correlate with alterations in cellular redox environments. Furthering our understanding of factors like oxidative stress and redox imbalance could elucidate potential therapies that could impact a man’s fertility.
The small biothiol glutathione (GSH) and its oxidized form, glutathione disulfide (GSSG), represents the predominant, most robust redox couple in most cells and, as such, is generally predictive of overall cellular redox environments. Glutathione is present within all cells of the body, as it regulates many cellular activities, including proliferation, differentiation, and apoptosis [2,3]. Central to GSH function is its cysteine residue, which can undergo reversible oxidation and is involved in reduction–oxidation (redox) reactions at the cellular level, allowing it to be an extremely powerful and useful antioxidant in the body [7]. GSH can be directly utilized in the detoxification of ROSs. For instance, hydrogen peroxide can be converted into water via the enzyme glutathione peroxidase (GPx), using two GSH molecules to yield a single GSSG [8]. Levels of GSSG are mitigated through the actions of glutathione disulfide reductase, using NADPH to convert GSSG back into two GSH molecules, allowing the cycle of ROS detoxification to continue.
Using the Nernst equation, GSH and GSSG concentrations can indicate the cellular redox status, where comparably high concentrations of GSH to lower GSSG levels would suggest more reducing cellular environments. When GSSG increases comparatively, cellular environments become more oxidizing [9]. Under normal conditions, the GSH:GSSG ratio can approach 100:1, but during periods of oxidative stress, that ratio may shift to 10:1 [8]. While the GSH/GSSG ratio is often reported, perhaps a more accurate measure of cellular redox status is the cell’s redox potential (Eh) [10].
With reproductive capacity declining over the past 50 years, and infertility in men becoming more common [11], we were interested to understand possible correlations between sperm GSH/GSSG Eh and various measures of sperm quality. Since oxidized redox states are correlated with poor somatic cellular function, we expect that shifts to more oxidizing GSH/GSSG Eh in sperm will correlate to lower sperm quality measures, with the converse being true with more reducing Eh. Understanding how redox states influence sperm quality may serve as a point of therapeutic intervention to improve reproductive outcomes. Thus, the focus of this project is to evaluate GSH/GSSG Eh in human sperm samples and correlate it with various measures of sperm quality. With greater knowledge of the correlation between redox potential and sperm function, a therapeutic target could be developed as an intervention for male infertility.
2. Materials and Methods
2.1. Sample Collection
Samples were collected from 98 patients at the Utah Fertility Center, located in Pleasant Grove, Utah, under a protocol evaluated through the Brigham Young University institutional review board (IRB). The ages of patients ranged from 22 to 52 years, with a mean age of 32 years. Fertility status, smoking status, and other health histories or demographics were not collected by study personnel to ensure patient privacy; however, some patients were likely living with infertility.
Samples were collected following clinical requirements for semen analysis, which included dry masturbation following three to five days of abstinence or intercourse using the Hygene seminal fluid collection kit or a sterile condom. Per World Health Organization 5 (WHO-5) guidelines [12], semen quality depends on factors that usually cannot be modified, such as sperm production by the testes and abstention time, permitting the use of either collection method if necessary to produce a sample for analysis. Prior to sample collection, the patient was given instructions on the proper collection of semen in accordance with the Utah Fertility Center. Following semen analysis, the remaining sample was de-identified and utilized for this study as opposed to being discarded.
Once the sample was collected, it was given 20 min to liquefy. If the sample did not liquefy fully in the initial 20 min, the timing was extended to up to 60 min. The viscosity, color, and pH were measured following the 20-minute waiting period. Motility assessment, morphology assessment, concentration, and count were performed for each sample manually by a trained observer following WHO-5 guidelines [12]. The researcher performing the semen analysis was aware of the study hypothesis but unaware of the redox status of the sperm samples. The sample was then centrifuged at 1800× g for 10 min. The pellet was resuspended in 325 μL of a perchloric acid solution (containing internal standards; see below) for GSH/GSSG analysis and then placed into 2 mL cryotubes for storage in a –20 °C freezer before being prepared for high-performance liquid chromatography (HPLC) quantification. The de-identified samples retained data on basic semen parameters including total count and motility.
2.2. HPLC Analysis of GSH and GSSG
Both GSH and GSSG can be quantified using established HPLC methods [13,14]. In brief, sperm samples were collected as described above and then prepared for HPLC via the derivatization of GSH and GSSG to S-carboxymethyl, N-dansyl derivatives. Using reverse-phase HPLC, samples were analyzed using an e2695 Separations Module (Waters) fitted with a Supelcosil LC-NH2 5 μm column (Sigma-Aldrich, Saint Louis, MO, USA), and the detection of peaks was determined using a 2474 FLR Detector (excitation 335 nm and emission 518 nm, Waters). A bicinchoninic acid (BCA; Genesee Scientific, El Cajon, CA, USA) protein assay was used to determine the protein concentration in each sample, which was then used to estimate cellular volumes. Using HPLC data (moles per sample) and protein assay data (to determine volumes), GSH and GSSG molarity was calculated. The GSH/GSSG Eh was calculated using the Nernst equation: , where E° is the standard electrode potential (−230 mV), R is the gas constant, T is the temperature in Kelvin, n is the number of electrons transferred (2), and F is the Faraday constant. The intracellular concentrations of GSH and GSSG obtained from the HPLC analysis were used in the equation [10]. The researcher performing HPLC analysis was blinded to the group allocations based on WHO-5 sperm parameter classifications.
2.3. Data Analysis
A total of 98 de-identified samples were compiled into .csv files with their associated GSH, GSSG, total GSH (GSH + 2(GSSG)), and GSH/GSSG Eh measurements, along with their sperm parameters and qualities, including morphology, total motile count, motility, total count, and other quantitative identifiers. R Processing Software (https://www.r-project.org) was utilized to analyze sperm total count and motility and their correlations with GSH/GSSG Eh via linear regression analyses and box and whisker plot t-tests. After determining the statistical significance between sperm measures and redox potential, we conducted additional linear regression models to determine which altered levels of protein, GSH, total GSH, or GSSG were impacting the correlational trends. Regression analyses were then conducted on age to determine its relationship to GSH, total GSH, and GSSG values. Using WHO-5 cutoff standards, we categorized the samples in the .csv file to have “normal” and “abnormal” sperm morphology (≥4% is normal), total count (>39 million sperm is normal), and motility (>40% motile is normal). Welch’s t-tests were then conducted to assess significance and visualized using box and whisker plots to examine any differences in redox potential, GSH, total GSH, or GSSG levels between sperm with normal or abnormal morphology, total count, or motility.
3. Results
3.1. Glutathione Redox State and Sperm Function
Extensive analyses with the intent of looking at the relationship between GSH/GSSG Eh and both sperm count and motility were conducted. Based on a linear regression model, a statistically significant inverse correlation between GSH/GSSG Eh and total sperm count was identified (p = 0.0046 and R2 = 0.071). As the ratio of oxidized sperm increased, with the GSH/GSSG ratio becoming more positive, total sperm count declined across the 98 samples (Figure 1). Analysis of the relationship between GSH/GSSG Eh and sperm motility failed to yield any significance (p = 0.11 and R2 = 0.02). Therefore, the only significant correlation between redox state and sperm function was between GSH/GSSG Eh and total sperm count (Figure 1).
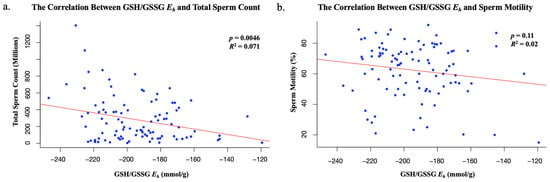
Figure 1.
(a) The statistically significant correlation between redox state and total sperm count; (b) the statistically insignificant correlation between redox state and sperm motility.
Protein quantification was then analyzed to determine whether individual GSH, total GSH, or GSSG levels impacted these sperm functions (Figure 2). GSH concentration was found to have a statistically significant positive correlation with total sperm count according to linear regression models (p < 0.001 and R2 = 0.11). As GSH levels increased in sperm samples, the total sperm count increased (Figure 2). When GSH was analyzed against sperm motility values, no statistically significant trends were found (p = 0.42 and R2 = −0.0036). Total GSH levels were also found to have a statistically significant positive correlation with total sperm count (p = 0.0039 and R2 = 0.074) with no additional correlation with sperm motility (p = 0.35 and R2 = −0.001). As total GSH levels increased, total sperm count increased (Figure 2). In contrast to GSH or total GSH, GSSG quantification had no direct impact on total sperm count (p = 0.90 and R2 = −0.01) or motility (p = 0.25 and R2 = 0.0035). The correlation between the age of donors and GSH was found to be insignificant (p = 0.99 and R2 = −0.010), as was the correlation between age and total GSH (p = 0.83 and R2 = −0.0099), and age and GSSG (p = 0.31 and R2 = 0.00027). Therefore, GSH and total GSH were the only statistically significant factors that contributed to total sperm count (Figure 2).
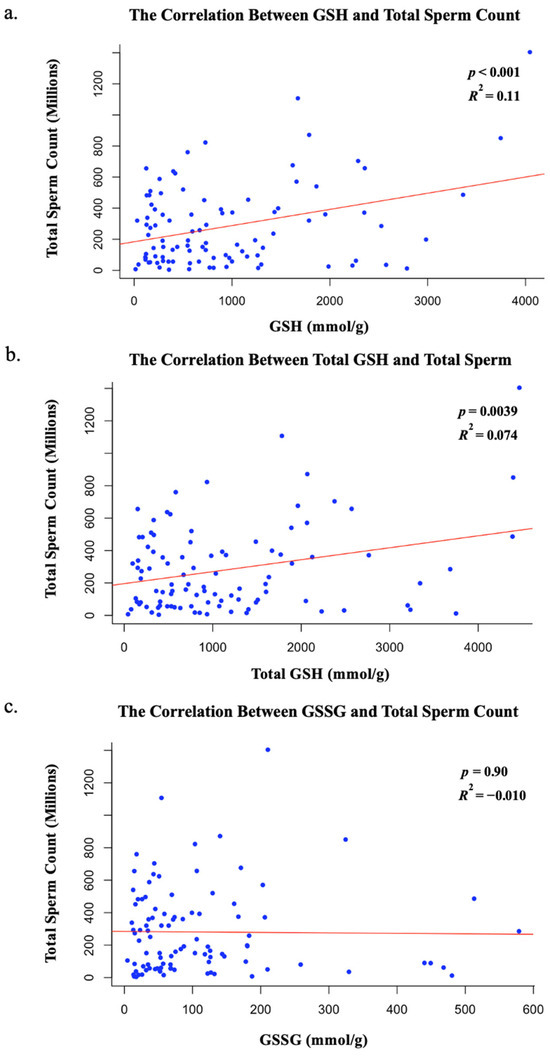
Figure 2.
(a) The statistically significant correlation between GSH and total sperm count; (b) the statistically significant correlation between total GSH and total sperm count; (c) the insignificant relationship between GSSG and total sperm count.
3.2. Redox State and Sperm Parameters
We next determined whether there was a statistically significant difference in GSH/GSSG Eh, GSH, total GSH, or GSSG for samples that were below (abnormal values) or above (normal values) the WHO-5 criteria for sperm morphology (size and shape), motility, and total count (Figure 3). No statistical significance was found between GSH/GSSG Eh and morphology, motility, or total count in terms of abnormal WHO-5 criteria. Additionally, no statistically significant correlation was found between GSH, total GSH, or GSSG, when compared to morphology, motility, or total count in regard to abnormal WHO-5 criteria (Table 1).
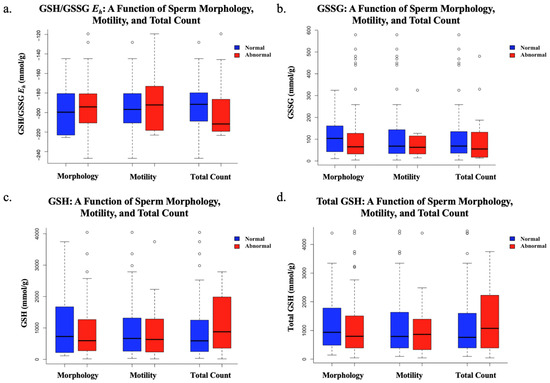
Figure 3.
The comparison of “normal” and “abnormal” sperm morphology (≥4% is normal), motility (>40% motile is normal), and total count (>39 million sperm is normal) based on WHO-5 cutoff standards using Welch’s t-tests, accounting for differences in sample size or variance. Sample sizes: normal morphology (n = 22), abnormal morphology (n = 76); normal motility (n = 85), abnormal motility (n = 13); normal total count (n = 83), abnormal total count (n = 15). (a) The insignificant findings regarding redox potential as a function of abnormal sperm morphology, motility, or total count; (b) the insignificant findings regarding GSSG as a function of abnormal sperm morphology, motility, or total count; (c) the insignificant findings regarding GSH as a function of abnormal sperm morphology, motility, or total count (d) the insignificant findings regarding total GSH as a function of abnormal sperm morphology, motility, or total count.

Table 1.
The statistical insignificance of glutathione protein quantification in sperm contributing to abnormal WHO-5 standard values of sperm morphology, motility, or total count.
4. Discussion
This study suggests that total sperm count decreases as redox potential increases. The inverse correlation between sperm total count and GSH/GSSG Eh in semen analyses indicates that more oxidized sperm, with more positive redox potentials, may contribute to male infertility. Despite this finding being statistically significant, this study suggests that GSH/GSSG Eh does not impact an individual’s total sperm motility or morphology. Additionally, while a statistically significant inverse correlation between GSH/GSSG Eh and total sperm count was identified (p = 0.0046), the relationship was relatively weak (R2 = 0.071), indicating that only a small portion of the variability in sperm count could be explained by changes in the redox state. This suggests that other biological or environmental factors most likely play a more prominent role in determining sperm count, and that GSH/GSSG Eh may not be a strong predictor in this context. This variation should be considered when interpreting these results, as using GSH/GSSG Eh is not the only predictor of redox potential, and past studies have found sperm parameters such as motility and viability to be highly sensitive to ROSs [15,16]. Future studies could explore additional indicators of redox status, including direct quantification of reactive oxygen species (ROSs). The evaluation of additional redox couples, such as cysteine/cystine (Cys/CySS), may also help to further elucidate the relationship between redox homeostasis and sperm function. Blinded research with larger sample sizes and a fertile (normal) control group is essential to confirm and strengthen this correlation. If validated, this correlation would provide a potential therapeutic target for intervention amongst patients who suffer from asthenozoospermia, as one could receive glutathione treatment to increase the levels of GSH, therefore shifting the glutathione redox couple to a more negative and reducing state. Studies have shown that glutathione has a protective effect on ROS-mediated DNA damage, and the protein can have a reversible effect on H2O2-induced cytotoxicity and ROS generation (Figure 4) [17]. This poses the idea that glutathione treatments could potentially reverse a decline in sperm count in men over the course of time, thus increasing one’s ability to conceive a viable embryo.
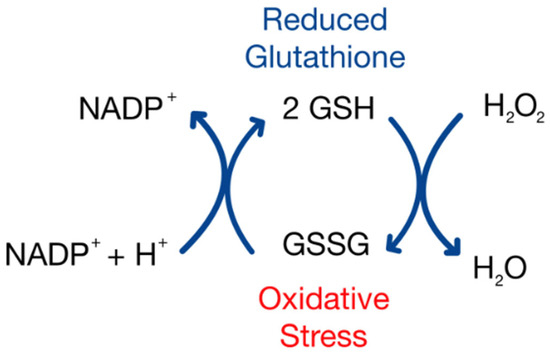
Figure 4.
A model of the glutathione oxidation reaction as a correlate to oxidative stress.
The average total sperm count has been found to have declined to around 62.3% in the past 45 years, with many possible causes contributing to this decline [11]. Sperm counts since 2000 have decreased at a rate −2.64%/year compared to the rates in 1972 of −1.16%/year. Our study elucidates the idea that the redox state of sperm could be a contributor to this outcome. With a statistically significant correlation being made between redox state and total sperm count but not sperm motility, our results suggest that redox state is more closely associated with spermatogenesis than sperm function. Using plasma redox states as a marker of systemic redox states, studies have identified plasma redox couple shifts that occur during aging (ages 19–85), of which GSH/GSSG Eh was a couple that was evaluated [13]. The CyS/CySS redox couple is the primary redox couple in plasma, mostly being derived as a breakdown product from GSH/GSSG secreted from cells. Interestingly, CyS/CySS Eh almost immediately becomes increasingly oxidized at a linear rate of nearly +0.16 mV/year throughout life. Conversely, the GSH/GSSG Eh showed a completely different pattern, where redox states were relatively stable until age 45, after which it becomes more rapidly oxidized at a rate of +0.7 mV/year. Because these two redox couples are related but appear independently regulated, it suggests that the individual couples may have specified function and control of cellular activities. Additional studies have shown that total sperm counts become significantly lower starting at ages between 41 and 50 [18], overlaying on periods where shifts in GSH/GSSG Eh begin to become increasingly oxidized in humans. These results are highly suggestive of a GSH/GSSG centric, homeostatic redox mechanism to support normal spermatogenesis.
Intriguingly, our data show that GSH and total GSH levels, not GSSG levels, correlate with total sperm count. Thus, these data suggest that a decline in GSH accumulation or absence of reduced glutathione within sperm cells, and not an increase in GSSG accumulation, may be associated with decreased sperm counts in ejaculated sperm samples. However, because our measurements were performed on ejaculated sperm, the redox status primarily reflects the extracellular environment of seminal plasma, not the intratesticular environment during spermatogenesis. While reduced antioxidants like GSH significantly contribute to ROS detoxification, we cannot conclude from our data that decreased GSH levels directly impair spermatogenesis. Nevertheless, Sertoli cells actively synthesize GSH, and adequate antioxidant protection from GSH may be required within the seminiferous epithelium for proper sperm development and maturation [19]. Future studies evaluating redox balance within testicular tissue itself are needed to clarify this relationship. Thus, while our findings reveal a correlation between seminal plasma GSH levels and sperm count, further mechanistic research is required to determine whether these associations extend to redox regulation during spermatogenesis. Alternatively, lowered levels of GSH and a more oxidizing redox state in the seminal environment may be reflective of broader oxidative stress that could contribute to impaired spermatogenesis, potentially resulting in decreased total sperm count, as observed in our cohort.
With an increase in the rate of total sperm count decline, it is difficult to determine a single factor that is responsible, but there are likely many environmental influences that are contributory, like pollution, alcohol consumption, or medications [20], many of which may promote oxidative stress and alterations to lower GSH levels. Numerous studies have shown that specific environmental/chemical influences that promote oxidative stress and injury are positively correlated with poor sperm quality and a decrease in positive reproductive outcomes. For example, cigarettes contain several chemicals that promote free radical production and oxidative injury, promoting changes to cellular redox states, including reproductive toxicants such as cadmium and oxidants such as various peroxides. Generally speaking, sperm concentrations in smokers are lower than in non-smokers [21]. In terms of smoking and infertility, human studies showed that infertile, non-smoking men had sperm concentrations that were decreased by nearly 60% (25.5 × 106/mL vs. 62.8 × 106/mL in controls), but in infertile, smoking men, sperm concentrations dropped even more, by 73% (17.0 × 106/mL). Interestingly, similar trends were noted when comparing spermatozoa GSH levels, with infertile, non-smoking samples showing significantly higher levels (35.4 pmol/106 spermatozoa) than infertile, smoking samples (31.4 pmol/106 spermatozoa). Additionally, markers of oxidative-stress-induced damage, such as malondialdehyde (MDA) and protein carbonyls, measures of lipid peroxidation and protein oxidation, respectively, and ROS itself, were all significantly higher in the spermatozoa from infertile, smoking men than those from infertile, non-smoking men [22].
Other studies on infertile human males also show similar trends, where ROS levels in smokers are significantly higher in smokers vs. non-smokers. However, when infertile non-smokers are compared to fertile men (normal donors), ROS levels are still significantly greater in infertile non-smokers [21], suggesting that smoking is not the sole rationale for ROS differences, but rather the observed ROS increase is connected to infertility in another way. Interestingly, in this same study, data show that infertile smokers have a decreased sperm concentration by approximately 40% from normal donors, but infertile non-smokers have an even greater decrease, a 57% decrease in sperm concentrations [21]. These data further support the possibility that sperm counts are, at least in part, correlated to changes in ROS availability and potential oxidation. DNA damage was higher in infertile smokers (p = 0.04) but not higher in infertile non-smokers (p = 0.06), even though comparisons between groups show that the amount of damage was essentially the same [21]. Glutathione addition to collected sperm improved a variety of parameters related to sperm quality, especially related to kinematic measures [23]. ATP levels were increased with supplementation, while ROS availability was significantly decreased. In vivo GSH supplementation (600 mg/day IM) for 60 days in infertile men also led to similar improvements on kinematic measurements, significantly increasing total motility, linear motility, and velocity [24]. This approach did not affect the total sperm concentration, however.
While not performed specifically in humans, mice with Wilson’s-disease-associated oligospermia that received GSH supplementation showed a significant increase in sperm counts. GSH as a supplement has already shown to improve the sperm count in mice while reducing ROS availability and diminishing MDA levels and occur concomitantly with corrections in mitochondrial health and a reduction in testicular apoptosis [25]. While these studies highlight the direct support for GSH in positive male reproductive outcomes, they do not directly address the role of redox states’ contributions to male infertility.
Our primary finding related GSH/GSSG Eh to changes in sperm concentrations in human subjects. Glutathione redox states have been highly studied in regard to cellular proliferation. Early work showed that fibroblasts, both normal, wild-type fibroblasts, which display contact inhibition, and fibrosarcoma cells, which do not exhibit contact inhibition, exhibited very reducing GSH/GSSG Eh at low confluency, where proliferation rates were relatively high. As confluency increased, normal fibroblast proliferation decreased, which coincided with oxidation in GSH redox states, but fibrosarcoma cells, which continued constant growth, did not show GSH redox state oxidation but rather remained unchanged, suggesting that proliferating cells prefer a reducing intracellular redox environment. Using wild-type fibroblasts, GSH/GSSG Eh was pharmacologically oxidized (+24 mV), and cells demonstrated a decrease in cellular proliferation by 54% compared to untreated cells [26]. Inversely, pharmacologically reducing GSH/GSSG Eh (−10 mV) in fibroblasts resulted in increased proliferation (26% increase from control). With more reducing systems, cells appear to proliferate at a much higher rate. While spermatozoa are not mitotic, precursors are actively dividing and proliferating. Lowered GSH and more oxidizing GSH/GSSG Eh at this level may cause a low sperm yield, reflected in lower sperm counts in the semen.
Our study shows that a more reduced redox state correlates highly with higher sperm counts. More oxidized GSH/GSSG Eh is associated with much lower counts, but, interestingly, shifts to more oxidizing GSH/GSSG Eh are not driven by increases in GSSG levels, which did not show a significant correlation, but rather strongly correlated to decreased GSH concentrations. As such, a decrease in total GSH in sperm, also correlated with lowered sperm counts, is reflective of a loss of the reduced form of glutathione, GSH. Direct supplemental GSH or other pharmacological means to increase GSH levels to increase sperm counts could be a potential approach to increase fertility. Clearly, further work is required to better understand meaningful therapies regarding GSH/GSSG Eh regulatory control of sperm concentrations in men, but these findings do support a feasible and potential point of intervention.
In conclusion, we believe our study provides data acknowledging that the GSH/GSSG Eh shift to a more positive, oxidizing redox potential in sperm drives lower sperm counts in men. Alternatively, more reduced GSH/GSSG Eh promotes increased sperm counts in men and is indicative of less oxidative stress and a healthier cellular environment. Thus, we infer that preconception lifestyle, including factors such as smoking, diet, and environmental exposures, can all contribute to cellular GSH/GSSG Eh, and ultimately sperm count or production. A greater understanding of both the culminating reasons behind the depletion of GSH levels in sperm, as well as more data pertaining to sperm production under oxidative stress, is needed to develop redox-based strategies to improve infertility.
Author Contributions
C.G.P. carried out the methodology of the study, compilation of data, and transportation of samples, performed all bioinformatic analyses of data, and wrote the manuscript. K.H. helped carry out benchwork and assays, and helped draft the manuscript. A.B. (Ammon Bayles) facilitated sample collection and coordination with Brigham Young University to facilitate data collection. A.B. (Adriana Burger) collected and performed semen analyses at Utah Fertility Center, then stored samples for the study. J.H. participated in the study’s design and coordination concerning redox information and methodology. T.J. conceived the study, participated in its design and coordination, and helped draft the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
A protocol was submitted to the Brigham Young University Institutional Review Board, and the committee determined that this work was exempt. Ethical review and approval were waived for this study due to the analyses of de-identified sperm parameters having no direct impact on patients (see below).
Informed Consent Statement
Patient consent was waived as this study involved only de-identified samples, posing minimal risk to participants. The study personnel did not have access to identifiable patient information, ensuring privacy and confidentiality. Additionally, the samples used for analysis were those that would have otherwise been discarded following routine clinical semen analysis. The study was conducted under an exempt protocol reviewed by the Brigham Young University Institutional Review Board (IRB), which determined that the use of de-identified, leftover clinical specimens met criteria for exemption from informed consent in accordance with ethical guidelines for human research.
Data Availability Statement
Redox data are available upon request to the corresponding author Tim Jenkins (tim_jenkins@byu.edu).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jakubczyk, K.; Dec, K.; Kaldunska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Pizzorno, J. Glutathione! Integr. Med. A Clin. J. 2014, 13, 8–12. [Google Scholar]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Bitto, A.; Pallio, G.; Mannino, F.; Arcoraci, V.; Aliquo, F.; Minutoli, L.; De Ponte, C.; D’Andrea, P.; et al. Cadmium-Induced Oxidative Stress Impairs Glycemic Control in Adolescents. Oxidative Med. Cell. Longev. 2017, 2017, 6341671. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Bayram, H.; Donmez Cakil, Y.; Sitar, M.E.; Demirel, G.; Selam, B.; Cincik, M. The Effects of Glutathione on Clinically Essential Fertility Parameters in a Bleomycin Etoposide Cisplatin Chemotherapy Model. Life 2023, 13, 815. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Chatterjee, A. Reduced glutathione: A radioprotector or a modulator of DNA-repair activity? Nutrients 2013, 5, 525–542. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Levine, H.; Jorgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Jones, D.P.; Carlson, J.L.; Mody, V.C.; Cai, J.; Lynn, M.J.; Sternberg, P. Redox state of glutathione in human plasma. Free Radic. Biol. Med. 2000, 28, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzym. 2002, 348, 93–112. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Nallella, K.P.; Thomas, A.J., Jr.; Alvarez, J.G.; Sikka, S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006, 86, 878–885. [Google Scholar] [CrossRef]
- Shi, T.Y.; Chen, G.; Huang, X.; Yuan, Y.; Wu, X.; Wu, B.; Li, Z.; Shun, F.; Chen, H.; Shi, H. Effects of reactive oxygen species from activated leucocytes on human sperm motility, viability and morphology. Andrologia 2012, 44 (Suppl. S1), 696–703. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Lee, H.; Hong, S.H.; Park, C.; Park, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Hwang, H.J.; et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef]
- Pino, V.; Sanz, A.; Valdes, N.; Crosby, J.; Mackenna, A. The effects of aging on semen parameters and sperm DNA fragmentation. JBRA Assist. Reprod. 2020, 24, 82–86. [Google Scholar] [CrossRef]
- Den Boer, P.J.; Mackenbach, P.; Grootegoed, J.A. Glutathione metabolism in cultured Sertoli cells and spermatogenic cells from hamsters. J. Reprod. Fertil. 1989, 87, 391–400. [Google Scholar] [CrossRef]
- Sciorio, R.; Tramontano, L.; Adel, M.; Fleming, S. Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. J. Pers. Med. 2024, 14, 198. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J., Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study. Fertil. Steril. 2002, 78, 491–499. [Google Scholar] [CrossRef]
- Kiziler, A.R.; Aydemir, B.; Onaran, I.; Alici, B.; Ozkara, H.; Gulyasar, T.; Akyolcu, M.C. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol. Trace Elem. Res. 2007, 120, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Brahmajosyula, M.; Morimoto, Y. Exogenous GSH Supplementation to Raw Semen Alters Sperm Kinematic Parameters in Infertile Patients. Reprod. Sci. 2023, 30, 2853–2865. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Lombardo, F.; Gandini, L.; Culasso, F.; Dondero, F. Glutathione therapy for male infertility. Arch. Androl. 1992, 29, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, L.; Liu, Q.; Tan, F.; Wang, L.; Zhao, D.; Fang, X.; Liu, X.; Liu, J.; Han, H. Glutathione improves testicular spermatogenesis through inhibiting oxidative stress, mitochondrial damage, and apoptosis induced by copper deposition in mice with Wilson disease. Biomed. Pharmacother. 2023, 158, 114107. [Google Scholar] [CrossRef]
- Hutter, D.E.; Till, B.G.; Greene, J.J. Redox state changes in density-dependent regulation of proliferation. Exp. Cell Res. 1997, 232, 435–438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).