Prevalence of Pathogenic Microbes within the Endometrium in Normal Weight vs. Obese Women with Infertility
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, G.K.; DiBari, J.N. Marked Disparities in Pre-Pregnancy Obesity and Overweight Prevalence among US Women by Race/Ethnicity, Nativity/Immigrant Status, and Sociodemographic Characteristics, 2012–2014. J. Obes. 2019, 2019, 2419263. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Travieso, J.L.; Carson, K.R.; Moley, K.H. Obesity and reproductive function. Obstet. Gynecol. Clin. N. Am. 2012, 39, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Talmor, A.; Dunphy, B. Female obesity and infertility. Best. Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 498–506. [Google Scholar] [CrossRef] [PubMed]
- García-Ferreyra, J.; Carpio, J.; Zambrano, M.; Valdivieso-Mejía, P.; Valdivieso-Rivera, P. Overweight and obesity significantly reduce pregnancy, implantation, and live birth rates in women undergoing in Vitro Fertilization procedures. JBRA Assist. Reprod. 2021, 25, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Wei, Y.S.; Niu, J.M.; Li, Y.; Miao, Z.L.; Wang, Z.N. Risk factors for unexplained recurrent spontaneous abortion in a population from southern China. Int. J. Gynaecol. Obstet. 2010, 108, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Lashen, H.; Fear, K.; Sturdee, D.W. Obesity is associated with increased risk of first trimester and recurrent miscarriage: Matched case-control study. Hum. Reprod. 2004, 19, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Park, S.K.; Shin, A.; Lee, S.A.; Choi, J.Y.; Hong, Y.C.; Yoo, K.Y.; Lee, J.K.; Kang, D. Body mass index at age 18–20 and later risk of spontaneous abortion in the Health Examinees Study (HEXA). BMC Pregnancy Childbirth 2015, 15, 228. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.; Ong, K.J.; Ledger, W.L.; Li, T.C. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil. Steril. 2008, 90, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Axelsson, D.; Brynhildsen, J.; Blomberg, M. Postpartum infection in relation to maternal characteristics, obstetric interventions and complications. J. Perinat. Med. 2018, 46, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Garcia-Grau, I.; Bau, D.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Vilella, F.; Romero, R.; Simón, C. The first glimpse of the endometrial microbiota in early pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 296–305. [Google Scholar] [CrossRef]
- Diaz-Martínez, M.D.C.; Bernabeu, A.; Lledó, B.; Carratalá-Munuera, C.; Quesada, J.A.; Lozano, F.M.; Ruiz, V.; Morales, R.; Llácer, J.; Ten, J.; et al. Impact of the Vaginal and Endometrial Microbiome Pattern on Assisted Reproduction Outcomes. J. Clin. Med. 2021, 10, 4063. [Google Scholar] [CrossRef]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Nagayoshi, M.; Sakuraba, Y.; Yamasaki, F.; Hata, K.; et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediators Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef]
- Moore, D.E.; Soules, M.R.; Klein, N.A.; Fujimoto, V.Y.; Agnew, K.J.; Eschenbach, D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil. Steril. 2000, 74, 1118–1124. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Kushnir, V.A.; Solouki, S.; Sarig-Meth, T.; Vega, M.G.; Albertini, D.F.; Darmon, S.K.; Deligdisch, L.; Barad, D.H.; Gleicher, N. Systemic Inflammation and Autoimmunity in Women with Chronic Endometritis. Am. J. Reprod. Immunol. 2016, 75, 672–677. [Google Scholar] [CrossRef]
- Liu, Y.; Ko, E.Y.; Wong, K.K.; Chen, X.; Cheung, W.C.; Law, T.S.; Chung, J.P.; Tsui, S.K.; Li, T.C.; Chim, S.S. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil. Steril. 2019, 112, 707–717.e1. [Google Scholar] [CrossRef]
- Allen, N.G.; Edupuganti, L.; Edwards, D.J.; Jimenez, N.R.; Buck, G.A.; Jefferson, K.K.; Strauss, J.F., 3rd; Wickham, E.P., 3rd; Fettweis, J.M. The vaginal microbiome in women of reproductive age with healthy weight versus overweight/obesity. Obesity (Silver Spring) 2022, 30, 142–152. [Google Scholar] [CrossRef]
- Hawkins, G.M.; Burkett, W.C.; McCoy, A.N.; Nichols, H.B.; Olshan, A.F.; Broaddus, R.; Merker, J.D.; Weissman, B.; Brewster, W.R.; Roach, J.; et al. Differences in the microbial profiles of early stage endometrial cancers between Black and White women. Gynecol. Oncol. 2022, 165, 248–256. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Olzomer, E.M.; Kosasih, M.; Martin, A.R.; Fargah, F.; Lambie, N.; Susic, D.; Hoehn, K.L.; Farrell, R.; Byrne, F.L. Differences in the Active Endometrial Microbiota across Body Weight and Cancer in Humans and Mice. Cancers 2022, 14, 2141. [Google Scholar] [CrossRef]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Naqvi, A.; MacKintosh, M.L.; Derbyshire, A.E.; Tsakiroglou, A.M.; Walker, T.D.J.; McVey, R.J.; Bolton, J.; Fergie, M.; Bagley, S.; Ashton, G.; et al. The impact of obesity and bariatric surgery on the immune microenvironment of the endometrium. Int. J. Obes. 2022, 46, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Cela, V.; Daniele, S.; Obino, M.E.R.; Ruggiero, M.; Zappelli, E.; Ceccarelli, L.; Papini, F.; Marzi, I.; Scarfò, G.; Tosi, F.; et al. Endometrial Dysbiosis Is Related to Inflammatory Factors in Women with Repeated Implantation Failure: A Pilot Study. J. Clin. Med. 2022, 11, 2481. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Vitagliano, A.; Laganà, A.S.; De Ziegler, D.; Cicinelli, R.; Santarsiero, C.M.; Buzzaccarini, G.; Chiantera, V.; Cicinelli, E.; Marinaccio, M. Chronic Endometritis in Infertile Women: Impact of Untreated Disease, Plasma Cell Count and Antibiotic Therapy on IVF Outcome—A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2250. [Google Scholar] [CrossRef] [PubMed]
- Pirtea, P.; Cicinelli, E.; De Nola, R.; de Ziegler, D.; Ayoubi, J.M. Endometrial causes of recurrent pregnancy losses: Endometriosis, adenomyosis, and chronic endometritis. Fertil. Steril. 2021, 115, 546–560. [Google Scholar] [CrossRef] [PubMed]
| Demographics | BMI < 25 (n = 53) | BMI 25–29 (n = 32) | BMI ≥ 30 (n = 47) | p Value |
|---|---|---|---|---|
| Age; years, Avg (Stdv) | 35.35 (4.21) | 36.53 (4.66) | 36.46 (5.05) | 0.39 |
| Age range; No (%) | ||||
| <35 years | 22 (41.5%) | 10 (33.3%) | 21 (42.9%) | |
| 35–37 years | 13 (24.5%) | 4 (13.3%) | 8 (16.3%) | |
| 38–39 years | 12 (22.6%) | 7 (23.3%) | 8 (16.3%) | |
| 40+ years | 6 (11.3%) | 9 (30.0%) | 12 (24.5%) | |
| Ethnicity; No (%) | ||||
| Caucasian/white | 48 (90.6%) | 27 (84.4%) | 39 (83.0%) | |
| African American/black | 0 | 2 (6.3%) | 1 (2.1%) | |
| Asian | 7 (13.2%) | 0 | 1 (2.1%) | |
| Hispanic | 0 | 0 | 3 (6.4%) | |
| Other/not specified | 3 (5.7%) | 1 (3.1%) | 1 (2.1%) | |
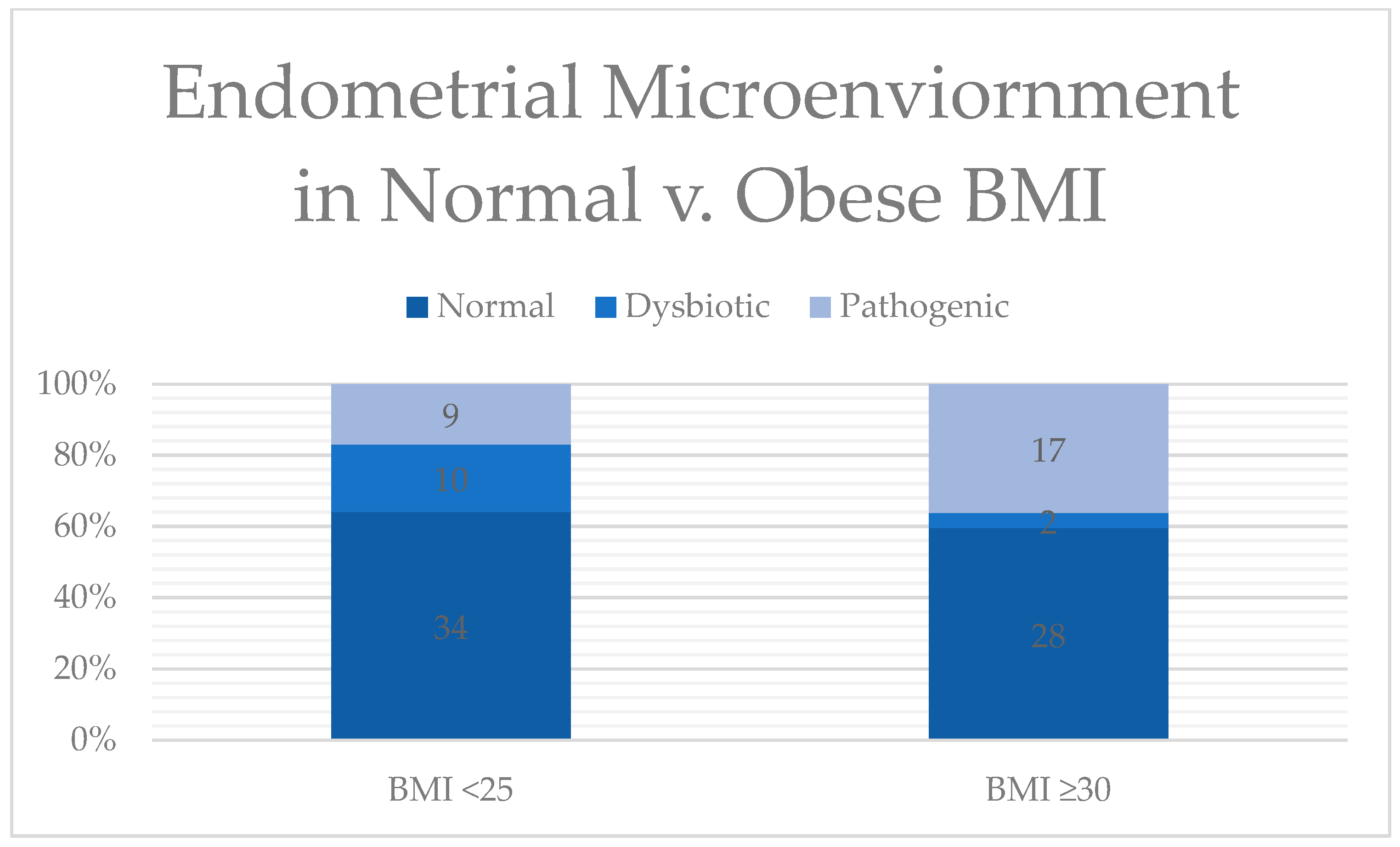
| Microbial Composition; No (%) | 0.08 | |||
| Normal | 34 (64.2%) | 17 (56.7%) | 28 (59.5%) | |
| Dysbiotic | 10 (18.9%) | 4 (13.3%) | 2 (4.1%) | |
| Pathogenic | 9 (16.9%) | 10 (33.3%) | 17 (35.4%) | |
| Infertility etiology; No. (%) | 0.28 | |||
| Number of patients with a history of failed implantation with IVF; No. (%) | 30 (56.6%) | 14 (34.4%) | 29 (66.0%) | |
| Number of patients with a history of RPL 1; No. (%) | 23 (43.4%) | 18 (65.6%) | 18 (34.0%) | |
| Mean number of failed ET cycles, Avg (Stdv) | 1.8 (1.37) | 1.54 (0.88) | 1.44 (0.75) | 0.46 |
| Lactobacillus spp. | RR 1 | Pathogens | RR 1 | Pathogens, Cont. | RR 1 |
|---|---|---|---|---|---|
| Normal vs. Dysbiotic | Pathogenic | Pathogenic | |||
| Lactobacillus crispatus | ≥3.71 | Actinomyces israelii | Absent | Prevotella disiens | ≤3.57 |
| Lactobacillus gasseri | ≥3.60 | Atopobium vaginae | ≤3.57 | Chlamydia trachomatis | Absent |
| Lactobacillus iners | >3.57 | Bacteriodes fragilis | ≤3.57 | Enterococcus faecalis | ≤3.63 |
| Lactobacillus jensenii | ≥3.70 | Bifidobacterium spp. | ≤4.22 | Escherichia coli | ≤3.58 |
| Clostridium sordellii | Absent | Klebsiella pneumoniae | ≤3.57 | ||
| Fusobacterium nucleatum | Absent | Mycoplasma genitalium | ≤3.57 | ||
| Gardnerella vaginalis | ≤3.72 | Mycoplasma hominis | ≤3.57 | ||
| Haemophilus ducreyi | Absent | Neisseria gonorrhoeae | Absent | ||
| Mycobacterium tuberculosis | Absent | Staphylococcus aureus | ≤3.57 | ||
| Mobiluncus spp. | ≤3.57 | Streptococcus viridans | ≤3.57 | ||
| Peptostreptococcus anaerobius | ≤3.57 | Ureaplasma urealyticum | ≤3.58 | ||
| Porphyromonas asaccharolytica | ≤3.57 | Sneathia spp. | ≤3.57 | ||
| Prevotella bivia | ≤3.57 | Treponema pallidum | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, S.; Osei, F.; Marsh, C. Prevalence of Pathogenic Microbes within the Endometrium in Normal Weight vs. Obese Women with Infertility. Reprod. Med. 2024, 5, 90-96. https://doi.org/10.3390/reprodmed5020010
King S, Osei F, Marsh C. Prevalence of Pathogenic Microbes within the Endometrium in Normal Weight vs. Obese Women with Infertility. Reproductive Medicine. 2024; 5(2):90-96. https://doi.org/10.3390/reprodmed5020010
Chicago/Turabian StyleKing, Sarah, Florence Osei, and Courtney Marsh. 2024. "Prevalence of Pathogenic Microbes within the Endometrium in Normal Weight vs. Obese Women with Infertility" Reproductive Medicine 5, no. 2: 90-96. https://doi.org/10.3390/reprodmed5020010
APA StyleKing, S., Osei, F., & Marsh, C. (2024). Prevalence of Pathogenic Microbes within the Endometrium in Normal Weight vs. Obese Women with Infertility. Reproductive Medicine, 5(2), 90-96. https://doi.org/10.3390/reprodmed5020010






