A New Bioreactor to Promote Human Follicular Growth with or without Activin A in Transgender Men
Abstract
1. Introduction
2. Methods
2.1. Ovarian Tissue Collection
2.2. Bioreactor Preparation
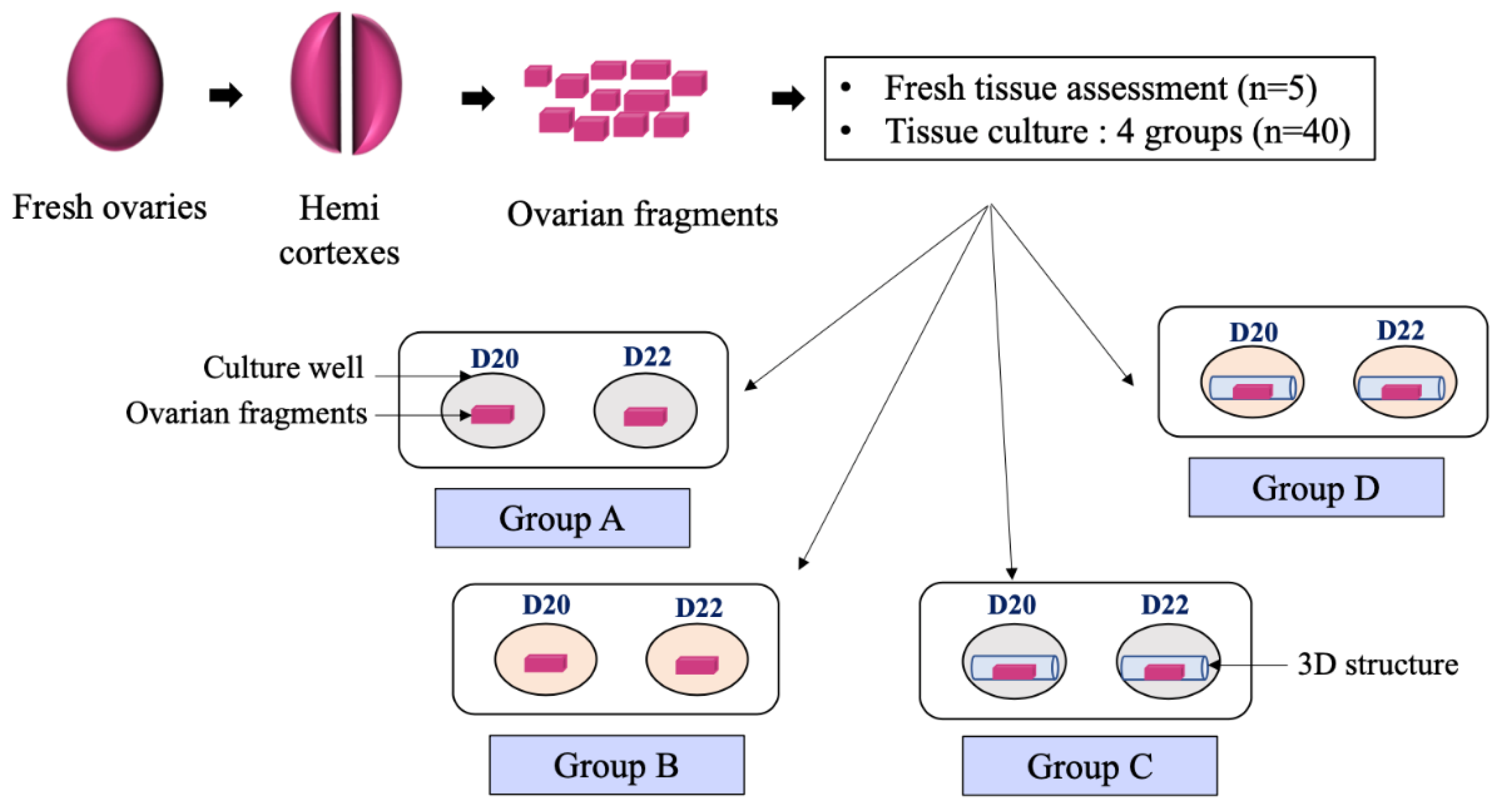
2.3. Tissue Preparation and Ovarian Fragments Culture
- -
- Group A (3D−/A−): Ovarian fragment placed in culture medium without activin A without 3D-structure
- -
- Group B (3D−/A+): Ovarian fragment placed in culture medium with activin A but without 3D-structure
- -
- Group C (3D+/A−): Ovarian fragment placed in culture medium without activin A but within a 3D-structure
- -
- Group D (3D+/A+): Ovarian fragment placed in culture medium with activin A within 3D-structure.
2.4. Histological Evaluation
2.5. Follicular Density
2.6. Follicular Growth
2.7. Follicular Quality and Morphology
2.8. Detection of Ki67 and Cleaved Caspase 3 Immunoreactivity in Cultured Follicles
2.9. Detection of Estradiol in Culture Medium
2.10. Statistical Analyses
3. Results
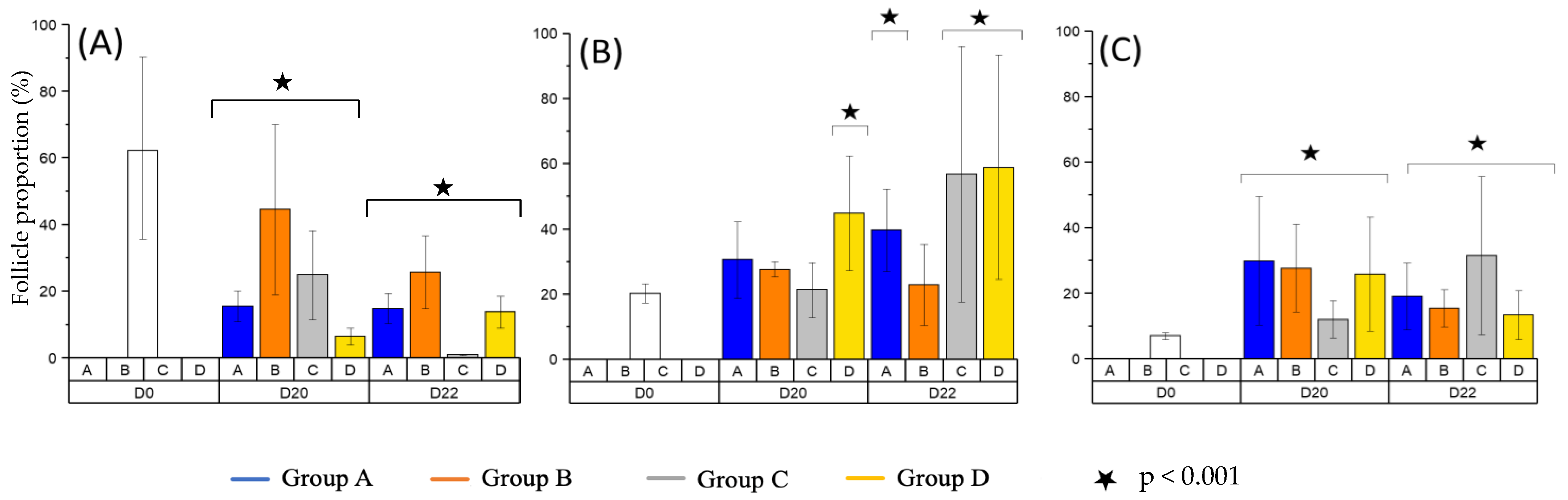
3.1. Follicular Density
3.2. Follicular Growth
3.3. Follicular Quality and Morphology
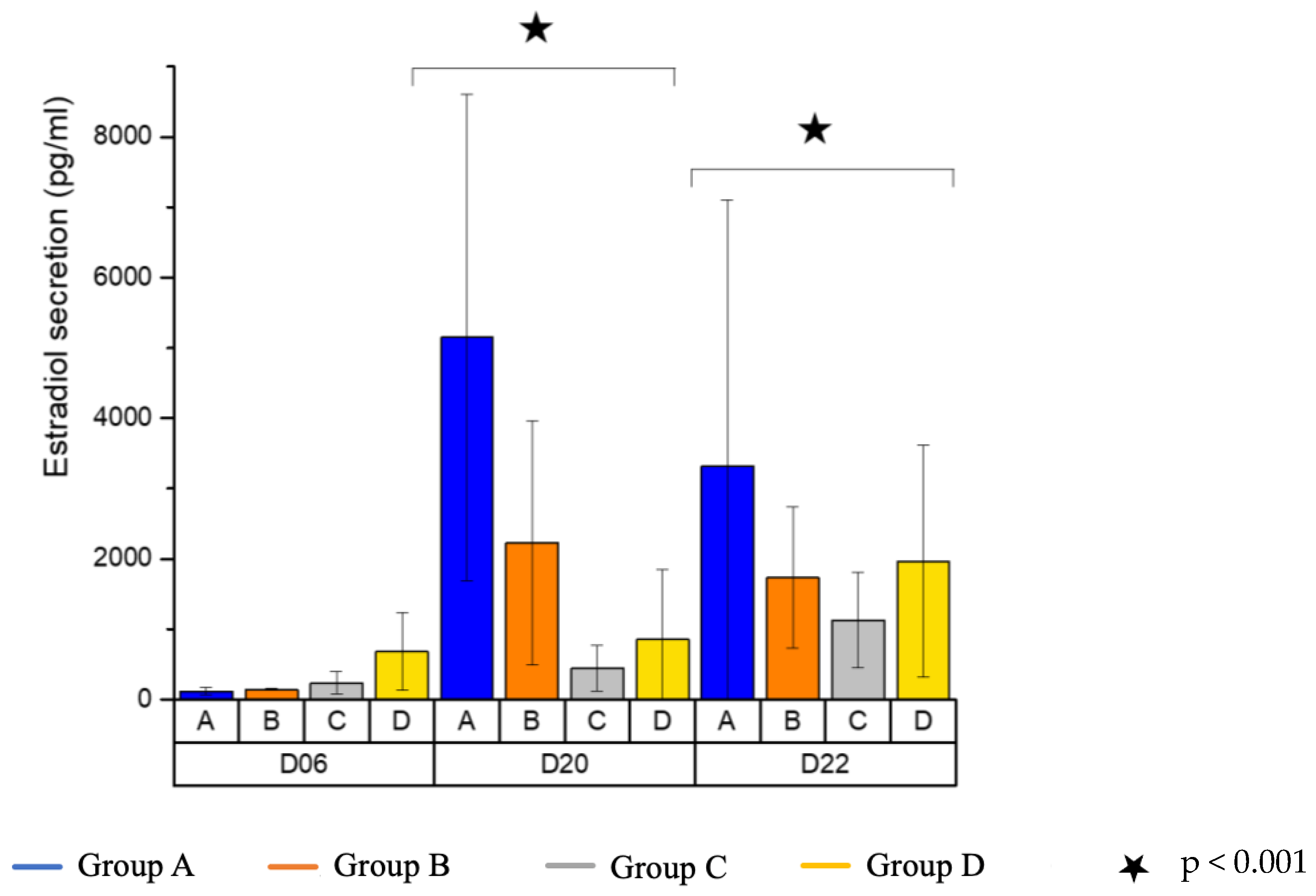
3.4. Estradiol Secretion
3.5. Detection of Ki67 Antigen
3.6. Detection of Cleaved Caspase 3 Antigen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lotz, L.; Dittrich, R.; Hoffmann, I.; Beckmann, M.W. Ovarian Tissue Transplantation: Experience From Germany and Worldwide Efficacy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119867357. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J.; O’Brien, M.; Wigglesworth, K. Mammalian Oocyte Growth and Development in Vitro. Mol. Reprod. Dev. 1996, 44, 260–273. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Pendola, J.K.; Eppig, J.J. A Revised Protocol for in Vitro Development of Mouse Oocytes from Primordial Follicles Dramatically Improves Their Developmental Competence. Biol. Reprod. 2003, 68, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- de Sá, N.A.R.; Ferreira, A.C.A.; Sousa, F.G.C.; Duarte, A.B.G.; Paes, V.M.; Cadenas, J.; Anjos, J.C.; Fernandes, C.C.L.; Rosseto, R.; Cibin, F.W.S.; et al. First Pregnancy after in Vitro Culture of Early Antral Follicles in Goats: Positive Effects of Anethole on Follicle Development and Steroidogenesis. Mol. Reprod. Dev. 2020, 87, 966–977. [Google Scholar] [CrossRef]
- Labrune, E.; Salle, B.; Lornage, J. An Update on In Vitro Folliculogenesis: A New Technique for Post-Cancer Fertility. Biomedicines 2022, 10, 2217. [Google Scholar] [CrossRef]
- McLaughlin, M.; Bromfield, J.J.; Albertini, D.F.; Telfer, E.E. Activin Promotes Follicular Integrity and Oogenesis in Cultured Pre-Antral Bovine Follicles. Mol. Hum. Reprod. 2010, 16, 644–653. [Google Scholar] [CrossRef]
- Brevini, T.A.L.; Pennarossa, G.; Gandolfi, F. A 3D Approach to Reproduction. Theriogenology 2020, 150, 2–7. [Google Scholar] [CrossRef]
- Joo, S.; Oh, S.-H.; Sittadjody, S.; Opara, E.C.; Jackson, J.D.; Lee, S.J.; Yoo, J.J.; Atala, A. The Effect of Collagen Hydrogel on 3D Culture of Ovarian Follicles. Biomed. Mater. 2016, 11, 065009. [Google Scholar] [CrossRef]
- Sadr, S.Z.; Fatehi, R.; Maroufizadeh, S.; Amorim, C.A.; Ebrahimi, B. Utilizing Fibrin-Alginate and Matrigel-Alginate for Mouse Follicle Development in Three-Dimensional Culture Systems. Biopreserv. Biobank. 2018, 16, 120–127. [Google Scholar] [CrossRef]
- Simon, L.E.; Kumar, T.R.; Duncan, F.E. In Vitro Ovarian Follicle Growth: A Comprehensive Analysis of Key Protocol Variables†. Biol. Reprod. 2020, 103, 455–470. [Google Scholar] [CrossRef]
- Zuccotti, M.; Merico, V.; Rebuzzini, P.; Belli, M.; Vigone, G.; Mulas, F.; Fassina, L.; Wruck, W.; Adjaye, J.; Bellazzi, R.; et al. 3D Culture of Ovarian Follicles: A System towards Their Engineering? Int. J. Dev. Biol. 2015, 59, 211–216. [Google Scholar] [CrossRef]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II Oocytes from Human Unilaminar Follicles Grown in a Multi-Step Culture System. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef]
- Bloise, E.; Ciarmela, P.; Dela Cruz, C.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in Mammalian Physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A Two-Step Serum-Free Culture System Supports Development of Human Oocytes from Primordial Follicles in the Presence of Activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef]
- Labrune, E.; Salle, B.; Lornage, J.; Montembault, A.; David, L. Procédés in vitro de culture de tissu ovarien. French patente number 3101356, 3 June 2022. [Google Scholar]
- Labrune, E.; Fournier, C.; Riche, B.; David, L.; Montembault, A.; Collardeau-Frachon, S.; Benchaib, M.; Lornage, J.; Iwaz, J.; Salle, B. Development and Survival of Human Ovarian Cells in Chitosan Hydrogel Micro-Bioreactor. Medicina 2022, 58, 1565. [Google Scholar] [CrossRef]
- Araiza, R.N.R.; Rochas, C.; David, L.; Domard, A. Interrupted Wet-Spinning Process for Chitosan Hollow Fiber Elaboration. Macromol. Symp. 2008, 266, 1–5. [Google Scholar] [CrossRef]
- David, L.; Domard, A.; Rivas-Araiza, R. Hollow, Notably Multi-Membrane Fibers, Method for Preparation Thereof by Spinning and Device for Applying Said Method. International Patent WO 200944053 A2n, 5 September 2008. [Google Scholar]
- Montembault, A.; Viton, C.; Domard, A. Physico-Chemical Studies of the Gelation of Chitosan in a Hydroalcoholic Medium. Biomaterials 2005, 26, 933–943. [Google Scholar] [CrossRef]
- Ding, C.C.; Thong, K.J.; Krishna, A.; Telfer, E.E. Activin A Inhibits Activation of Human Primordial Follicles in Vitro. J. Assist. Reprod. Genet. 2010, 27, 141–147. [Google Scholar] [CrossRef]
- Gougeon, A. Dynamics of Follicular Growth in the Human: A Model from Preliminary Results. Hum. Reprod. 1986, 1, 81–87. [Google Scholar] [CrossRef]
- Fortune, J.E.; Cushman, R.A.; Wahl, C.M.; Kito, S. The Primordial to Primary Follicle Transition. Mol. Cell. Endocrinol. 2000, 163, 53–60. [Google Scholar] [CrossRef]
- Hovatta, O.; Silye, R.; Abir, R.; Krausz, T.; Winston, R.M. Extracellular Matrix Improves Survival of Both Stored and Fresh Human Primordial and Primary Ovarian Follicles in Long-Term Culture. Hum. Reprod. 1997, 12, 1032–1036. [Google Scholar] [CrossRef]
- Wandji, S.A.; Srsen, V.; Voss, A.K.; Eppig, J.J.; Fortune, J.E. Initiation in Vitro of Growth of Bovine Primordial Follicles. Biol. Reprod. 1996, 55, 942–948. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo Signaling Disruption and Akt Stimulation of Ovarian Follicles for Infertility Treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef]
- Higuchi, C.M.; Maeda, Y.; Horiuchi, T.; Yamazaki, Y. A Simplified Method for Three-Dimensional (3-D) Ovarian Tissue Culture Yielding Oocytes Competent to Produce Full-Term Offspring in Mice. PLoS ONE 2015, 10, e0143114. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Wildt, D.E.; Travis, A.J.; Songsasen, N. Activin Promotes Growth and Antral Cavity Expansion in the Dog Ovarian Follicle. Theriogenology 2019, 129, 168–177. [Google Scholar] [CrossRef]
- Cossigny, D.A.; Findlay, J.K.; Drummond, A.E. The Effects of FSH and Activin A on Follicle Development in Vitro. Reproduction 2012, 143, 221–229. [Google Scholar] [CrossRef]
- Martins da Silva, S.J.; Bayne, R.A.L.; Cambray, N.; Hartley, P.S.; McNeilly, A.S.; Anderson, R.A. Expression of Activin Subunits and Receptors in the Developing Human Ovary: Activin A Promotes Germ Cell Survival and Proliferation before Primordial Follicle Formation. Dev. Biol. 2004, 266, 334–345. [Google Scholar] [CrossRef]
- Kinnear, H.M.; Constance, E.S.; David, A.; Marsh, E.E.; Padmanabhan, V.; Shikanov, A.; Moravek, M.B. A Mouse Model to Investigate the Impact of Testosterone Therapy on Reproduction in Transgender Men. Hum. Reprod. 2019, 34, 2009–2017. [Google Scholar] [CrossRef]
- Kinnear, H.M.; Hashim, P.H.; Dela Cruz, C.; Rubenstein, G.; Chang, F.L.; Nimmagadda, L.; Brunette, M.A.; Padmanabhan, V.; Shikanov, A.; Moravek, M.B. Reversibility of Testosterone-Induced Acyclicity after Testosterone Cessation in a Transgender Mouse Model. F&S Sci. 2021, 2, 116–123. [Google Scholar] [CrossRef]
- Bartels, C.B.; Uliasz, T.F.; Lestz, L.; Mehlmann, L.M. Short-Term Testosterone Use in Female Mice Does Not Impair Fertilizability of Eggs: Implications for the Fertility Care of Transgender Males. Hum. Reprod. 2021, 36, 189–198. [Google Scholar] [CrossRef]
- Xu, F.; Lawson, M.S.; Bean, Y.; Ting, A.Y.; Pejovic, T.; De Geest, K.; Moffitt, M.; Mitalipov, S.M.; Xu, J. Matrix-Free 3D Culture Supports Human Follicular Development from the Unilaminar to the Antral Stage in Vitro Yielding Morphologically Normal Metaphase II Oocytes. Hum. Reprod. 2021, 36, 1326–1338. [Google Scholar] [CrossRef] [PubMed]

| Group | Total | Primordial Stage | Intermediate Stage | Primary Stage | Secondary Stage | Tertiary Stage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intact | Altered | Intact | Altered | Intact | Altered | Intact | Altered | Intact | Altered | ||||
| Fresh | Total number of follicles | 637 | 22 | 375 | 5 | 61 | 27 | 102 | 17 | 27 | 0 | 1 | |
| Follicular stage by group (%) | 3.5 | 58.9 | 0.8 | 9.6 | 4.2 | 16.0 | 2.7 | 4.2 | 0 | 0.2 | |||
| D20 | A | Total number of follicles | 265 | 3 | 38 | 4 | 59 | 25 | 56 | 61 | 18 | 1 | 0 |
| Follicular stage by group (%) | 1.1 | 14.3 | 1.5 | 22.2 | 9.4 | 21.1 | 23.0 | 6.8 | 0.4 | 0 | |||
| B | Total number of follicles | 301 | 16 | 118 | 10 | 41 | 12 | 17 | 37 | 46 | 1 | 3 | |
| Follicular stage by group (%) | 5.3 | 39.2 | 3.3 | 13.6 | 4.0 | 5.6 | 12.3 | 15.2 | 0.3 | 1.0 | |||
| C | Total number of follicles | 423 | 2 | 103 | 1 | 172 | 24 | 66 | 28 | 23 | 2 | 2 | |
| Follicular stage by group (%) | 0.5 | 24.3 | 0.2 | 40.7 | 5.7 | 15.6 | 6.6 | 5.4 | 0.5 | 0.5 | |||
| D | Total number of follicles | 315 | 11 | 9 | 33 | 36 | 85 | 56 | 47 | 34 | 2 | 2 | |
| Follicular stage by group (%) | 3.5 | 2.9 | 10.5 | 11.4 | 27.0 | 17.8 | 14.9 | 10.8 | 0.6 | 0.6 | |||
| D22 | A | Total number of follicles | 535 | 13 | 66 | 35 | 107 | 123 | 89 | 84 | 17 | 1 | 0 |
| Follicular stage by group (%) | 2.4 | 12.3 | 6.5 | 20.0 | 23.0 | 16.6 | 15.7 | 3.2 | 0.2 | 0 | |||
| B | Total number of follicles | 347 | 5 | 85 | 14 | 109 | 50 | 29 | 33 | 20 | 1 | 1 | |
| Follicular stage by group (%) | 1.4 | 24.5 | 4.0 | 31.4 | 14.4 | 8.4 | 9.5 | 5.8 | 0.3 | 0.3 | |||
| C | Total number of follicles | 229 | 0 | 2 | 10 | 14 | 71 | 59 | 52 | 20 | 0 | 1 | |
| Follicular stage by group (%) | 0.0 | 0.9 | 4.4 | 6.1 | 31.0 | 25.8 | 22.7 | 8.7 | 0 | 0.4 | |||
| D | Total number of follicles | 467 | 5 | 59 | 21 | 42 | 150 | 125 | 49 | 13 | 1 | 2 | |
| Follicular stage by group (%) | 1.1 | 12.6 | 4.5 | 9.0 | 32.1 | 26.8 | 10.5 | 2.8 | 0.2 | 0.4 | |||
| Group | D20 | D22 | ||
|---|---|---|---|---|
| % Intact Follicles (Mean ± SD) | n | % Intact Follicles (Mean ± SD) | n | |
| A (3D−/A−) | 42.7 ± 34.7 | 265 | 54.3 ± 33.6 | 535 |
| B (3D−/A+) | 27.7 ± 16.4 | 301 | 35.7 ± 33.0 | 347 |
| C (3D+/A−) | 17.5 ± 29.1 | 423 | 62.9 ± 32.7 | 229 |
| D (3D+/A+) | 54.5 ± 5.0 | 315 | 54.1 ± 31.4 | 467 |
| Group | D6 | D20 | D22 | |||
|---|---|---|---|---|---|---|
| A (3D−/A−) | 120.0 | ±55.43 | 5151.0 | ±3463.23 | 3323.6 | ±3780.42 |
| B (3D−/A+) | 146.0 | ±10.39 | 2227.2 | ±1736.04 | 1738.6 | ±1002.98 |
| C (3D+/A−) | 237.3 | ±162.24 | 446.8 | ±326.12 | 1130.2 | ±683.07 |
| D (3D+/A+) | 680.3 | ±551.95 | 854.2 | ±990.17 | 1965.0 | ±1650 |
| Number of Counted Follicles | Number of Positive Follicles (Ki67+) | ||
|---|---|---|---|
| Ki67 | 3D− | 379 | 33 |
| 3D+ | 384 | 12 | |
| A− | 413 | 53 | |
| A+ | 350 | 17 |
| Number of Counted Follicles | Number of Positive Follicles | ||
|---|---|---|---|
| Caspase | 3D− | 379 | 55 |
| 3D+ | 384 | 39 | |
| A− | 413 | 42 | |
| A+ | 350 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovet, C.; Fraison, E.; Lornage, J.; Journel, N.M.; Gavoille, A.; David, L.; Montembault, A.; Fournier, C.; Salle, B.; Labrune, E. A New Bioreactor to Promote Human Follicular Growth with or without Activin A in Transgender Men. Reprod. Med. 2023, 4, 14-27. https://doi.org/10.3390/reprodmed4010003
Jovet C, Fraison E, Lornage J, Journel NM, Gavoille A, David L, Montembault A, Fournier C, Salle B, Labrune E. A New Bioreactor to Promote Human Follicular Growth with or without Activin A in Transgender Men. Reproductive Medicine. 2023; 4(1):14-27. https://doi.org/10.3390/reprodmed4010003
Chicago/Turabian StyleJovet, Cynthia, Eloïse Fraison, Jacqueline Lornage, Nicolas Morel Journel, Antoine Gavoille, Laurent David, Alexandra Montembault, Cyrielle Fournier, Bruno Salle, and Elsa Labrune. 2023. "A New Bioreactor to Promote Human Follicular Growth with or without Activin A in Transgender Men" Reproductive Medicine 4, no. 1: 14-27. https://doi.org/10.3390/reprodmed4010003
APA StyleJovet, C., Fraison, E., Lornage, J., Journel, N. M., Gavoille, A., David, L., Montembault, A., Fournier, C., Salle, B., & Labrune, E. (2023). A New Bioreactor to Promote Human Follicular Growth with or without Activin A in Transgender Men. Reproductive Medicine, 4(1), 14-27. https://doi.org/10.3390/reprodmed4010003






