Kisspeptin Is Upregulated at the Maternal-Fetal Interface of the Preeclamptic-like BPH/5 Mouse and Normalized after Synchronization of Sex Steroid Hormones
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
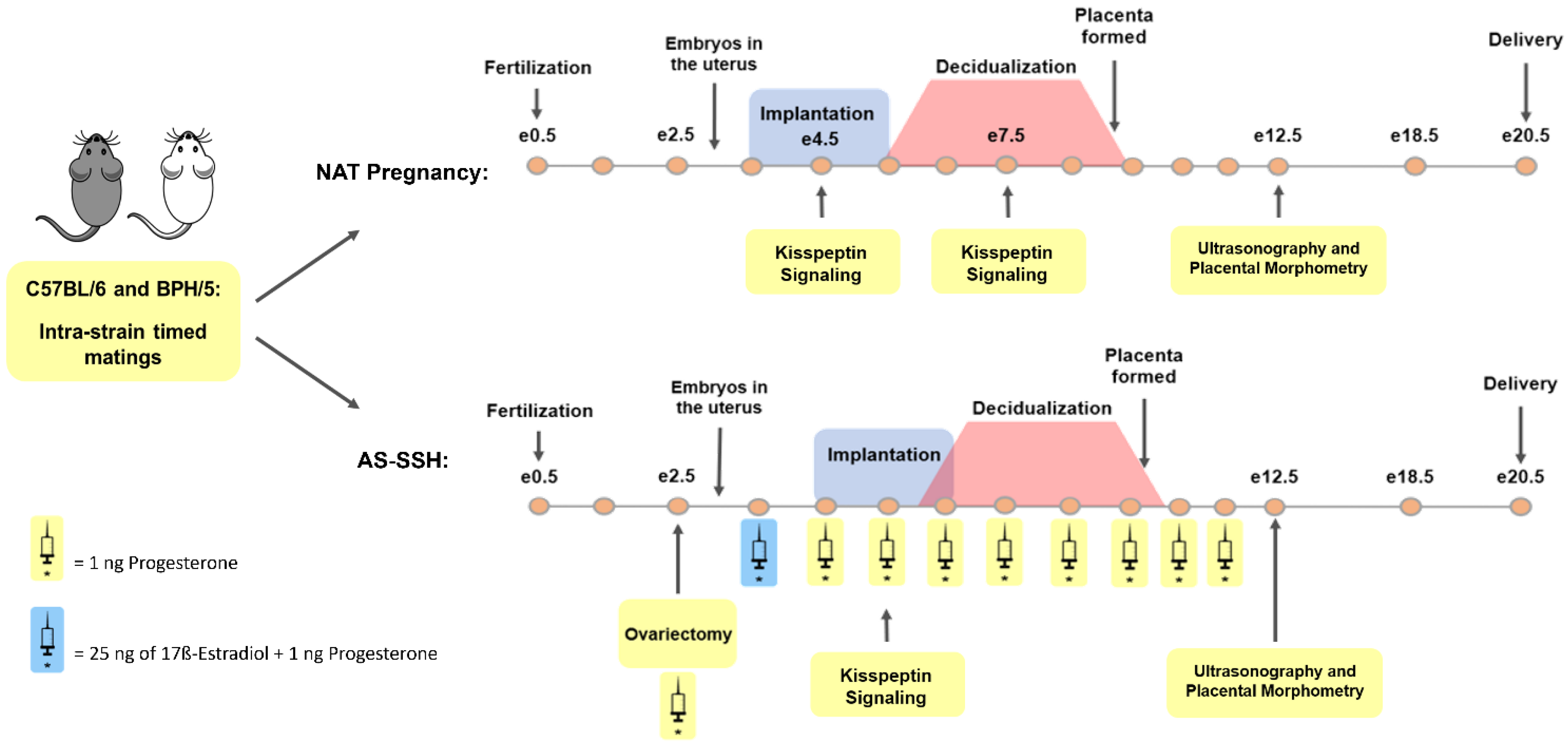
2.2. Reproductive Management and Sample Collection
2.3. Artificial Synchronization of Sex Steroid Hormones
2.4. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.5. Immunohistochemistry
2.6. Ultrasonography
2.7. Placental Morphometry
2.8. Statistical Analysis
3. Results
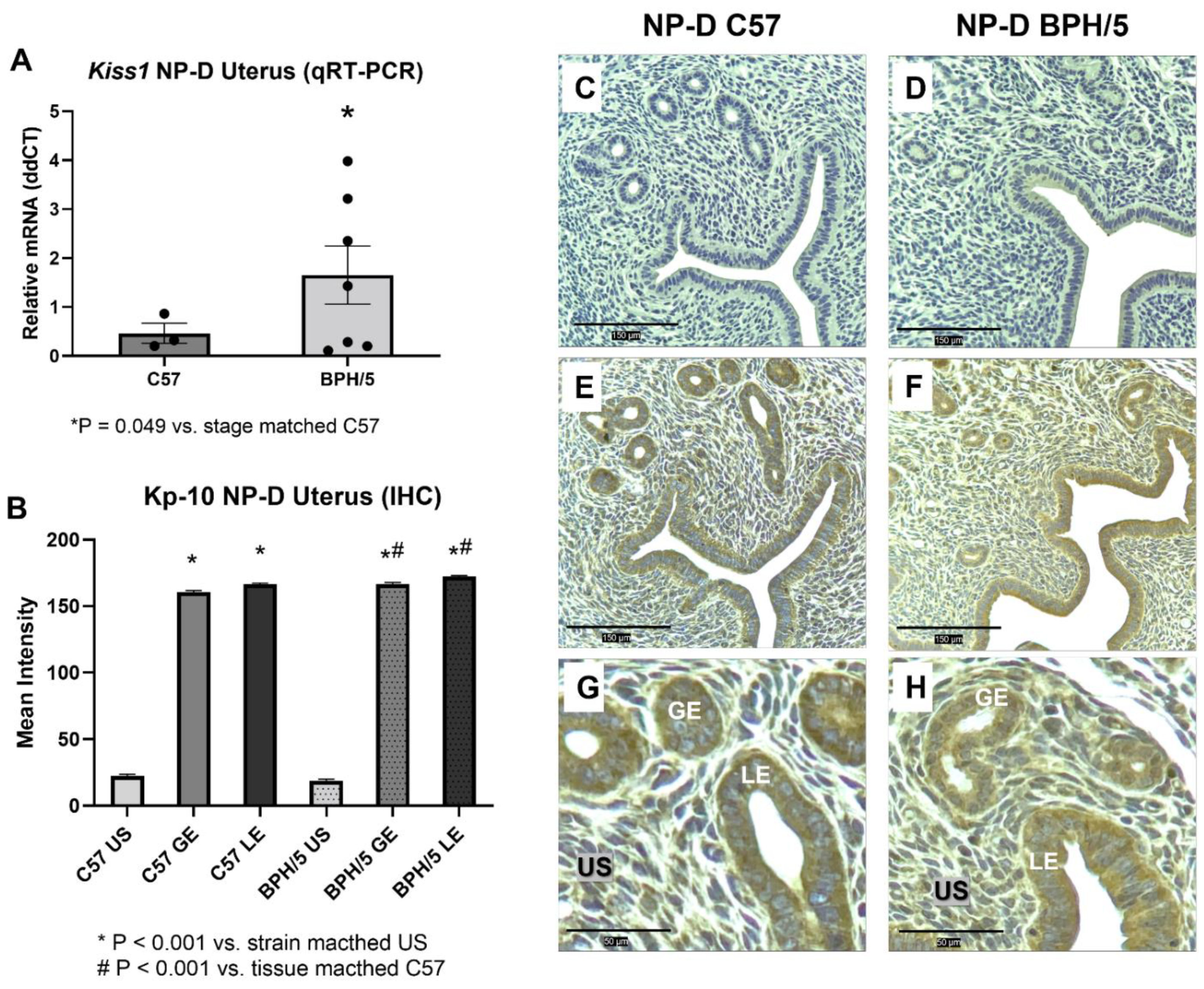
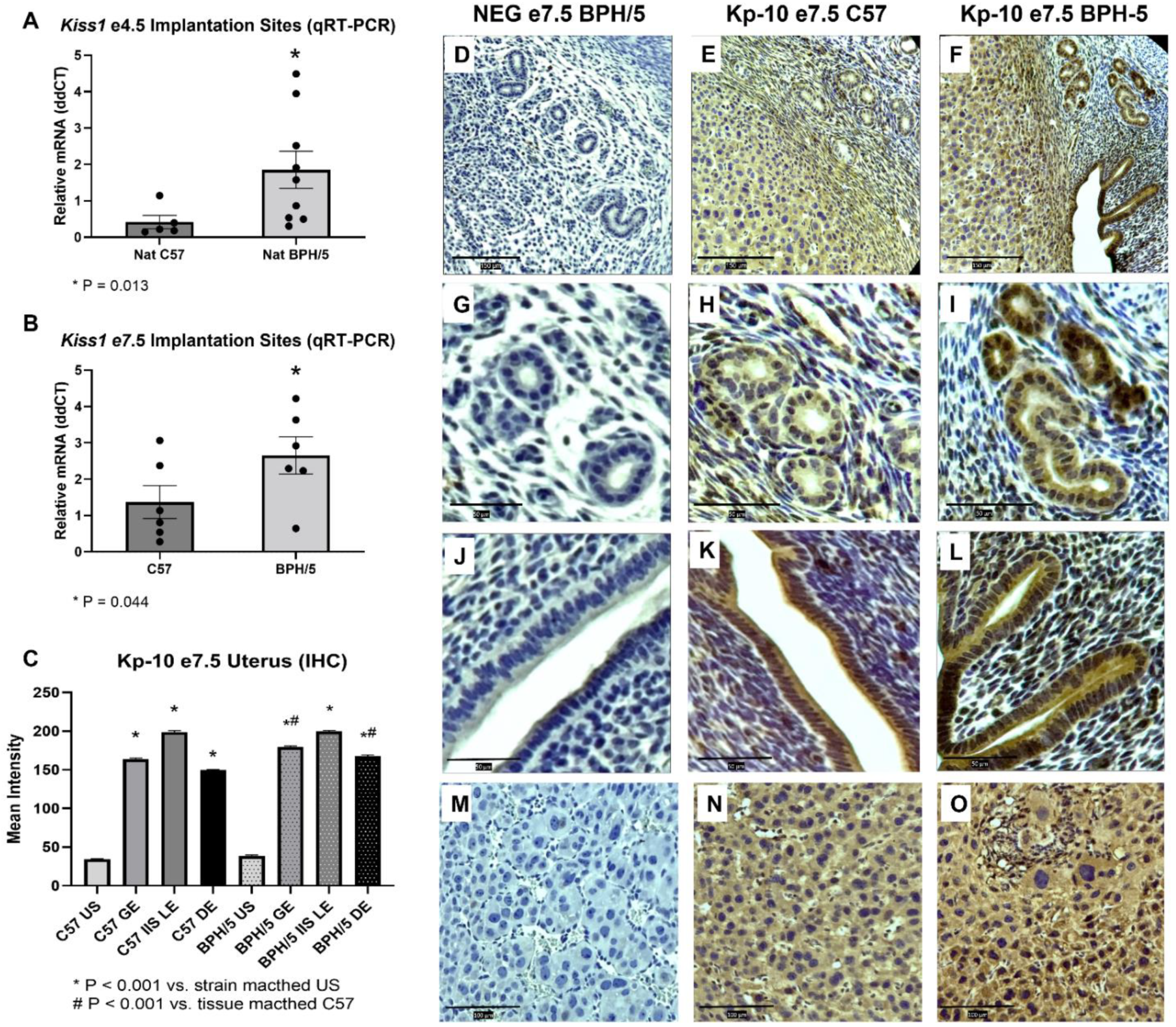
3.1. Kiss1 Is Upregulated in BPH/5 Non-Pregnant Uterus and Maternal-Fetal Interface
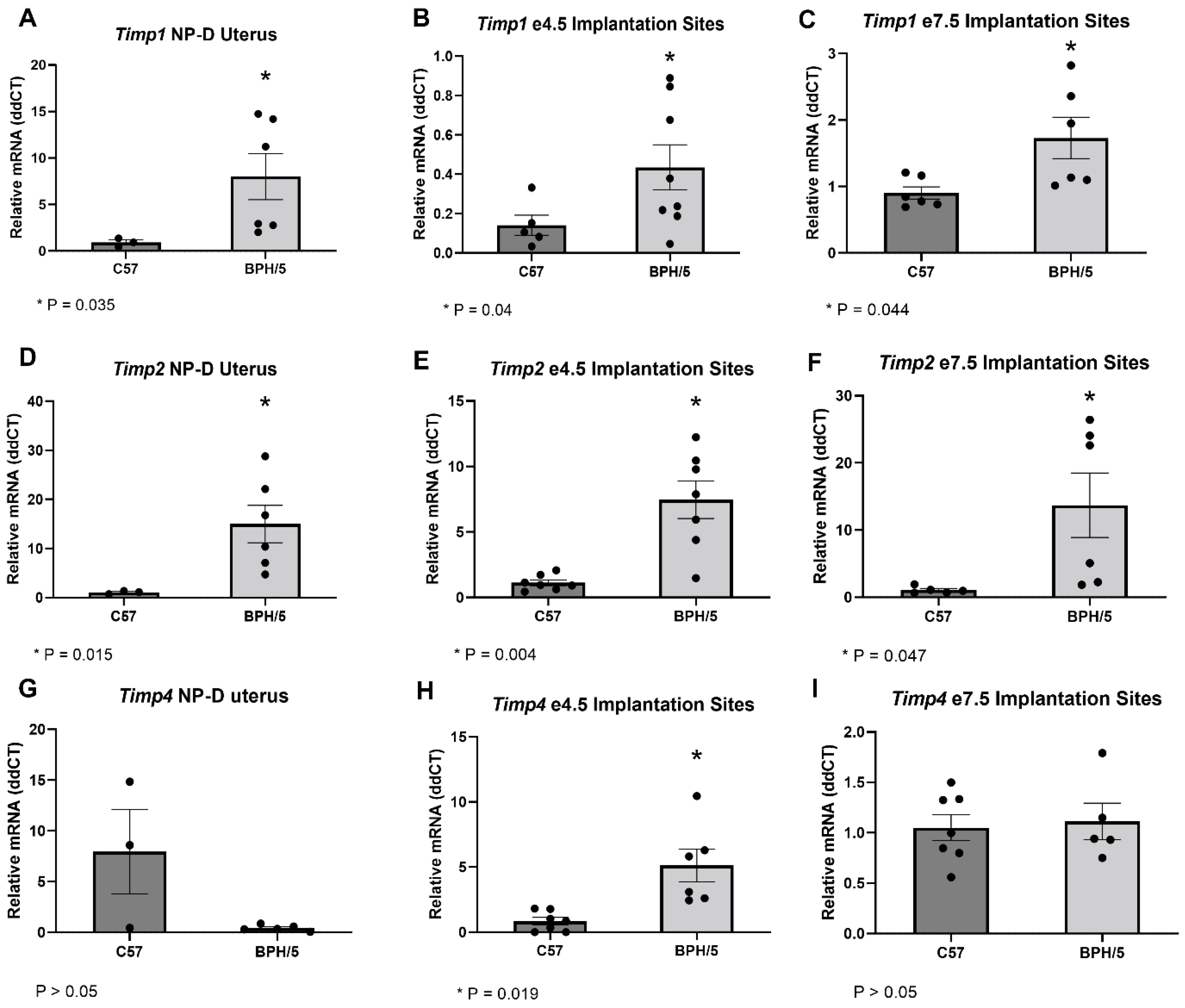
3.2. Timps Are Upregulated in the BPH/5 Non-Pregnant Uterus and Maternal-Fetal Interface
3.3. Artificial Synchronization of SSH Normalizes the Expression of Kiss1 and Downstream Molecules in the BPH/5 Mouse
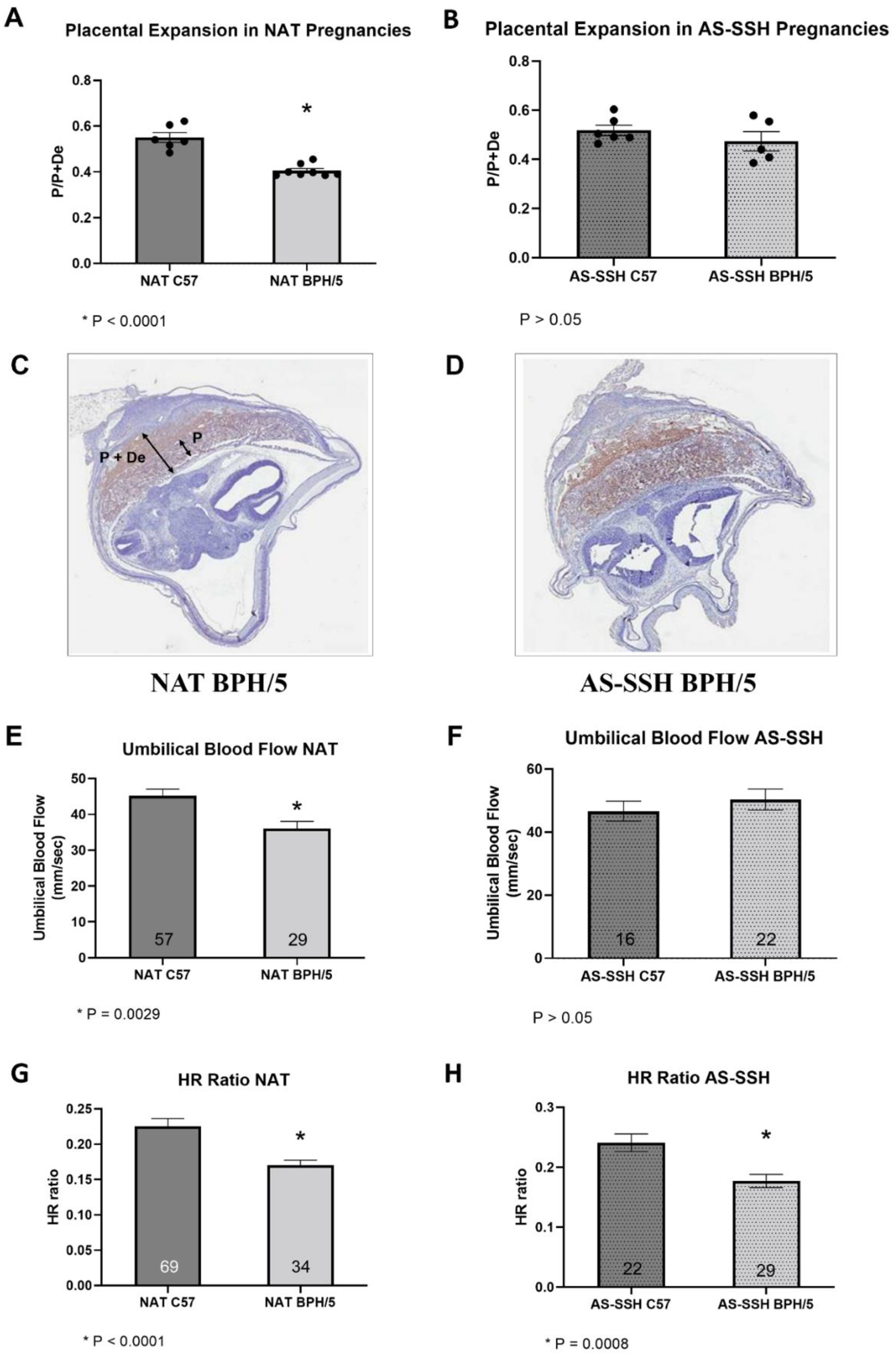
3.4. Placentation and Umbilical Cord Blood Flow Are Improved in BPH/5 Females after AS-SSH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, O.; Moller, A.B.; Daniels, J.; Gulmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Tarca, A.L.; Kekesi, K.A.; Xu, Y.; Xu, Z.; Juhasz, K.; Bhatti, G.; Leavitt, R.J.; Gelencser, Z.; et al. Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia. Front. Immunol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.; Zembala-Szczerba, M.; Babczyk, D.; Kolodziejczyk-Pietruszka, M.; Lawaczynska, O.; Huras, H. Early- and Late-Onset Preeclampsia: A Comprehensive Cohort Study of Laboratory and Clinical Findings according to the New ISHHP Criteria. Int. J. Hypertens. 2019, 4108271. [Google Scholar] [CrossRef]
- Raymond, D.; Peterson, E. A Critical Review of Early-Onset and Late-Onset Preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef]
- Naicker, T.; Khedun, S.M.; Moddley, J.; Pijnenborg, R. Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet. Gynecol. Scand. 2003, 82, 722–729. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S895–S906. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Bischof, P.; Meisser, A.; Campana, A. Paracrine and autocrine regulators of trophoblast invasion—A review. Placenta 2000, 21 (Suppl. A), S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.G.; Cao, Y.J.; Sang, Q.X.; Duan, E.K. Expression and implications of tissue inhibitor of metalloproteinases-4 in mouse embryo. Mol. Hum. Reprod. 2003, 9, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Chang, H.M.; Zhao, H.C.; Yu, Y.; Li, R.; Qiao, J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum. Reprod. Update 2019, 25, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Bilban, M.; Ghaffari-Tabrizi, N.; Hintermann, E.; Bauer, S.; Molzer, S.; Zoratti, C.; Malli, R.; Sharabi, A.; Hidden, U.; Graier, W.; et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J. Cell Sci. 2004, 117 Pt 8, 1319–1328. [Google Scholar] [CrossRef]
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.; Weissman, B.E.; Welch, D.R. KISS-1, a Novel Human Malignant Melanoma Metastasis-Supressor Gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef]
- Janneau, J.L.; Maldonado-Estrada, J.; Tachdjian, G.; Miran, I.; Motte, N.; Saulnier, P.; Sabourin, J.-C.; Cote, J.-F.; Simon, B.; Frydman, R.; et al. Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J. Clin. Endocrinol. Metab. 2002, 87, 5336–5339. [Google Scholar] [CrossRef] [PubMed]
- Francis, V.A.; Abera, A.B.; Matjila, M.; Millar, R.P.; Katz, A.A. Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells. PLoS ONE 2014, 9, e99680. [Google Scholar]
- Baba, T.; Kang, H.S.; Hosoe, Y.; Kharma, B.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; et al. Menstrual cyclic change of metastin/GPR54 in endometrium. Med. Mol. Morphol. 2015, 48, 76–84. [Google Scholar] [CrossRef]
- Qiao, C.; Wang, C.; Zhao, J.; Liu, C.; Shang, T. Elevated expression of KiSS-1 in placenta of Chinese women with early-onset preeclampsia. PLoS ONE 2012, 7, e48937. [Google Scholar] [CrossRef] [PubMed]
- Matjila, M.; Millar, R.; van der Spuy, Z.; Katz, A. Elevated placental expression at the maternal-fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens. 2016, 6, 79–87. [Google Scholar] [CrossRef]
- Gomes, V.C.L.; Sones, J.L. From inhibition of trophoblast cell invasion to proapoptosis: What are the potential roles of kisspeptins in preeclampsia? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R41–R48. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, M.; Zhong, T.; Lin, Y.; Zong, T.; Zhong, C.; Zhang, B.; Ren, M.; Kuang, H. Expression and function of kisspeptin during mouse decidualization. PLoS ONE 2014, 9, e97647. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.; Vilos, A.G.; Vilos, G.A.; Bhattacharya, M.; Babwah, A.V. Uterine kisspeptin receptor critically regulates epithelial estrogen receptor alpha transcriptional activity at the time of embryo implantation in a mouse model. Mol. Hum. Reprod. 2021, 27, 1–13. [Google Scholar] [CrossRef]
- Davisson, R.L.; Hoffmann, D.S.; Butz, G.M.; Aldape, G.; Schlager, G.; Merrill, D.C.; Sethi, S.; Weiss, R.M.; Bates, J.N. Discovery of a Spontaneous Genetic Mouse Model of Preeclampsia. Hypertension 2002, 39, 337–342. [Google Scholar] [CrossRef]
- Dokras, A.; Hoffmann, D.S.; Eastvold, J.S.; Kienzle, M.F.; Gruman, L.M.; Kirby, P.A.; Weiss, R.M.; Davisson, R.L. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol. Reprod. 2006, 75, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Gelber, S.E.; Brent, E.; Redecha, P.; Perino, G.; Tomlinson, S.; Davisson, R.L.; Salmon, J.E. Prevention of Defective Placentation and Pregnancy Loss by Blocking Innate Immune Pathways in a Syngeneic Model of Placental Insufficiency. J. Immunol. 2015, 195, 1129–1138. [Google Scholar] [CrossRef]
- Sones, J.L.; Cha, J.; Woods, K.A.; Bartos, A.; Heyward, C.Y.; Lob, H.E.; Isroff, C.E.; Butler, S.D.; Shapiro, S.E.; Dey, S.K.; et al. Decidual Cox2 inhibition improves fetal and maternal outcomes in a preeclampsia-like mouse model. JCI Insight 2016, 1, e75351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sutton, E.F.; Lob, H.E.; Song, J.; Xia, Y.; Butler, S.; Liu, C.C.; Redman, L.M.; Sones, J.L. Adverse metabolic phenotype of female offspring exposed to preeclampsia in utero: A characterization of the BPH/5 mouse in postnatal life. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R485–R491. [Google Scholar] [CrossRef] [PubMed]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009, S48, A-4I. [Google Scholar] [CrossRef] [PubMed]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Su, R.W.; Lei, W.; Liu, J.L.; Zhang, Z.R.; Jia, B.; Feng, X.H.; Ren, G.; Hu, S.-J.; Yang, Z.-M. The Integrative Analysis of microRNA and mRNA Expression in Mouse Uterus under Delayed Implantation and Activation. PLoS ONE 2010, 5, e15513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Flores, D.; Madhavan, M.; Wright, S.; Arora, R. Mechanical and signaling mechanisms that guide pre-implantation embryo movement. Development 2020, 147, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ramathal, C.Y.; Bagchi, I.C.; Taylor, R.N.; Bagchi, M.K. Endometrial decidualization: Of mice and men. Semin. Reprod. Med. 2010, 28, 17–26. [Google Scholar] [CrossRef]
- Karthikeyan, V.J.; Lane, D.A.; Beevers, D.G.; Lip, G.Y.; Blann, A.D. Matrix metalloproteinases and their tissue inhibitors in hypertension-related pregnancy complications. J. Hum. Hypertens. 2013, 27, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Calder, M.; Chan, Y.M.; Raj, R.; Pampillo, M.; Elbert, A.; Noonan, M.; Gillio-Meina, C.; Caligioni, C.; Berube, N.G.; Bhattacharya, M.; et al. Implantation failure in female Kiss1-/- mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology 2014, 155, 3065–3078. [Google Scholar] [CrossRef]
- Arany, Z.; Hilfiker-Kleiner, D.; Karumanchi, S.A. Animal Models of Cardiovascular Complications of Pregnancy. Circ. Res. 2022, 130, 1763–1779. [Google Scholar] [CrossRef]
- Sones, J.L.; Yarborough, C.C.; O’Besso, V.O.; Lemenze, A.; Douglas, N.C. Genotypic analysis of the female BPH/5 mouse, a model of superimposed preeclampsia. PLoS ONE 2021, 16, e0253453. [Google Scholar] [CrossRef]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef]
- Margioula-Siarkou, G.; Margioula-Siarkou, C.; Petousis, S.; Margaritis, K.; Vavoulidis, E.; Gullo, G.; Alexandratou, M.; Dinas, K.; Sotiriadis, A.; Mavromatidis, G. The role of endoglin and its soluble form in pathogenesis of preeclampsia. Mol. Cell. Biochem. 2022, 477, 479–491. [Google Scholar] [CrossRef]
- Thomopoulos, C.; Tsioufis, C.; Michalopoulou, H.; Makris, T.; Papademetriou, V.; Stefanadis, C. Assisted reproductive technology and pregnancy-related hypertensive complications: A systematic review. J. Hum. Hypertens. 2013, 27, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Ling, Z.; Hou, X.; Fan, Y.; Xie, K.; Shen, R. In vitro fertilization is associated with the onset and progression of preeclampsia. Placenta 2020, 89, 50–57. [Google Scholar] [CrossRef]
- Prapas, Y.; Ravanos, K.; Petousis, S.; Panagiotidis, Y.; Papatheodorou, A.; Margioula-Siarkou, C.; Iuliano, A.; Gullo, G.; Prapas, N. GnRH antagonist administered twice the day before hCG trigger combined with a step-down protocol may prevent OHSS in IVF/ICSI antagonist cycles at risk for OHSS without affecting the reproductive outcomes: A prospective randomized control trial. J. Assist. Reprod. Genet. 2017, 34, 1537–1545. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; D’Indinosante, M.; Turrini, I.; Giusti, M.; Gullo, G.; Vizzielli, G.; Mattei, A.; Scambia, G.; et al. Fertility sparing treatments in endometrial cancer patients: The potential role of the new molecular classification. Int. J. Mol. Sci. 2021, 22, 12248. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Etrusco, A.; Cucinella, G.; Perino, A.; Chiantera, V.; Lagana, A.S.; Tomaiuolo, R.; Vitagliano, A.; Giampaolino, P.; Noventa, P.; et al. Fertility-sparing approach in women affected by stage I and low-grade endometrial carcinoma: An updated overview. Int. J. Mol. Sci. 2021, 22, 11825. [Google Scholar] [CrossRef] [PubMed]
- Tanos, P.; Dimitriou, S.; Gullo, G.; Tanos, V. Biomolecular and genetic prognostic factors that can facilitate fertility-sparing treatment (FST) decision making in early stage endometrial cancer (ES-EC): A systematic review. Int. J. Mol. Sci. 2022, 23, 2653. [Google Scholar] [CrossRef]
- Leon, S.; Fernandois, D.; Sull, A.; Sull, J.; Clader, M.; Hayashi, K.; Battacharya, M.; Power, S.; Vilos, G.A.; Vilos, A.G.; et al. Beyond the brain-Peripheral kisspeptin signaling is essential for promoting endometrial gland development and function. Sci. Rep. 2016, 6, 29073. [Google Scholar] [CrossRef]
- Garrido-Gomez, T.; Castillo-Marco, N.; Cordero, T.; Simon, C. Decidualization resistance in the origin of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S886–S894. [Google Scholar] [CrossRef]
- Zhou, Y.; Gormley, M.J.; Hunkapiller, N.M.; Kapidzic, M.; Stolyarov, Y.; Feng, V.; Nishida, M.; Drake, P.M.; Bianco, K.; Wang, F.; et al. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J. Clin. Invest. 2013, 123, 2862–2872. [Google Scholar] [CrossRef]
- Herreboudt, A.M.; Kyle, V.R.; Lawrence, J.; Doran, J.; College, W.H. Kiss1 mutant placentas show normal structure and function in the mouse. Placenta 2015, 36, 52–58. [Google Scholar] [CrossRef][Green Version]
- Ashkar, A.A.; di Santo, J.P.; Croy, B.A. Interferon Y Contributes to Initiation of Uterine Vascular Modification, Decidual Integrity, and Uterine Natural Killer Cell Maturation during Normal Murine Pregnancy. J. Exp. Med. 2000, 192, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ain, R.; Canham, L.N.; Soares, M.J. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: Novel endocrine phenotype and regulation. Dev. Biol. 2003, 260, 176–190. [Google Scholar] [CrossRef]
- Sakamuri, S.S.; Watts, R.; Takawale, A.; Wang, X.; Hernandez-Anzaldo, S.; Bahitham, W.; Fernandez-Patron, C.; Lehner, R.; Kassiri, Z. Absence of Tissue Inhibitor of Metalloproteinase-4 (TIMP4) ameliorates high fat diet-induced obesity in mice due to defective lipid absorption. Sci. Rep. 2017, 7, 6210. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, V.C.L.; Woods, A.K.; Crissman, K.R.; Landry, C.A.; Beckers, K.F.; Gilbert, B.M.; Ferro, L.R.; Liu, C.-C.; Oberhaus, E.L.; Sones, J.L. Kisspeptin Is Upregulated at the Maternal-Fetal Interface of the Preeclamptic-like BPH/5 Mouse and Normalized after Synchronization of Sex Steroid Hormones. Reprod. Med. 2022, 3, 263-279. https://doi.org/10.3390/reprodmed3040021
Gomes VCL, Woods AK, Crissman KR, Landry CA, Beckers KF, Gilbert BM, Ferro LR, Liu C-C, Oberhaus EL, Sones JL. Kisspeptin Is Upregulated at the Maternal-Fetal Interface of the Preeclamptic-like BPH/5 Mouse and Normalized after Synchronization of Sex Steroid Hormones. Reproductive Medicine. 2022; 3(4):263-279. https://doi.org/10.3390/reprodmed3040021
Chicago/Turabian StyleGomes, Viviane C. L., Ashley K. Woods, Kassandra R. Crissman, Camille A. Landry, Kalie F. Beckers, Bryce M. Gilbert, Lucas R. Ferro, Chin-Chi Liu, Erin L. Oberhaus, and Jenny L. Sones. 2022. "Kisspeptin Is Upregulated at the Maternal-Fetal Interface of the Preeclamptic-like BPH/5 Mouse and Normalized after Synchronization of Sex Steroid Hormones" Reproductive Medicine 3, no. 4: 263-279. https://doi.org/10.3390/reprodmed3040021
APA StyleGomes, V. C. L., Woods, A. K., Crissman, K. R., Landry, C. A., Beckers, K. F., Gilbert, B. M., Ferro, L. R., Liu, C.-C., Oberhaus, E. L., & Sones, J. L. (2022). Kisspeptin Is Upregulated at the Maternal-Fetal Interface of the Preeclamptic-like BPH/5 Mouse and Normalized after Synchronization of Sex Steroid Hormones. Reproductive Medicine, 3(4), 263-279. https://doi.org/10.3390/reprodmed3040021







