Abstract
Objectives—To conduct a secondary analysis of prediction accuracy of biophysical markers for suspected Preeclampsia (PE), Fetal Growth Restriction (FGR) and the two combined near delivery in a Slovenian cohort. Methods—This was a secondary analysis of a database of a total 125 Slovenian pregnant women attending a high-risk pregnancy clinic due to suspected PE (n = 31), FGR (n = 16) and PE + FGR (n = 42) from 28–39 weeks gestation and their corresponding term (n = 21) and preterm (PTD, n = 15) controls. Data for Mean Arterial blood Pressure (MAP) and Uterine artery pulsatility index (UtA PI) estimated by Doppler sonography were extracted from the database of patients who were tested at admission to the high-risk clinic with the suspected complications. The reactive hyperemia index (RHI), and the Augmentation Index (AIX%) were extracted from the patient database using measured values obtained with the assistance of the Endo PAT, a device set to measure the signal of the peripheral arterial tone (PAT) from the blood vessels endothelium. Linear regression coefficients, Box and Whisker plots, Area under the Curve (AUC) of receiver Operation Characteristic (ROC) curves, and multiple regression were used to assess the marker accuracy using detection rate (DR) and false-positive rate (FPR) and previously reported cut-offs for estimating the positive and negative predictive value (NPV and PPV). The SPSS non-parametric statistics (Kruskal Wallis and Mann–Whitney) and Spearman’s regression coefficient were used to assess marker accuracy; p < 0.05 was considered significant. Results—MAP values reached diagnostic accuracy (AUC = 1.00, DR = 100%) for early PE cases delivered < 34, whereas UtA Doppler PI values yielded such results for early FGR < 34 weeks and the two combined reached such accuracy for PE + FGR. To reach diagnostic accuracy for all cases of the complications, the Endo PAT markers with values for MAP and UtA Doppler PI were required for cases near delivery. Multiple regression analyses showed added value for advanced maternal age and gestational week in risk assessment for all cases of PE, FGR, and PE + FGR. Spearman’s regression coefficient yielded r > 0.6 for UtA Doppler PI over GA for PE and FGR, whereas for RHI over BMI, the regression coefficient was r > 0.5 (p < 0.001 for each). Very high correlations were also found between UtA Doppler PI and sFlt-1/PlGF or PlGF (r = −0.495, p < 0.001), especially in cases of FGR. Conclusion—The classical biophysical markers MAP and UtA Doppler PI provided diagnostic accuracy for PE and FGR < 34 wks gestation. A multiple biophysical marker analysis was required to reach diagnostic accuracy for all cases of these complications. The UtA Doppler PI and maternal serum sFlt-1/PlGF or PlGF were equally accurate for early cases to enable the choice of the markers for the clinical use according to the more accessible method.
1. Introduction
In the last two decades, challenges have been made to the classical use of blood pressure measurements in the diagnosis of preeclampsia (PE), and it was suggested that near delivery (birth), the diagnosis can reach higher accuracy by adding certain biomarkers, particularly the pro- and anti-angiogenic markers placental growth factor (PLGF) and soluble forms, like tyrosine kinase-1 (sFlt-1) [1,2,3,4,5,6]. Preeclampsia is a leading cause of maternal and neonatal morbidity and mortality worldwide [7,8,9,10]. The International Society for the Study of Hypertension in Pregnancy (ISSHP) [8] recommended adding the measurements of angiogenic markers in the diagnosis of PE near delivery [8]. The American College of Obstetricians and Gynecologists (ACOG) [9], and the National Institute of Clinical Excellence (NICE) [10] included recommendations to use various biochemical and biophysical markers to improve the accurate diagnosis and prediction of PE near delivery.
In a previous set of publications [11,12,13,14], our team examined the accuracy of levels of maternal blood biochemical pro- and angiogenic markers in the prediction and diagnosis of PE using a Slovenian cohort of women attending the high-risk pregnancy clinic with the suspected complication. Indeed, we were able to show, like many previous studies [1,2,15,16,17,18,19,20], that near delivery, PlGF and the ratio of sFlt-1/PlGF reached high accuracy in the prediction and diagnosis of early PE, where delivery would be required before 34 weeks (<34 wks gestation), but were less accurate in predicting late cases of PE [13]. In addition, we showed the added accuracy offered when we combined the levels of the said biomarkers with the level of maternal serum Inhibin A, especially in the diagnosis of PE developed around term [14]. The accuracy was estimated using the area under the curve (AUC) of the receiver operating characteristic (ROC) curves, from which we extracted the detection rates (DR) and false positive rates (FPR) using continuous and cut-off models [1,15,19].
Prior cardiovascular disorders (CVD) were found to contribute to the development of PE [21]. Although the etiology of PE remained an enigma, impaired placentation and inappropriate remodeling and expansion of the uterine arteries are considered as important causality as they are accompanied by reduced supply of nutrients and oxygen to the placenta especially in early cases of the disorder [22,23]. One measure for the narrower arteries supplying blood to the pregnancy is the increased uterine arteries pulsatility index (UtA PI) for the blood flow through the uterine arteries that is measured by abdominal Doppler sonography [24,25]. Other features of blood vessel changes include increased blood vessel stiffness [26] and insufficiency of the endothelium layer [27], that are contributing to reduced oxygen and nutrient supply to the pregnancy.
Fetal growth restriction (FGR) is often accompanied by PE, creating a combined complication (PE + FGR). The FGR can also be developed without PE symptoms. Typical features of FGR combined with PE are reduced blood circulation to the fetus without hypertension, that can be assessed as a reduced impedance of the fetal middle cerebral artery (MCA), increased umbilical cord artery’s PI, and reduced fetal biometry as the fetus does not grow to its expected biological potential in utero [28]. The newborn has fetal distress, low birthweight, and an un-optimized APGAR score at delivery. The FGR could also be developed due to genetic abnormalities alone or in conjunction with placental insufficiency [28,29,30]. Pure FGR as well as FGR coupled with PE, especially the early cases, are often correlated with impaired cognitive and motor disabilities of the newborn, and long-term developmental disorders [30].
The distrust in using blood pressure measurements for PE prediction and diagnosis is probably derived from using old manometers or uncalibrated automated devices [1,2,3,4,5]. Subsequently, the Fetal Medicine Foundation (FMF) has introduced rigorous guidelines for blood pressure measurements based on the use of arm adjusted cuffs placed on both arms and the double measurements from both arms, 20 min apart, after pre-calibrating the measuring devices. These are followed by calculating the mean arterial blood pressure as a better measure than the use of diastolic and/or systolic values [31]. The values of MAP and uterine artery PI (UtA PI, also measured according to the FMF guidelines [24,25]) are widely used in the prediction and diagnosis of PE, FGR, and PE + FGR.
Endo PAT is an additional tool to evaluate biophysical features [32]. We and others have previously showed the efficacy of using this device in evaluating the risk to develop hypertension disorders in pregnancy, as was previously applied to monitoring cardiovascular diseases (CVDs). The device enables the evaluation of the peripheral arteries’ tone (PAT) [33,34,35,36,37], and the assessment of the impaired function of the endothelial layer of the blood vessels. Measures involve the determination of fingertips hyperemia index (RHI) elicited by arterial pulse after a short occlusion of the brachial artery, and the comparison of responses between the occluded and free arms. Arterial stiffness is measured from the response to PAT signal that augment the blood pressure over the peripheral vessels walls and enables calculation of the augmentation index (AIX) of peripheral vascular resistance [37].
Our aim in this study was to evaluate the accuracy of each biophysical marker on its own and in combination for the prediction of PE, FGR and PE + FGR near delivery to direct clinical decision making in managing the patients admitting to the hospital delivery clinic near labor with the suspected complication.
While such studies have already been performed for large cohorts and medical centers, here we conducted the study and analysis in a relatively small medical center with restricted staff time and resources. Our study aimed to evaluate how the biophysical markers could be best utilized to assist in reaching clinical excellence despite limited resources and personnel.
The biophysical markers used were divided into two groups: those we routinely use, including mean arterial blood pressure (MAP) and uterine artery pulsatility index (PI), and two additional markers generated using a relatively new tool, the Endo PA, the AIX (%) and RHI. This analysis could help verify the added value of the new tools compared to the routinely available ones.
The performance of each of these markers on its own was evaluated using standard tools such as Box and Whisker plots to identify means, medians, and interquartile distribution, and Receiver Operation Characteristic (ROC) curve analysis to assess the detection rate (DR) and false positive rate (FPR), as well as to extract the positive (PPV) and negative (NPV) predictive value for each complication group.
These standard tools provide accuracy to the use of markers to direct clinical work as has been shown elsewhere [15,19]. These were calculated for each clinical group as a whole and for early cases before 34 wks gestation, to separate their future necessity for clinical management.
Markers were evaluated either singly, or in combined analyses of two, three and four markers, in the context of early and all cases. This way we could verify whether such combined analysis was adding accuracy to the clinical work up.
A multiple regression model was subsequently developed to evaluate the Odds Ratio of the prediction accuracy to create a tool to support differential diagnosis.
Finally, the performance of the biophysical markers was compared to the performance of the angiogenic markers as previously published by us (11–14) for this cohort. We did this to assess the apparent advantages for a relatively small clinical setting like ours, and to help clinical sites like ours to choose the most suitable methods of testing (according to available resource and staff expertise) to reach clinical decision, and whether one should prefer biophysical vs. biochemical tests to maintain high standards of clinical care.
2. Sample and Methods
2.1. Sample
Our dataset for the secondary analysis was previously described elsewhere [11,12,13,14] based on data collected between 2012 and 2015 as ethically approved (No. 104/04/12) by the National Medical Ethics Committee of the Republic of Slovenia. The dataset included patient medical records from the outpatient high-risk clinics at the Department of Perinatology of the University Medical Centre of Ljubljana, Slovenia. Women with the suspected PE, FGR and PE + FGR complications, or with a history of these complications, were enrolled after signing off their informed consent. No patient was in labor at the time of enrolment. Included, were women aged 18 years and above who were pregnant with a singleton fetus for at least 24 weeks. Women who had a twin demise to a singleton, major fetal anomalies, pre-existing renal, hematological, autoimmune conditions, or chorioamnionitis, were excluded along with those with mental disorders jeopardizing the reliability of their informed consent.
Data extracted included maternal age, weight and height, parity, years of education, medical and pregnancy history, and mode of conception. All patients were Caucasian. The biochemical and biophysical markers were recorded at enrolment as detailed below and a full dataset was obtained for each patient as was previously described [11,12,13,14].
What was known and what is new in the approach taken in this study?
This is a secondary analysis of the dataset of a cohort that has been partially used before [11,12,13,14]. Same patients were previously analyzed, and their basic characteristic were similar. However, the purpose of that study was different. In reference [12] the same patients were used but we extracted the values of their means, medians, and inter-quartiles to establish the values of nine different biochemical and biophysical markers, each found to provide different accuracy. The aim of that study was to establish a differential marker profile to assist in the clinical management of PE, FGR, PE + FGR, and PTD. Among other things, the study showed that it was hard to differentiate between PE, FGR, and PE + FGR according to the angiogenic markers alone, and that the Endo-PAT markers were important for separating PE from pure FGR cases. It indicated the importance of the Endo-PAT markers in developing a differential diagnosis of PE and FGR and of the early PE and FGR versus unrelated cases of PTD.
Reference [13] analyzed in depth the pro- and anti-angiogenic markers. In addition to Box and Whisker plots, it also included ROC curves of each marker and their combination for the prediction of PE, FGR and PE + FGR. The study indicated that out of all the pro- and anti-angiogenic markers (PlGF, sFlt-1, their ratio, and sEndoglin), PlGF was the best marker for FGR, and reached diagnostic accuracy for the cases developed before 34 weeks of gestation, whereas the ratio of sFlt-1/PlGF reached this accuracy for the PE cases.
Reference [14] focused on the assessment of the add value of Inhibin-A, and how it could add to the accuracy of prediction by the pro-and-anti-angiogenesis markers. It showed that when inhibin-A was combined with PlGF, it offered a similar accuracy to the prediction obtained combining PlGF with the ratio of sFlt-1/PlGF.
Reference [11] was an earlier study, and not all cases that are now available in the cohort were included. Among all markers that are now available in the cohort, it analyzed the pro- and anti-angiogenesis markers and the Endo PAT markers. And showed their value in the prediction of all cases of PE.
Here we performed an in-depth analysis of the biophysical markers, and in certain points we draw comparison to the performance of the pro- and anti-angiogenesis markers.
2.2. Immunodiagnostic Test of Angiogenic Markers
Details of the measurement of placental growth factor (PlGF) and soluble FMS-Like tyrosine kinase (sFlt-1) have been provided before and the same dataset was used here [11,12,13,14].
2.3. Biophysical Markers
Data for all biophysical markers were extracted from the database.
2.3.1. Blood Pressure
Blood pressure was measured according to the guidelines of the Fetal Medicine Foundation (FMF) from both arms with arm adjusted cuffs, using pre-calibrated automated device (OMRON M6 Comfort, Omron Healthcare Co., Ltd., Kyoto, Japan). We measured the diastolic and systolic blood pressure twice, 20 min apart, and calculated the mean arterial blood pressure (MAP), according to (systolic + diastolic * 2)/3 [31].
2.3.2. Endo PAT
Measurements by the Endo PAT 2000 device (Itamar Medical, Caesarea, Israel) were conducted at enrolment according to the manufacturer’s instructions. In a supine position in a quiet, temperature-controlled room (21–24 °C), the measurements were made after a 15-min rest to ensure a steady state of a relaxed cardiovascular function. Patients with untangled legs were asked to remove jewelry and remain still and silent throughout the test.
Measuring the hyperemia index (RHI)—A plethysmography probe was placed on the index finger of each hand., and after brachial artery occlusion, blood pressure was measured following a 5-min pause and the value was corrected for the systemic changes in the vascular tone measured simultaneously from the un-occluded arm. After 10 min, measurement of RHI was made, after which the cuff was inflated 60 mmHg above systolic blood pressure and no less than 200 mm Hg, and a transient ischemia was provoked by up to 300 mmHg for exactly 5 min. The release of the cuff led to increased blood flow that caused an endothelium-dependent dilation of the vascular bed recorded continuously for 5–10 min [37]. The ratio of the post-(O21) to pre-(O11) occlusion PAT amplitude of the tested arm divided by the post (O22) to pre-occlusion (O12) ratio of the control arm was used to calculate the RHI. Values were issued by the Endo PAT software.
Measuring the augmentation index (AIX (%))—The peripheral vascular resistance was estimated using the AIX % of arterial stiffness. A pulse-waveform was expressed as a percentage of the relative value of the difference between the late systolic peaks of the waveform (P2) and the early value (P1) divided by the early pick (P1). The Endo PAT software used values below 2, which were defined as increased arterial stiffness between −10% and 10% [38].
2.3.3. Ultrasound
For ultrasound analysis, we used a GE Voluson U6 and GE Voluson 8Expert with 2–7 MHz GE RAB6-D probe (GE Healthcare GmbH, Solingen, Germany). Pregnancy dating was included in the first trimester records extracted for each patient from which gestational age was determined according to the last menstrual period and the sonographic measurements of the fetal crown rump length [39]. Fetal biometry was determined at admission according to the femur length and the fetal head and abdominal circumference (guidelines of the International Society of Ultrasound in Obstetrics and Gynecology, ISUOG, [28]). Hadlock’s formula was used to estimate fetal weight (EFW) [40].
A Doppler transducer placed on the mother’s abdomen was used to measure the uterine artery pulsatility index (UtA Doppler PI) at a sagittal cervical section view. The transducer was tilted from side to side to identify the uterine arteries at the level of the internal os. The A pulsed Doppler sampling gate of 2 mm was used to cover each vessel, and an angle of insonation < 30 with peak systolic velocity of >60 cm/s was used to obtain the necessary waveforms before calculating the average of the pulsatility index in the left and right uterine arteries [24,25,28].
2.4. Clinical Definition of the Study Groups
Preeclampsia (PE)—In this paper we used the updated criteria for the definition of preeclampsia as published by the ACOG [9]. Preeclampsia was defined as systolic blood pressure of 140 mm Hg or more or diastolic blood pressure of 90 mm Hg or more on two occasions at least 4 h apart after 20 weeks of gestation in a woman with a previously normal blood pressure. Severe PE was defined according to systolic blood pressure of 160 mm Hg or more or diastolic blood pressure of 110 mm Hg or more. For the latter, the blood pressure was confirmed within a shorter interval (minute) to facilitate timely management. The new onset proteinuria was defined as 300 mg or more per 24 h urine collection (or this amount extrapolated from a timed collection) or protein/creatinine ratio of 0.3 mg/dL or more or dipstick reading of 2+ (used only if other quantitative methods was not available). In the absence of proteinuria, the definition included new-onset hypertension with any of the following: (a) thrombocytopenia defined as platelet count less than 100 × 109/L; (b) renal insufficiency determined as serum creatinine concentrations greater than 1.1 mg/dL or (c) a doubling of the serum creatinine concentration in the absence of other renal disease, (d) an impaired liver function: defined as elevated blood concentrations of liver transaminases to twice normal concentration. Other symptoms included pulmonary edema, new-onset headache unresponsive to medication and not accounted for by alternative diagnoses or visual symptoms [7,8,9,10].
References [9,10] included an updated definition of PE by the ACOG [9] and by the International Society for the Study of Hypertension Disorders in Pregnancy (ISSHP) [10] that were published after all patients had delivered, and thus, each patient was re-evaluated according to these guidelines [9] to confirm the clinical definitions, and luckily no discrepancy was determined compared to the former hospital guidelines.
Fetal growth restriction (FGR) was defined according to the ISUOG criteria as fetal weight estimated by biometric evaluation with ultrasound to be at the 10-precentile lower growth scale and abnormal blood flow patterns demonstrated by Doppler ultrasound in the uterine, umbilical, or middle cerebral arteries [28].
Preterm delivery (PTD)—was defined as delivery < 37 weeks gestation due to short cervix, after spontaneous onset of contraction, spontaneous preterm pre labor rupture of membranes (PPROM) but not due to any of PE, FGR or PE + FGR, fetal abnormalities or chorioamnionitis [41,42].
2.5. Statistical Analyses
Comparison between the outcome groups was performed by Mann–Whitney non-parametric U test for continuous variables presented as median with 95% Confidence Interval (95% CI). Values of each group were compared to the unaffected term delivery control group (all cases) and the early complication cases (<34 weeks) were compared to the group who delivered <34 not due to PE, FGR or PE + FGR. A Kruskal–Wallis analysis was performed for multiple groups following Bonferroni post hoc corrections for multiple comparisons, and Mann–Whitney comparisons were made for group pairs. Chi-square tests were used for categorical values presented as n (%).
Box and Whisker plots were depicted for the marker medians and quartile distributions. The areas under the curve (AUC) were calculated under the receiver operating characteristic (ROC) curves with 95% CI prepared from the detection rate (DR) at 10% false positive rate (10%FPR). True cases were subdivided by all cases to obtain the positive predictive value PPV. All true negative cases were divided by all cases to calculate the negative predictive value (NPV). Combined analysis was performed by combining percentiles of individual marker values for each FPR.
Multiple regression was calculated with this function as it incorporated in the SPSS software. The non-parametric Spearman’s rank correlation coefficient or Pearson correlation were used to evaluate statistical dependence between the rankings of two variables, depending on whether regressions were linear (Pearson) or non-linear (Spearman’s).
All the calculation and the analysis were performed using the SPSS software, version 28.0 (IBM) with a statistical significance threshold of p < 0.05.
3. Results
3.1. Cohort Characteristics
As our previous secondary analysis showed, our dataset included 125 patients: 31 PE cases (10 < 34 wks of gestation), 16 FGR (12 < 34 wks of gestation), 42 PE + FGR (28 < 34 wks of gestation), and 15 PTD < 37 wks of gestation (6 < 34 wks of gestation).
Patients were enrolled as they attended the delivery and high-risk clinics. Among singleton pregnancies, in Slovenia, PE (with and without FGR) has higher prevalence than FGR, and accounts for 2–3% of all patients, whereas FGR is less common and accounts for 0.7–1.2% of the deliveries, predicting a ratio of 1:3–1:4.3 in the number of anticipated cases. Thus, it was not surprising that we had 16 FGR cases compared to 73 PE (with and without FGR, 42 + 31), a ratio of 1:4.5. In this respect, the study represented a population of 2400–3650 patients (approximately 20% of all deliveries in Slovenia).
In terms of basic characteristics, most of the women were in their early thirties, they conceived spontaneously, parity was ~1.5 across all groups, and the median gestational age at enrolment was 31–32 weeks. The BMI was higher in the PE and PE + FGR groups. The gestational week at delivery (GA) for all cases was three weeks earlier for the complication groups vs. the unaffected one. In the early groups delivered <34 wks of gestation, no difference in gestational week at delivery was found. Delivery by cesarean section was significantly different in the PE, FGR and PE + FGR groups (Table 1, Delivery results). Significant reduction in baby birth weights were also recorded for the FGR and the FGR + PE groups (Table 1).

Table 1.
Enrolment and delivery characteristics and biophysical Markers.
3.2. Biophysical Marker, Medians and Inter Quartiles for the Outcome Groups
A Box and Whisker plot was used to display the data to evaluate the performance of the biophysical markers in predicting the risk to develop PE, FGR and PE + FGR. A cut-off of UTPI = 0.85, MAP = 98, RHI = 1.6 and AIX = 4.0 was derived from ours and other publications as an initial tool to estimate marker accuracy in the prediction (Figure 1).
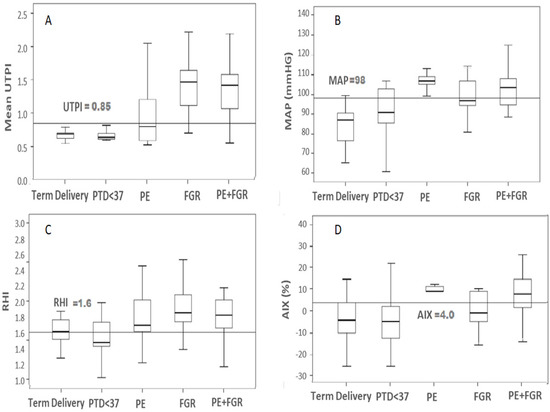
Figure 1.
Box and Whisker plots comparing values for the Biophysical Markers by case type. The figure shows median and interquartile spread of the biophysical markers across all cases in each complication group. (A) Uterine Artery Pulsatility Index (UTPI), (B) Mean Arterial Blood Pressure (MAP), (C) Reactive Hyperemia Index (RHI), (D) Percent Augmentation Index of tonometry (AIX). PE (n = 31), FGR (n = 16) and PE + FGR (n = 42). Cut-offs marked for each biophysical marker are placed as a horizontal line.
Figure 1 shows that for the marker UtA Doppler PI, the cut-off separates cases of FGR and PE + FGR from the control. Also, MAP on its own works quite well for PE, while AIX appears useful for PE and less for PE + FGR but not to FGR, and RHI was good for characterizing any of PE, FGR and PE + FGR. Yet, for each complication group, there were many values that were beyond the cut-off and for each complication group, a single marker was insufficient to generate an accurate prediction (Figure 1, Table 1). Accurate prediction of any of the complication required data of all four biomarkers (UtA Doppler PI, MAP, vascular stiffness (AIX), and endothelial insufficiency (RHI).
Mean Arterial blood pressure (MAP) was significantly higher for the group of All PE (106 [95% CI 102–110]) and the group of all PE + FGR (102 [95% CI 95–108]) compared to the term control (85 [80–90], p ≤ 0.05) (Table 1). At the MAP cut-off = 98, 95% of cases that delivered at term without complications, and 90% of PTD < 37 weeks of gestation had MAP < 90 mm Hg, whereas all the PE cases, and most of the cases of PE + FGR were above the cut-off, providing very good separation between the cases and the control (Figure 1).
The UtA Doppler PI values were significantly higher (p < 0.01) in the FGR (1.35 [1.05–1.66]) and FGR + PE groups (1.42 [1.25–1.56]) compared to term delivery (0.68 [0.66–0.70]). At a UtA Doppler PI cut-off = 0.85, all cases of unaffected term delivery control and PTD < 37 weeks were below the cut-off, whereas >95% of all cases of FGR and of PE + FGR were above it (Figure 1)
For the Endo PAT markers, the arterial stiffness (AIX) was significantly higher for PE and PE + FGR, while the endothelial dysfunction marker RHI was significantly higher in FGR and FGR + PE (Table 1). The cut-off for the stiffness marker (AIX) was 4.0 and all cases of PE were above it, while for the groups of FGR and PE + FGR, the division of cases above and below it was less significant. The value of RHI = 1.6 places >90% of the cases in all three groups above the cut-off (Figure 1).
The sub-groups of PE +FGR who delivered <34 wks of gestation had values of UtA Doppler PI > 1.35 and MAP > 100 mm Hg corresponding to the upper scale of values (lower part of Table 1), indicating a derivation of placental insufficiency and reduced blood supply to the uterus, and the pregnancy linked to hypertension. Late cases may be derived of other causes.
3.3. Spearman’s Coefficient of the Biophysical Markers against Gestational Week and Body Mass Index
Biophysical markers are often standardized against various confounders, mainly the gestational week (GA) and body mass index (BMI) and values ae often converted to multiple of the medians (MoM). Our cohort was not large enough for obtaining such conversion, but we used Spearman’s coefficient analysis to evaluate the impact of these confounders on the biophysical markers, comparing the correlation of each clinical group with the unaffected control combined with the PTD group.
3.3.1. Gestational Week (GA)
The UtA Doppler PI—The analysis revealed that when all cases in each of the clinical complication groups were analyzed, values of this marker decrease with GA, indicating that for the earlier occurring complications, UtA Doppler PI is a better predictor (Table 2, Figure 2). The marker is particularly efficient with FGR (r = −0.811, p < 0.001), and with FGR + PE (r = −0.41, p < 0.05).

Table 2.
Spearman’s correlation coefficient (r) between biophysical markers and patients.
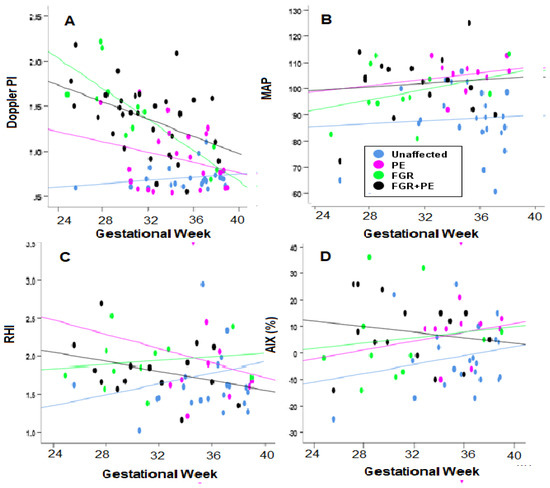
Figure 2.
Evaluating the Correlation of Biophysical Markers over Gestational Age. (A,B) UtA PI—Uterine artery pulsatility index (Doppler PI in the figure) and MAP; (C,D) RHI and AIX. Blue—unaffected group, purple—PE, green—FGR, and black—PE + FGR.
The MAP—In the case of this marker, it was poorly correlated with gestational week (Table 2, Figure 2).
Endo-PAT Markers- Arterial Stiffness (AIX) was increased with GA in the group of All PE (r = 0.485, p < 0.05) and All FGR (r = 0.305, p < 0.05) groups, indicating it was a better marker for the late cases of these groups. AIX had negligible correlation with GA for the group of All PE + FGR. Endothelial insufficiency (RHI) had poor correlation with GA.
3.3.2. Body Mass Index (BMI)
The UtA Doppler PI—This marker was poorly correlated with BMI for the groups of All FGR and FGR + PE, but significantly increased with BMI (r = 0.367, p < 0.01).
The MAP—This marker was highly correlated with BMI for the groups PE (r = −0.487, p < 0.05) and FGR (r = −0.392, p < 0.05), indicating that the severity was a better predictor among non-obese women (Table 2, Figure 3).
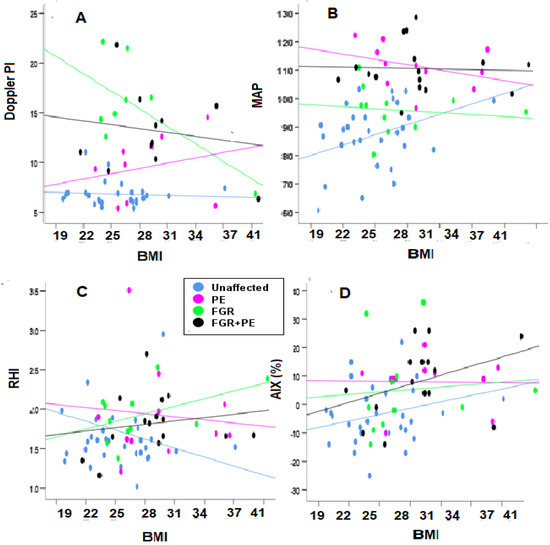
Figure 3.
Evaluating the correlation of biophysical markers over BMI. (A,B) Mean UtA Doppler PI (Doppler PI in the figure) and MAP; (C,D) RHI and AIX%. Blue—unaffected group, purple—PE, green—FGR, and black—PE + FGR.
3.3.3. Angiogenic Markers
For the groups of All PE, All FGR and All PE + FGR, the correlations of UtA Doppler PI with PlGF had Spearman’s coefficients of r = −0.354, 0.317, and −0.484, respectively (p < 0.05 for each), indicating that the values of UtA Doppler PI increase with PlGF for the group of All FGR and decrease for the other two.
A similar analysis for the Sperman’s correlation of UtA Doppler PI with the ratio of sFlt-1/PlGF generated r = −0.367 and r = 0.550 (p < 0.01, for each) for the group of All GFR and All FGR + PE, respectively, indicating that the ratio decreased against UtA Doppler PI for the first and increased for the other (Table 2).
Figure 4 and Table 2 shows that for the subgroups delivered < 34 wks gestation, if all cases are combined, the values of UtA Doppler PI decreased with PlGF, with a Sperman’s correlation r = −0.328 indicated that the larger the value of UtA Doppler PI, the lower was that of PlGF.
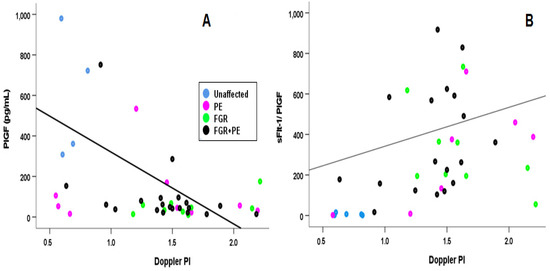
Figure 4.
Correlation of the UtA Doppler PI with the Angiogenic Marker. A Spearman’s correlation for between UTA Doppler PI (Doppler PI in the Figure) and (A) PlGF and (B) sFlt-1/PlGF ratio. Blue—unaffected group, purple—PE, green—FGR, and black—PE + FGR.
For the same analysis of the early cases, the ratio of sFlt-1/PlGF increased with increased values of UtA Doppler PI, both considered indications of the severity of the complication. The calculated Spearman’s correlation for this ratio was −0.497 (p < 0.001).
The correlations between the angiogenic markers and all the other biophysical markers were negligible.
3.4. AUC Analysis
We evaluated the accuracy of each biophysical marker using the area under the curve (AUCs) for the receiver operation characteristic (ROC) curves. The detection rate (DR, Sensitivity) over the False Positive rate (FPR, Specificity) were depicted for each biophysical marker evaluating a value of FPR = 10% to estimate the accuracy.
As shown in Table 3 and in Figure 5, very poor accuracy was found for any of the individual markers when evaluated for the groups of All cases. The exceptions were for MAP and PI that performed well in the group of All PE, and for UtA Doppler PI concerning the groups of All FGR and All PE + FGR. It was found that the AUCs were >0.9 and the DRs for 10% FPR were >80% (Figure 5, Table 3). All other markers performed less accurately when examined individually against all cases in each of the complication groups, except the Endo PAT marker RHI, that reached a DR = 80% at 15% FPR for All PE cases.

Table 3.
Accuracy of Receiver Operation Characteristic Curves (ROC) for Single Markers in Each Complication Group.
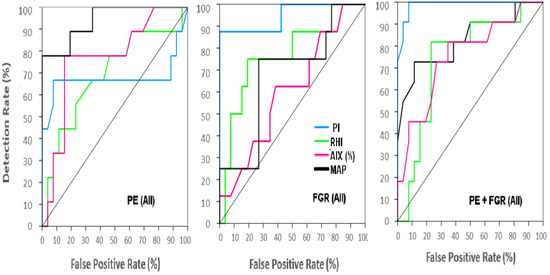
Figure 5.
ROC for single biophysical markers for all patients in each complication groups. A continuous model was used to depict the ROC curves and evaluate the detection rate (DR) and the False Positive Rate (FPR). (Left)—All cases of preeclampsia (PE), (Middle)—All cases of Fetal Growth Restriction (FGR), (Right)—All cases of PE + FGR. Turquoise—UtA Doppler PI, Green—MAP, Purple—AIX (%), Green—RHI, Black—MAP. The area below the line crossing from the bottom left to the upper right corner for each ROC curve represent AUC = 0.5 of a randomized accuracy.
Results of a detailed ROC analysis are depicted in Table 3, where we compared the results with the continuous model versus the cut-off models for each individual marker. It appears that usually the continuous model yielded a little higher DR for a 10% FPR although the differences were not large (Table 3).
For the early cases (before gestational week 34), elevated values of MAP and of UtA Doppler PI provided a diagnostic accuracy for PE and FGR, respectively (AUC = 1.00, DR = 100%) (Table 3).
When the positive and the negative predicted values (PPV and NPV) were evaluated according to the cut-off model (Table 3), for all cases of any of the complications, very high NPV was found, and these values were consistent with those currently used in Germany and other places to send patients with the suspected pathology home for subsequent ambulatory evaluation, as the complications were unlikely to require delivery in the next few days [2,11].
For the early cases (before 34 weeks of gestations), the UtA Doppler PI yielded a detection rate of 100% and AUCs were near 1.0. In addition, the PPV were also 1.0, corresponding with the diagnostic accuracy yielded by this marker.
Combined Biophysical Marker Analysis
We also conducted a combined analysis as shown in Table 4 and Figure 6. For the group of all cases of PE + FGR, diagnostic accuracy (AUC = 1.00 and DR = 100%) was reached when the MAP and UtA Doppler PI were combined, and the other markers added no additional effect (Table 4).

Table 4.
Combined analyses of the biophysical markers.
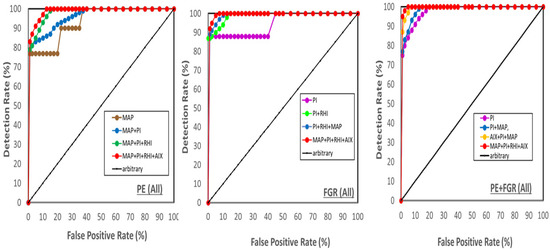
Figure 6.
Receiver Operation Characteristic (ROC) curves. ROCs of the area under the curve (AUC) depicted from the detection rate (DR) and the False Positive False Positive Rate (FPR) for All cases of Preeclampsia (PE), FGR, and PE + FGR. (Left)—PE, (Middle)—FGR, (right) PE + FGR.
For each of the groups of All cases of PE and FGR, there was a need to combine all four markers to reach near diagnostic accuracy (Table 4, Figure 6). For the group of All PE + FGR, elevated values of MAP and UtA Doppler PI already reached AUC = 0.98 (Table 4) but it was necessary to combine all markers to reach a DR = 95% (Table 4, Figure 6).
For the group of All PE (Figure 6 left), the serial combined analysis was made of mean arterial blood pressure (MAP), MAP combined with UtA Doppler PI (PI in the figures for the sake of keeping the index short), the two combined with RHI, and the three combined with AIX. For the group of All FGR (Figure 6 middle), the combination was made from UtA Doppler PI alone, UtA Doppler PI combined with RHI, the two combined with MAP, and the three combined with AIX. For group of All PE + FGR (Figure 6 right), the analysis was built of UtA Doppler PI alone, UtA Doppler PI + MAP, the two combined with AIX, and the three combined with RHI. For each complication, the order of marker combination depicted in Figure 6 presented the best fit. The dark line from DR and FPR = 0 to DR and FPR = 100, is the arbitrary line of AUC = 0.5. There were 31 cases of PE, 16 FGR cases and 42 cases of PE + FGR.
3.5. Multiple Regression
Multiple regression analysis was conducted with the biophysical parameters for all cases of the complications (tope part of Table 5) and also for the early cases alone (lower part of Table 5).

Table 5.
Multiple regression model.
3.5.1. All Cases
The equations for each marker made using all participants versus PE, FGR and PE + FGR were as follows:
- MAP = 92.82 − 0.41 × MA + 0.20 × GA + 19.31 × PE + 12.63 × FGR +17.33 × (FGR + PE)
- UtA PI = 2.42 + 0.01 × MA − 0.05 × GA + 0.17 × PE + 0.47 × FGR + 0.44 × (FGR + PE)
- RHI = 1.51 + 0.01 × MA − 0.01 × GA + 0.19 × PE + 0.19 × FGR + 0.05 × (FGR + PE)
- AIX = 2.36 − 0.19 × MA + 0.01 × GA + 11.58 × PE + 8.71 × FGR + 12.19 × (FGR + PE)
The upper part of Table 5 shows the values of the multiple regression for each biophysical marker for the groups of All cases.
MAP were R2 = 0.36, F(6,63) = 7.19 (p < 0.001), and standardized coefficients (β) of 0.53, 0.35 and 0.55 for any of PE, FGR and PE + FGR, respectively, p < 0.001 for each.
For UtA Doppler PI (UtA PI), the values were R2 = 0.57, F= 24.74 p < 0.001, and β’s of 0.16 (p < 0.05) for PE, and 0.33 and 0.44 (p < 0.001) for FGR and FGR + PE, respectively.
For AIX, R2 was 0.17, F = 2.53 and p < 005, and the values of the β’s were 0.32 and 0.39 (p < 0.001) for PE and PE + FGR, respectively but insignificant for FGR alone.
The results for RHI were poor: R2 = 0.03 and F = 0.39 with all β’s non-significant for any of the complications. (Bottom of all cases, Table 5).
3.5.2. Early Cases (<34 wks of Gestation) (Equations Not Shown)
The lower part of Table 5 shows the multiple regression statistics for the early cases as follows:
- MAP: R2 = 0.46, F(6,63)= 6.16 (p < 0.001), β’s = 0.49, 0.29 and 0.38 for PE, FGR and PE + FGR, respectively, p < 0.001 for any.
- UtA Doppler PI: R2 = 0.45, F = 7.29, p < 0.001, β’s = 0.62 for PE + FGR, and non-significant for other complications. (p < 0.001).
- AIX: R2 = 0.27, F = 2.63 and p < 0.05, and β = 0.38 and 0.35 for PE and PE + FGR (p < 0.001) but insignificant for FGR alone.
- RHI: R2 = 0.1703, F = 1.52 and all β’s were non-significant for any of the complications.
4. Discussion
The aims of this study were to conduct a secondary analysis of our cohort data to evaluate a set of biophysical markers measured at the time of suspected complications and evaluate how accurately they were in the prediction and the diagnosis of PE, FGR and PE + FGR near delivery. If such were to be successful, it may assist in directing clinical management. In previous studies [13,14], such evaluation involved mainly biochemical markers. We have estimated that having data for both types of markers could help in establishing our internal guidelines for clinical management of PE and FGR according to test availability and accuracy.
Our main findings were as follows: (a) In cases that required delivery < 34 weeks, high values of MAP and of UtA Doppler PI appeared each extremely accurate in the diagnosis of PE (MAP > 110) or FGR (UtA Doppler PI > 1.35), respectively, with AUC = 1.00 and DR = 100% at 10% FPR (for each). (b) In each of these cases the high values of MAP and UtA Doppler PI provided results that were near diagnostic accuracy for the group of PE + FGR developed before 34 wks gestation and reached diagnostic accuracy when they were combined. The underlying pathology was most likely placental insufficiency and reduced blood supply to the placenta and the fetus, leading to shortage of oxygen, and those should be managed according to the protocol for PE or FGR or both [23,24,27]. (c) To reach high accuracy in clinical diagnosis (and management) of late complications, it was necessary to have data for multiple markers, reflecting the potential involvement of different parameters such as systemic arterial stiffness and endothelial insufficiency that in this study were provided using the Endo PAT.
One needs to keep in mind that we were dealing with multi-factorial syndromes, with many unknowns in their etiology. A recent publication by Erez et al. [43] reviewed the broad additional pathways underlying the risk to develop PE with and without FGR. Factors like poor nutrition, obesity, inappropriate diet composition (such as shortage of calcium or anti-oxidants), genetics, cardio-vascular disorders or other confounders were listed along additional contributors [43]. Practically, it imposed the use of complicated testing protocols for reaching accurate prediction and diagnosis when late complications, which are most of the cases, are to be evaluated [1,2,3,4,5,6,7,8,9,10,15,16,17,18,19,20,21].
The MAP—The accuracy of blood pressure measurement (systolic, diastolic, or MAP) as a diagnosis measure of PE has been challenged frequently [1,2,3,4,5,44]. Difficulties in obtaining correct blood pressure values may have been derived from using devices that were not properly calibrated, the lack of cuff size adjustment to arm size, or other low precision steps while handling the measurements [31,44]. Here, where we adhered to a strict application of the FMF guidelines for blood pressure measurements, the problem was diminished and provided us with the good prediction accuracy in the cases of PE and PE + FGR requiring delivery <34 wks gestation. The MAP was found in our study to be a very good marker for predicting hypertension disorders. In the ASPRE and SPREE studies, members of our team have found that such early hypertension disorders of pregnancy associated with preterm delivery can be used to screen for and prevent the development of PE by the daily use of aspirin, starting from gestational week 12 and lasting until 36 weeks [45,46]. Aspirin causes reversible endothelium-dependent vasodilation of resistance arteries in pregnancy [47], and thus the repeated daily administration of aspirin may be required to renew the daily expansion of the uterine arteries [45,46,47]. The selection of cases to be treated with aspirin to prevent PE is based on the introduction of first trimester screening to identify patients at high risk of developing PE [45,46,48,49,50]. If aspirin treatment is not introduced and in the cases that the prophylactic use of aspirin is ineffective, additional frontier of risk assessment near delivery is offered by the use of pro-and anti-angiogenic markers as others have already demonstrated [1,2,3,4,5,6,7,15,16,17,18,19], and we have confirmed in this cohort [11,12,13,14]. The near-delivery assessment of cases with suspected complications enabled improved management of PE and of FGR, and offered the proper selection for the time of delivery.
In this study, we found that for All cases of PE, measuring MAP alone was indeed an insufficient measure, and multiple markers (MAP, UtA Doppler PI, RHI and AIX) were required to reach accuracy. The latter two markers indicate that systemic complications, such as endothelial insufficiency and arterial stiffness [37,38], must be taken into consideration when choosing the clinical management. We have previously found that there was an increased accuracy in the differential diagnosis of PE combining peripheral arterial tonometry with MAP (and with angiogenic markers [11]). Interestingly, tonometry and endothelial dysfunction (AIX and RHI) are also known to predict later development of cardiovascular disorders [35,36,37]. Having PE in pregnancy was identified as a major risk factor for developing cardiovascular disorder ten years later [50,51,52]. Hence, the American College of Cardiology has recommended to conduct periodic post pregnancy testing of cardiac function among women with a history of PE for evaluating their risk for developing cardiovascular disorders, given the finding that PE in pregnancy is a major risk for morbidity and mortality from cardiovascular disorder and 10-years of shortening of life expectancy [27,53,54]. Here, we found that the impaired values of these markers are present not only in the early PE cases but also in the late and term cases. Accordingly, it may be important to include all women who have developed PE in pregnancy and not only the early and preterm cases in the high-risk groups, as recommended by the American College of Cariology.
The uterine artery pulsatility Index (UtA PI) is certainly shown to be a powerful way to predict the risk and to diagnose FGR, particularly in cases of early FGR (<34 wks of gestation), who are in the top 20-percentile of values of UtA Doppler PI [28,29,30]. In these cases, our analysis showed that values of UtA Doppler PI > 1.35 have a diagnostic accuracy (ROC = 1.00, DR = 100%, and PPV and NPV = 100%). The UtA Doppler PIs have also provided us with high accuracy for the prediction of combined PE + FGR pathology, but not for the groups of All cases of PE without FGR, or for the FGR group without PE, mainly for cases near term delivery. In the latter, approximately 20% of the cases have normal values of UtA Doppler PI. For these near-term cases, a bimodal distribution of newborn birth weight was found here, with some cases in the higher range whereas others were in the lower range of the growth scale. This is consistent with findings of others [50,53]. This may explain the poor performance of UtA Doppler PI and of MAP in the prediction of these complications in the groups of All PE and All FGR, that include many near term cases. Our study showed that reaching accurate prediction in these cases necessitates the use of multiple biophysical markers and with an important impact for RHI and AIX.
Reduced PlGF has been broadly found as a useful marker to predict PE in the first and third trimester and near delivery [1,2,3,4,5,6,7,8,9,10,15,16,17,18,19, 45]. In previous publications, we have already shown the accuracy of reduced PlGF in the near delivery diagnosis of early but also of all cases of FGR in this cohort [13,14]. Hence, we now have two very good markers for the accurate prediction of FGR—UtA Doppler PI > 1.35 and PlGF < 150 pg/mL. Each of these two could individually provide accurate diagnosis of early FGR. In this regard, it is remarkably interesting to report that our Spearman’s regression coefficient of UtA Doppler PI over PlGF (r > −0.497, p < 0.001), or over the ratio of sFlt-1/PlGF (r = 0.327, p < 0.05), provided very high correlations. Similar results were reported by Schlembach et al. [55]. These findings highlighted the markers included in the toolbox for FGR prediction and diagnosis. Professionals could now use either UtA Doppler PI or PlGF for their clinical work and choose the marker that is most suitable for their setting according to local availabilities, resources, and professional expertise.
Endothelial insufficiency—The endothelium is estimated to be the larger organ in our body, covering the internal layers of all our blood vessels [56]. It is the source of vascular endothelial growth factors (VEGF), and of placental growth factor (PlGF) [55,57], although there are other tissues (the placenta, the kidney, etc.) that also generate these factors. Here RHI on its own was not a good biophysical marker for predicting the complication of All cases, or even of early PE, or early FGR, or the two combined. However, when added to UtA Doppler PI, the pair offered near diagnostic accuracy for the prediction of these complications.
Arterial Stiffness assessed by AIX—On its own, AIX is mainly higher in the cases requiring delivery <34 wks of gestation. Yet, among the early cases, there were alternative markers as the MAP and UtA PI provided accurate prediction and they are also more routinely used. However, when AIX was used to predict and diagnose all cases of PE, mainly when a large proportion of late cases are included, especially for the groups of PE + FGR near term, it helped improve the accuracy to diagnostic levels, especially in advanced maternal age and in obese women. Perry et al. [26] conducted a longitudinal analysis of arterial stiffness measured from the aorta and showed its increase throughout pregnancy in cases of hypertensive disorders. Our measurements near delivery reflect the worsening of arterial stiffness in cases developed near term. The high AIX was associated with a faster pulse wave that moves away from the heart and then returns earlier [58,59] especially in cases developed at advanced maternal age as we found here. Indeed, high AIX and MAP and a faster heart rate are all indicators of PE, and are linked to an increased vascular resistance, and the findings that a history of PE and high AIX are both risk factors for CVDs later in life show the importance of frequent life monitoring for women with a history of any PE [59].
5. Limitations of the Study
This study had several limitations. The first was the small sample size. It limited the conversion of the markers into MoMs. Yet, we conducted regression analysis of the biophysical markers against gestational age and BMI that may have partially corrected for both the lack of conversion to MoM, and for the fact that we measured marker values at enrolment as they appeared in the clinic, and not in a fixed gestational week. Indeed, we found much larger differences in the marker values between the unaffected and the complication groups in the early cases who delivered <34 weeks gestation and were all enrolled at about the same gestational age than in the cases developed around term, where the range of gestational weeks at measurements were larger. Another limitation was the lack of repeated testing, that in principle helps increase the accuracy of a marker.
Our study was not a screening one, but given that the prevalence of PE in Slovenia (pure PE and PE + FGR combined) accounts for 2–3% of all deliveries, having a cohort of 73 cases of PE (combining 31 cases of only PE and 42 cases of PE + FGR) accounted for an approximate sample size of 2433–3650 pregnant women This corresponded to 15–20% of all deliveries in Slovenia, and around 40% of the deliveries in the medical center of Ljubljana. In this respect, our study had a power of 0.85–0.90. This appears reasonable, given we also included control groups of preterm delivery that were unrelated to PE and FGR (mainly PPROM and spontaneous preterm delivery cases), and had also a group of unaffected cases of term delivery who were used as an unaffected control. Of course, larger studies are warranted.
6. Conclusions
This study shows that for early cases delivering >34 weeks gestation, mean arterial blood pressure (MAP) provides a perfectly accurate measure to predict PE. The Doppler Pulsatility Index of the blood flow through the maternal uterine arteries (UtA-PI) was an excellent measure for predicting early FGR. Combining both markers enabled accurate prediction of PE + FGR. Any of these markers was much less accurate when the evaluated cases were developed near term. In these late cases, adding measures of tonometry and endothelial dysfunctions offered when using the Endo PAT were required for the accurate diagnosis and prediction.
Summarizing all four studies of our cohort [11,12,13,14], it now appears we have a rich toolbox containing biophysical and biochemical markers. The markers PlGF, sFlt-1 and the sFlt-1/PlGF ratio have equal accuracy for the diagnosis and prediction of the early complications [13,14] as was found here for the biophysical markers of UtA Doppler PI and for MAP. Inhibin-1 augments the accuracy of PlGF, and of sFlt-1/PlGF ratio for the late complications [14]. At the same time tonometry and endothelial dysfunction do this job when combined with UtA Doppler PI and MAP. If repeated in larger cohorts, the finding enables medical centers to choose from many tools those that are available and affordable. A different study will be required for comparing the accuracy of these markers against other diagnostic techniques, which was out of the scope of this study.
Author Contributions
This publication reflects the extended cooperation among all the co-authors. J.O., K.K., T.P.S. and N.T. designed the study protocol, and applied for and obtained the ethical approval. T.P.S., V.F.V. and N.T., enrolled the patients in the ObGyn clinics, obtained patient signatures for the informed consent, and conducted all the clinical evaluations and management. K.K., T.F. and J.O. performed the immunodiagnostic tests. Blood pressure, the ultrasound examination and standard blood biochemistry were performed by T.P.S., V.F.V. and N.T. The database was constructed by K.K., A.S.-N. and H.M. The accuracy analysis by Box Plots and ROCs, single and multiple regression analysis and mathematical modeling were carried out by K.K., A.S.-N. and H.M. together with K.H.N. and J.O. Funding was secured by K.H.N., H.M., J.O. and K.K. All the authors were involved in writing the manuscript, its editing, and revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the ASPRE project (EU, FP7 # 601852, H.M., KH.N.), and by the Graduate School of the University Medical Center, Ljubljana, Slovenia (K.K., J.O.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki for Human subjects involved in clinical research, and was approved by The National Medical Ethics Committee of the Republic of Slovenia involved in regulating human subjects’ participation in clinical trials approved the study on 4 December 2012 (Approval No. 104/04/12).
Informed Consent Statement
A written informed consent was obtained from each pregnant woman involved in the study.
Data Availability Statement
Data are available at the websites of Tel Hai College and the University Medical Center, Ljubljana, Slovenia. Due to cyber security issues, links to the websites should be pre-arranged in advanced from A.S.-N. (Tel Hai College) and from K.K. or J.O. (University Medical Center, Ljubljana).
Conflicts of Interest
The authors report no conflict of interest. The authors alone were responsible for the content and writing of the paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| ACOG | American College of Obstetricians and Gynecologists |
| AIX (%) | Augmentation index (percent). |
| APGAR | Newborn score of Appearance (skin color), Pulse (heart rate), Grimace (reflex irritability), Activity (tone), and Respiration |
| AUC- | Area under the c of the receiver operation characteristic curve |
| BMI | Body mass index |
| BP | Blood pressure |
| dBP | diastolic blood pressure |
| DR | Detection rate (sensitivity) |
| FGR | Fetal growth restriction |
| FPR | False positive rate (1-specificity) |
| ISSHP | International Society for the Study of Hypertension Disorders in Pregnancy |
| ISUOG | International Society for Ultrasound in Obstetrics and Gynecology |
| IVF | In-vitro fertilization |
| MAP | Mean arterial blood pressure |
| MCA | Middle cerebral artery |
| MoM | Multiples of the medians |
| NICE | National Institute of Clinical Excellence |
| NPV | Negative predictive value |
| PE | Preeclampsia |
| UtA PI | Uterine artery pulsatility index assessed by Doppler |
| PPV | Positive predictive value |
| PlGF | Placental growth factor |
| PTD | Preterm delivery |
| sFlt-1 | Soluble FMS (oncogene for Feline McDonough Sarcoma) like tyrosine kinase 1 |
References
- Chappell, L.C.; Duckworth, S.; Seed, T.; Griffin, M.; Myers, J.; Mackillop, L.; Simpson, N.; Waugh, J.; Anumba, D.; Kenny, L.C.; et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia—A prospective multicenter study. Circulation 2013, 128, 2121–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlohren, S.; Dröge, L.-A. The diagnostic value of angiogenic and antiangiogenic factors in differential diagnosis of preeclampsia. Am. J. Obstet. Gynecol. 2020, 226, S1048–S1058. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.A.; Woolley, J.J.; Cleary, K.L.; Falzon, L.; Alpert, B.S.; Oparil, S.; Cutter, G.; Wapner, R.; Muntner, P.; Tita, A.T.; et al. Accuracy of Blood Pressure Measurement Devices in Pregnancy. Hypertension 2018, 71, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Mayrink, J.; Souza, R.T.; Feitosa, F.E.; Rocha Filho, E.A.; Leite, D.F.; Vettorazzi, J.; Calderon, I.M.; Costa, M.; Kenny, L.; Baker, P.; et al. Mean arterial blood pressure: Potential predictive tool for preeclampsia in a cohort of healthy nulliparous pregnant women. BMC Pregnancy Childbirth 2019, 19, 460. [Google Scholar] [CrossRef]
- Tagetti, A.; Fava, C. Diagnosis of hypertensive disorders in pregnancy: An update. J. Lab. Precis. Med. 2020, 5, 8. Available online: https://jlpm.amegroups.com/article/view/5239/html (accessed on 20 January 2020). [CrossRef]
- Wojtowicz, A.; Zembala-Szczerb, M.; Babczyk, D.; Kołodziejczyk-Pietruszka, M.; Lewaczynska, O.; Huras, H. Early- and Late-Onset Preeclampsia: A Comprehensive Cohort Study of Laboratory and Clinical Findings according to the New ISHHP Criteria. Int. J. Hypertens. 2019, 2019, 4108271. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2021, 27, 148–169. [Google Scholar] [CrossRef] [PubMed]
- WHO. Managing Complications in Pregnancy and Childbirth. Available online: http:///www.who.int/publications/i/item/9789241565493 (accessed on 1 February 2017).
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy: Diagnosis and Management NICE Guideline [NG133]. Published Date: 25 June 2019. Available online: https://www.nice.org.uk/guidance/ng133/resources/hypertension-in-pregnancy-diagnosis-and-management-pdf-66141717671365 (accessed on 25 June 2019).
- Kumer, K.; Premru-Srsen, T.; Fabjan-Vodušek, V.; Tul, N.; Fabjan, T.; Osredkar, J. Peripheral arterial tonometry and angiogenic biomarkers in preeclampsia. Hypertens. Pregnancy 2018, 37, 197–203. [Google Scholar] [CrossRef]
- Sharabi-Nov, A.; Kumer, K.; Vodušek, V.F.; Sršen, T.P.; Tul, N.; Fabjan, T.; Meiri, H.; Nicolaides, K.H.; Osredkar, J. Establishing a differential marker profile for pregnancy complications near delivery. Fetal Diagn. Ther. 2019, 47, 471–484. [Google Scholar] [CrossRef]
- Kumer, K.; Sharabi-Nov, A.; Fabjan Vodušek, V.; Premru Sršen, T.; Tul, N.; Fabjan, T.; Meiri, H.; Nicolaides, K.H.; Osredkar, J. Pro- and Anti-Angiogenic Markers as Clinical Tools for Suspected Preeclampsia with and without FGR near Delivery—A Secondary Analysis. Reprod. Med. 2021, 2, 12–25. [Google Scholar] [CrossRef]
- Sharabi-Nov, A.; Premru Sršen, T.; Kumer, K.; Fabjan Vodušek, V.; Fabjan, T.; Tul, N.; Meiri, H.; Nicolaides, K.H.; Osredkar, J. Maternal Serum Inhibin-A Augments the Value of Maternal Serum PlGF and of sFlt-1/PlGF Ratio in the Prediction of Preeclampsia and/or FGR Near Delivery—A Secondary Analysis. Reprod. Med. 2021, 2, 35–49. [Google Scholar] [CrossRef]
- Saleh, L.; Vergouwe, Y.; van den Meiracker, A.H.; Verdonk, K.; Russcher, H.; Bremer, H.A.; Versendaal, H.J.; Steegers, E.A.P.; Danser, J.A.H.; Visser, W. Angiogenic markers predict pregnancy complications and prolongation in preeclampsia continuous versus cut-off values. Hypertension 2017, 70, 1025–1033, Epub 28 August 2017. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Zeisler, J.; Llurba, E.; Chantraine, F.; Vatish, M.; Phil, D.; Staff, A.D.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H. Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Schlembach, D.; Hund, M.; Schroer, A.; Wolf, C. Economic assessment of the use of the sFlt-1/PlGF ratio test to predict preeclampsia in Germany. BMC Health Serv. Res. 2018, 18, 603, PMCID: PMC6080558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034, Epub 20 October 2020. PMCID: PMC888416. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Mueller, A.; Salahuddin, S.; Naseem, H.; Dhir, R.; Rana, S. Evaluation of angiogenic factors in the decision to admit women with suspected preeclampsia. Pregnancy Hypertens. 2020, 21, 124–131. [Google Scholar] [CrossRef]
- Thilaganathan, B. Maternal Cardiac Dysfunction Precedes Development of Preeclampsia. Hypertension 2020, 76, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. Fetal-maternal conflict, trophoblast invasion, preeclampsia, and the red queen. Hypertension 2008, 27, 183–196. [Google Scholar] [CrossRef]
- Osol, G.; Ko, N.L.; Mandalà, M. Plasticity of the Maternal Vasculature During Pregnancy. Annu. Rev. Physiol. 2019, 81, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Valiño, N.; Giunta, G.; Gallo, D.M.; Akolekar, R.; Nicolaides, K.H. Biophysical and biochemical markers at 30–34 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet. Gynecol. 2016, 47, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciobanu, A.; Rouvali, A.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of small for gestational age neonates: Screening by maternal factors, fetal biometry, and biomarkers at 35–37 weeks’ gestation. Am. J. Obstet. Gynecol. 2019, 220, 486.e1–486.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, H.; Gutierrez, J.; Binder, J.; Thilaganathan, B.; Khalil, A. Maternal arteria stiffness in hypertensive pregnancies with and without small-for-gestational-age neonate. Ultrasound Obstet. Gynecol. 2020, 56, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2022, 226, S907–S927, Epub 2021 Feb 2. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.; Alfirevic, Z.; Costa, F.D.S.; Deter, R.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG practice guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef]
- Khalil, A.; Gordijn, S.J.; Beune, I.M.; Wynia, K.; Ganzevoort, W.; Figueras, F.; Kingdom, J.; Marlow, N.; Papageorghiou, A.; Sebire, N.; et al. Essential variables for reporting research studies on fetal growth restriction: A Delphi consensus. Ultrasound Obstet. Gynecol. 2019, 53, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Figueras, F.; Gratacos, E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.; Kametas, N.A.; Valencia, C.; Chelemen, T.; Nicolaides, K.H. Hypertensive disorders in pregnancy: Screening by systolic diastolic and mean arterial pressure at 11–13 weeks. Hypertens. Pregnancy 2010, 30, 93–107. [Google Scholar] [CrossRef]
- Hamburg, N.M.; Benjamin, E.J. Assessment of Endothelial Function Using Digital Pulse Amplitude Tonometry. Trends Cardiovasc. Med. 2009, 19, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Moerland, M.; Kales, A.J.; Schrier, L.; van Dongen, M.G.; Bradnock, D.; Burggraaf, J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int. J. Vasc. Med. 2012, 2012, 904141, Epub 14 February 2012. PMCID: PMC3303545. [Google Scholar] [CrossRef] [PubMed]
- Arrebola-Moreno, A.L.; Laclaustra, M.; Kaski, J.C. Non-invasive Assessment of Endothelial Function in Clinical Practice. Rev. Esp. Cardiol. 2012, 65, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Carty, D.M.; Anderson, L.A.; Duncan, C.N.; Baird, D.P.; Rooney, L.K.; Dominiczak, A.F.; Delles, C. Peripheral arterial tone: Assessment of microcirculatory function in pregnancy. J. Hypertens. 2012, 30, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kuvin, J.; Patel, A.; Sliney, K.; Pandian, N.; Sheffy, J. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003, 146, 168–174. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Koo, B.K.; Chung, W.Y.; Moon, M.K. Peripheral arterial endothelial dysfunction predicts future cardiovascular events in diabetic patients with albuminuria: A prospective cohort study. Cardiovasc Diabetol. 2020, 13, 82, PMCID: PMC7293773. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Shah, Y.P.; Kanon, D.J.; Lindsey, J.V. Fetal crown-rump length: Reevaluation of relation to menstrual age (5–18 weeks) with high-resolution real-time US. Radiology 1992, 182, 501–505. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Meertens, L.J.; Van Montfort, P.; Scheepers, H.C.; Van Kuijk, S.M.; Aardenburg, R.; Langenveld, J.; Van Dooren, I.M.; Zwaan, I.M.; Spaanderman, M.E.; Smits, L.J. Prediction models for the risk of spontaneous preterm birth based on maternal characteristics: A systematic review and independent external validation. Acta Obstet. Gynecol. Scand. 2018, 97, 907–920. [Google Scholar] [CrossRef]
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Gallo, D.M.; Gotsch, F. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S786–S803. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, J.S.; Vollebregt, K.C.; de Vrieze, N.; ter Riet, G.; Mol, B.W.; Franx, A.; Khan, K.S.; van der Post, J.A. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: Systematic review and meta-analysis. BMJ 2008, 336, 1117–1120, Epub 2008 May 14. PMCID: PMC2386627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolnik, D.A.; Wright, D.; Poon, L.L.; O’Gorman, N.N.; Syngelaki, A.A.; Matallana, C.C.D.P.; Akolekar, R.R.; Cicero, S.S.; Janga, D.D.; Singh, M.M.; et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Tan, M.Y.; Wright, D.; Syngelaki, A.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Greco, E.; Wright, A.; Maclagan, K.; et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: Results of SPREE. Ultrasound Obstet. Gynecol. 2018, 51, 743–750, Epub 14 March 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helgadottir, H.; Tropea, T.; Gizurarson, S.; Meiri, H.; Mandalà, M. Aspirin causes endothelium-dependent vasodilation of resistance arteries from non-gravid and gravid rats. Pregnancy Hypertens. 2019, 15, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S844–S866. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Romero, R.; Benshalom-Tirosh, N.; Than, N.G.; Gudicha, D.W.; Done, B.; Pacora, P.; Chaiworapongsa, T.; Panaitescu, B.; Tirosh, D.; et al. The prediction of early preeclampsia: Results from a longitudinal proteomics study. PLoS ONE 2019, 14, e0217273, PMCID: PMC6548389. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Malinowski, A.; Zhou, Q.; Yusuf, S.; Devereaux, P.J. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am. Heart J. 2008, 156, 918–930. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, J.J.; Martinez-Garza, L.E.; Sepulveda-Gonzalez, G.; Hernandez-Castro, F.; Gaston-Locsin, T. Serum biomarkers and Doppler pulsatile index increases likelihood ratio for prediction of preeclampsia in the second trimester of pregnancy. J. Obstet. Gynaecol. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, K.; Thilaganathan, B.; Giorgione, V.; Ridder, A.; Memmo, A.; Khalil, A. Hypertensive Disorders of Pregnancy and Future Cardiovascular Health. Front. Cardiovasc. Med. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwala, A.; Michos, E.D.; Samad, Z.; Ballantyne, C.M.; Virani, S.S. The Use of Sex-Specific Factors in the Assessment of Women’s Cardiovascular Risk. Circulation 2020, 141, 592–599, PMCID: PMC7032610. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Khalil, R.A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H661–H681, PMCID: PMC7509272. [Google Scholar] [CrossRef] [PubMed]
- Schlembach, D.; Wallner, W.; Sengenberger, R.; Stieglere, E.; Mortl, M.; Beckmann, M.W.; Lang, U. Angiogenic growth factor levels in maternal and fetal blood: Correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2007, 29, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Melchiorre, K.; Khalil, A.; Thilaganathan, B. Uterine artery Doppler, birth weight and timing of onset of pre-eclampsia: Providing insights into the dual etiology of late-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2014, 44, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Klabunde, R.E. Cardiovascular Physiology Concepts, 2nd ed.; Walter Kluwer, Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; 256p. [Google Scholar]
- Riise, H.K.; Sulo, G.; Tell, G.S.; Igland, J.; Nygård, O.; Vollset, S.E.; Iversen, A.; Austgulen, R.; Daltveit, A.K. Incident coronary heart disease after preeclampsia: Role of reduced fetal growth, preterm delivery, and parity. J. Am. Heart Assoc. 2017, 6, e004158. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).