Efficacy of Low Doses of Acetylsalicylic Acid in the Prevention of Preeclampsia in Women with Type 1 and 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- type 1 DM (n = 506)
- −
- taking ASA (n = 100),
- −
- not taking ASA (n = 406).
- type 2 DM (n = 229)
- −
- taking ASA (n = 96),
- −
- not taking ASA (n = 133).
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and Diabetes. Curr. Diabetes Rep. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Sahota, D. Screening and Prevention of Preeclampsia. Matern. Med. 2019, 1, 25–30. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy: Diagnosis and Management. NICE Guideline. 2019. Available online: www.nice.org.uk/guidance/ng133 (accessed on 30 March 2021).
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynecol. Obstet. 2019, 145 (Suppl. 1), 1–33. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: http://www.diabetesatlas.org/ (accessed on 30 March 2021).
- Huynh, J.; Dawson, D.; Roberts, D.; Bentley-Lewis, R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta 2015, 36, 101–114. [Google Scholar] [CrossRef]
- Li, X.; Milosavljevic, A.; Elsea, S.H.; Wang, C.C.; Scaglia, F.; Syngelaki, A.; Nicolaides, K.H.; Poon, L.C. Effective Aspirin Treatment of Women at Risk for Preeclampsia Delays the Metabolic Clock of Gestation. Hypertension 2021, 78, 1398–1410. [Google Scholar] [CrossRef]
- Beaufils, M.; Donsimoni, R.; Uzan, S.; Colau, J. Prevention of pre-eclampsia by early antiplatelet therapy. Lancet 1985, 1, 840–842. [Google Scholar] [CrossRef]
- ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet. Gynecol. 2018, 132, e44–e52. [CrossRef]
- Loussert, L.; Vidal, F.; Parant, O.; Hamdi, S.M.; Vayssiere, C.; Guerby, P. Aspirin for prevention of preeclampsia and fetal growth restriction. Prenat. Diagn. 2020, 40, 519–527. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.N.; Syngelaki, A.A.; Matallana, C.C.D.P.; Akolekar, R.R.; Cicero, S.S.; Janga, D.D.; Singh, M.M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Meher, S.; Duley, L.; Hunter, K.; Askie, L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: An individual participant data meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 121–128.e2. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Nicolaides, K.; Demers, S.; Villa, P.; Bujold, E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet. Gynecol. 2013, 41, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.; Rivard, G.-É.; Michon, N.; Morin, F.; Pilon, D.; Moutquin, J.-M.; Rey, É. Low-dose ASA response using the PFA-100 in women with high-risk pregnancy. J. Obstet. Gynaecol. Can. 2009, 31, 1022–1027. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120.e6. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Tan, M.Y.; Wright, D.; Syngelaki, A.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Greco, E.; Wright, A.; Maclagan, K.; et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: Results of SPREE. Ultrasound Obstet. Gynecol. 2018, 51, 743–750. [Google Scholar] [CrossRef]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G.; High Risk of Pre-Eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef]
- Poon, L.C.; Wright, D.; Rolnik, D.L.; Syngelaki, A.; Delgado, J.L.; Tsokaki, T.; Leipold, G.; Akolekar, R.; Shearing, S.; De Stefani, L.; et al. Aspirin for Evidence-Based Preeclampsia Prevention trial: Effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am. J. Obstet. Gynecol. 2017, 217, 585.e1–585.e5. [Google Scholar] [CrossRef]
- Caritis, S.; Sibai, B.; Hauth, J.; Lindheimer, M.D.; Klebanoff, M.; Thom, E.; VanDorsten, P.; Landon, M.; Paul, R.; Miodovnik, M.; et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 1998, 338, 701–705. [Google Scholar] [CrossRef]
- Lah, S.; Cheung, N.W.; Lee, V.; Athayde, N.; Inglis, E.; Padmanabhan, S. Aspirin and preeclampsia prevention in women with pre-existing diabetes: A retrospective study. Intern. Med. J. 2021. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e1. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Allshouse, A.A.; Post, A.; Galan, H.L.; Heyborne, K. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: A secondary analysis of the MFMU High-Risk Aspirin Study. J. Perinatol. 2015, 35, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Adkins, K.; Allshouse, A.A.; Metz, T.D.; Heyborne, K.D. Impact of aspirin on fetal growth in diabetic pregnancies according to White classification. Am. J. Obstet. Gynecol. 2017, 217, 465.e1–465.e5. [Google Scholar] [CrossRef][Green Version]
- Klemetti, M.M.; Laivuori, H.; Tikkanen, M.; Nuutila, M.; Hiilesmaa, V.; Teramo, K. White’s classification and pregnancy outcome in women with type 1 diabetes: A population-based cohort study. Diabetologia 2015, 59, 92–100. [Google Scholar] [CrossRef]
- Sacks, D.A.; Metzger, B.E. Classification of Diabetes in Pregnancy: Time to reassess the alphabet. Obstet. Gynecol. 2013, 121, 345–348. [Google Scholar] [CrossRef]
- Lin, L.; Li, G.; Zhang, W.; Wang, Y.; Yang, H. Low-dose aspirin reduces hypoxia-induced sFlt1 release via the JNK/AP-1 pathway in human trophoblast and endothelial cells. J. Cell. Physiol. 2019, 234, 18928–18941. [Google Scholar] [CrossRef]
- Li, C.; Raikwar, N.; Santillan, M.; Santillan, D.; Thomas, C. Aspirin inhibits expression of sFLT1 from human cytotrophoblasts induced by hypoxia, via cyclo-oxygenase 1. Placenta 2015, 36, 446–453. [Google Scholar] [CrossRef]
- Xu, B.; Shanmugalingam, R.; Chau, K.; Pears, S.; Hennessy, A.; Makris, A. The effect of acetyl salicylic acid (Aspirin) on trophoblast-endothelial interaction in vitro. J. Reprod. Immunol. 2017, 124, 54–61. [Google Scholar] [CrossRef]
- Murtoniemi, K.; Vahlberg, T.; Hämäläinen, E.; Kajantie, E.; Pesonen, A.; Räikkönen, K.; Taipale, P.; Villa, P.; Laivuori, H. The effect of low-dose aspirin on serum placental growth factor levels in a high-risk PREDO cohort. Pregnancy Hypertens. 2018, 13, 51–57. [Google Scholar] [CrossRef]

| Type 1 DM (n = 506) | Type 2 DM (n = 229) | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | ASA (n = 100) | No ASA (n = 406) | F/U/χ2 | p-Value | ASA (n = 96) | No ASA (n = 133) | F/U/χ2 | p-Value |
| Age (years, IQR) | 28.1 (27.2; 29.0) | 28.5 (27.9; 28.9) | 19669 | 0.63 | 33.0 (30.0; 38.0) | 35.0 (31.5; 38.0) | 5862 | 0.29 |
| Body-mass index (kg/m2, IQR) | 27.4 (26.6–28.1) * | 27.1 (26.7–27.4) * | 2.30 | 0.13 | 35.1 (30.2; 38.6) | 32.4 (27.9; 36.8) | 4807 | 0.007 |
| Cigarette smoking (n, %) | 0 | 0 | 5 (5.2) | 4 (3) | 0.42 | 0.51 | ||
| Primiparous (n, %) | 29 (29.0) | 110 (31.8) | 1.58 | 0.82 | 15 (15.6) | 36 (27.5) | 1.40 | 0.22 |
| Chronic arterial hypertension (n, %) | 5 (5.0) | 28 (6.9) | 0.47 | 0.49 | 25 (26.0) | 29 (21.8) | 0.56 | 0.46 |
| Vascular complications (n, %) | 26 (26.0) | 79 (19.5) | 1.60 | 0.23 | 54 (56.3) | 83 (62.4) | 0.88 | 0.35 |
| Excess body weight (n, %) § | 39 (39.0) | 219 (53.9) | 7.20 | 0.007 | 17 (17.7) | 34 (26.8) | 2.54 | 0.11 |
| Obesity (n, %) ‡ | 27 (27.0) | 76 (18.7) | 1.75 | 0.19 | 75 (78.1) | 82 (61.7) | 2.30 | 0.1 |
| Type 1 DM (n = 506) | Type 2 DM (n = 229) | |||||||
|---|---|---|---|---|---|---|---|---|
| ASA (n = 100) | No ASA (n = 406) | F/U/χ2 | p-Value | ASA (n = 96) | No ASA (n = 133) | F/U/χ2 | p-Value | |
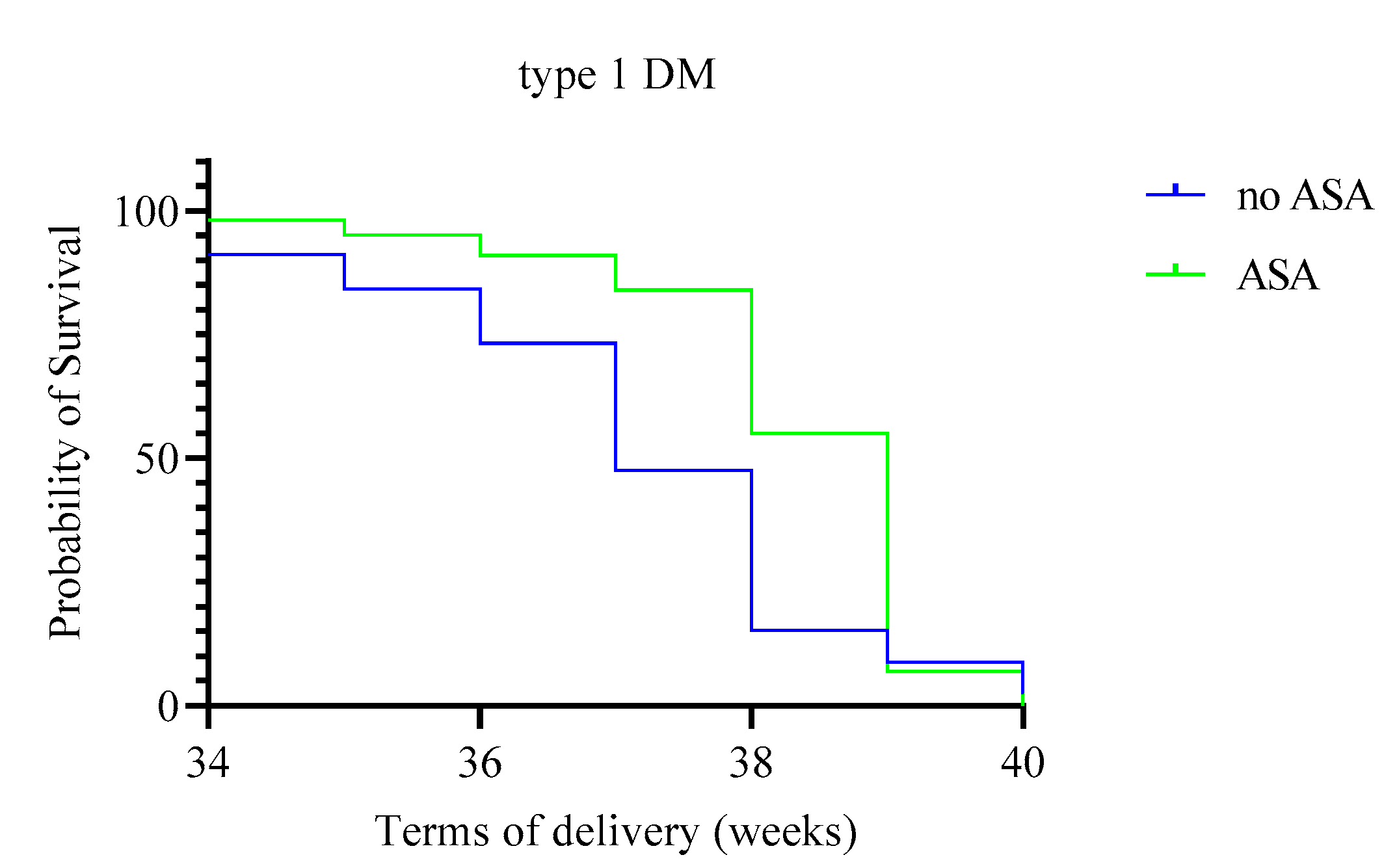
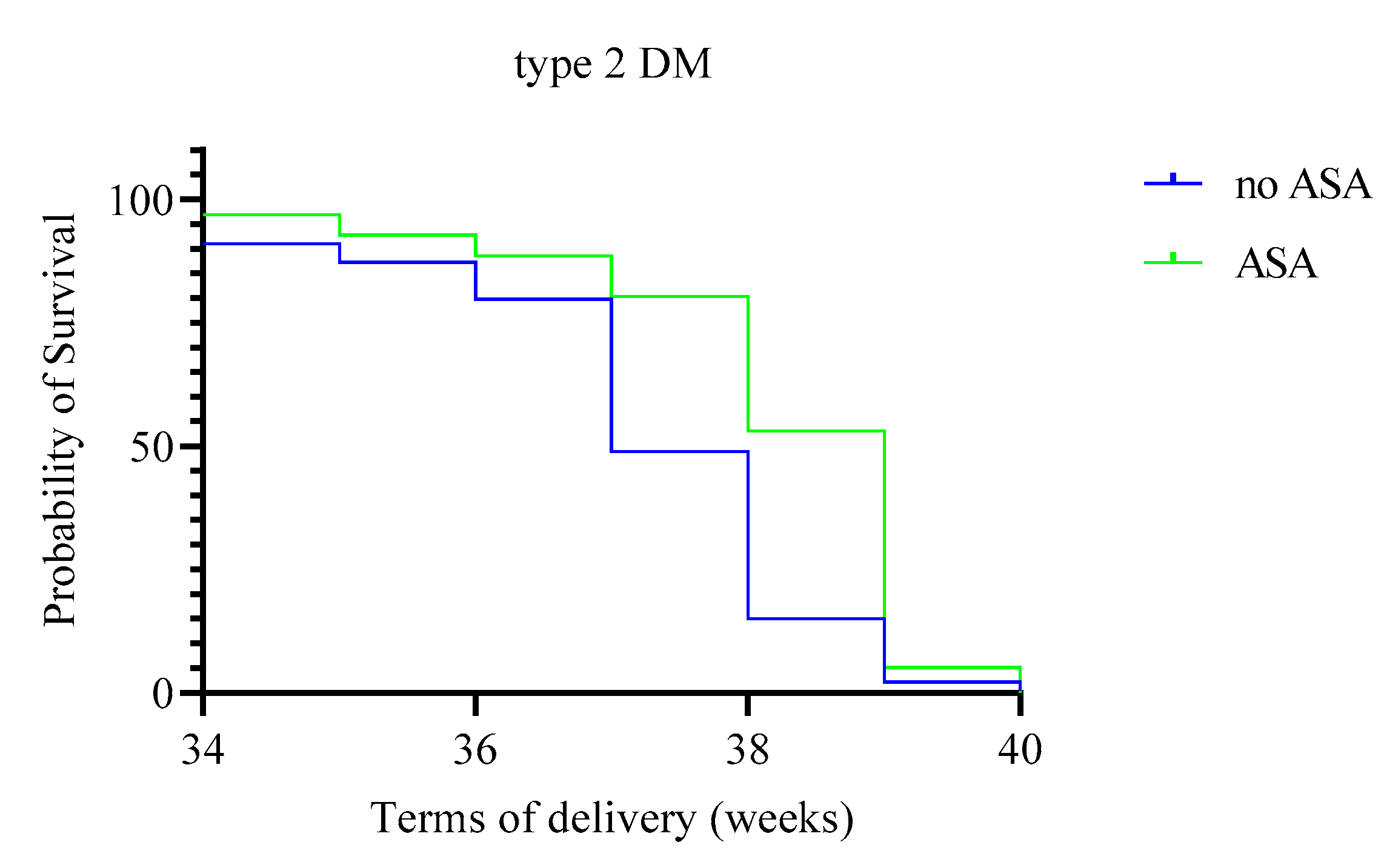
| Due date (weeks, 95% CI) | 38.3 (38.1–38.5) | 37.1 (36.8–37.3) | 9.50 | 0.002 | 38.1 (37.7–38.4) | 37.1 (36.8–37.4) | 0.56 | 0.46 |
| Weight of newborns (g, 95% CI) | 3825 (3408–4200) | 3550 (3045–3955) | 14820 | <0.0001 | 3640 (3160–3840) | 3200 (2578–3785) | 4545 | <0.001 |
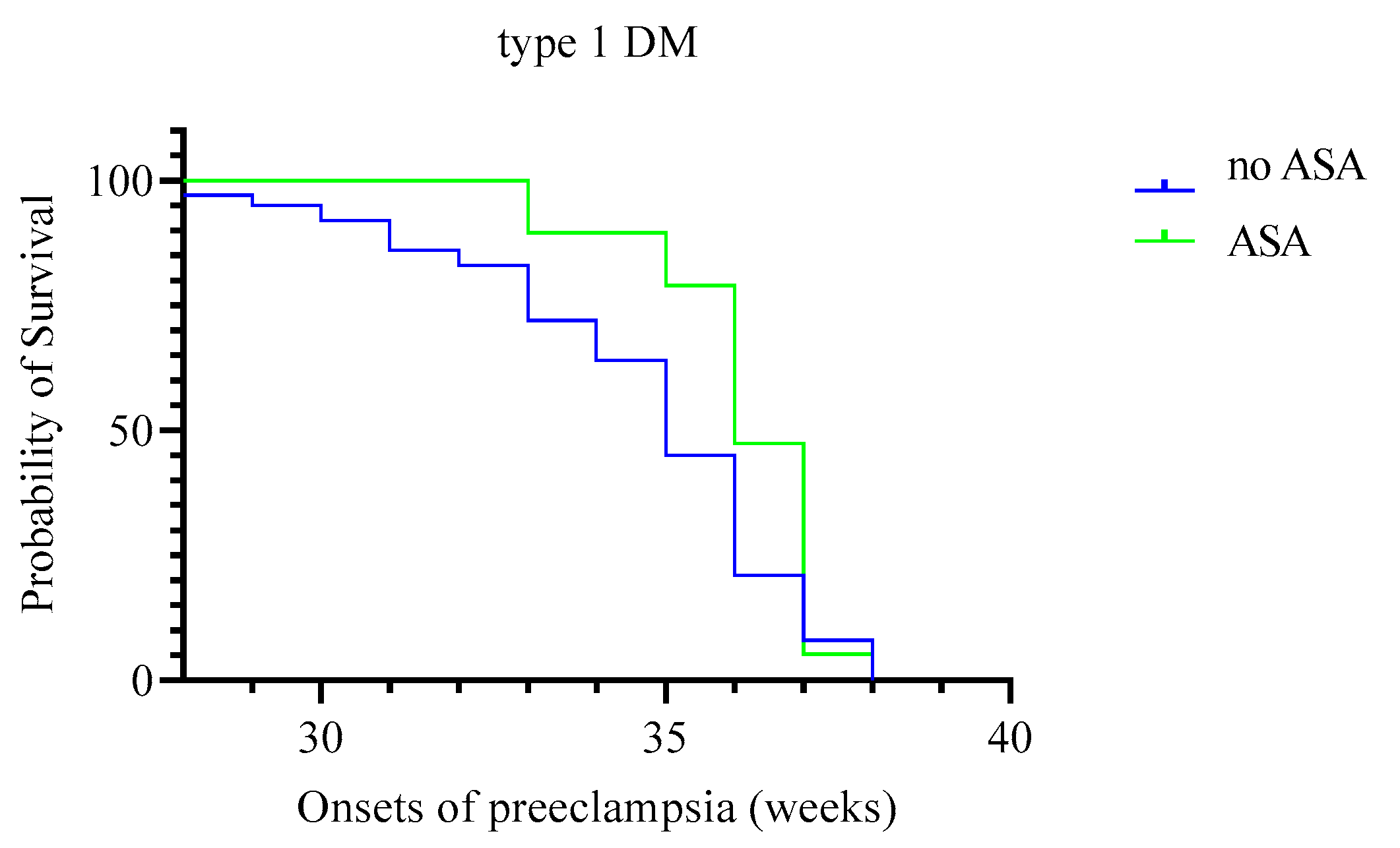
| Onset of preeclampsia (weeks) | 35.5 (35; 36) | 34 (33.5; 34) | 1 | 0.004 | 36 (34.5; 36) | 35 (34; 35) | 9.5 | 0.08 |
| Preeclampsia (n, %) | 29 (29) | 159 (39.2) | 3.55 | 0.037 | 19 (19.8) | 72 (54.1) | 27.46 | <0.0001 |
| Moderate (n, %) | 29 (29) | 106 (26.1) | 0.34 | 0.32 | 6 (6.3) | 53 (39.8) | 32.9 | <0.0001 |
| Severe (n, %) | 0 | 53 (13) | 14.58 | <0.0001 | 13 (13.5) | 19 (14.3) | 0.03 | 0.52 |
| Premature birth (n, %) | 9 (9.0) | 110 (27.1) | 14.6 | <0.0001 | 11 (11.5) | 27 (20.3) | 3.15 | 0.07 |
| <30 weeks (n, %) | 0 | 6 (1.5) | 1.50 | 0.22 | 2 (2.1) | 2 (1.5) | 0.11 | 0.74 |
| 30–34 weeks (n, %) | 0 | 15 (3.7) | 3.80 | 0.05 | 0 | 6 (4.5) | 4.45 | 0.035 |
| 34–36 weeks (n, %) | 5 (5.0) | 44 (10.8) | 3.10 | 0.08 | 5 (5.2) | 9 (6.8) | 0.24 | 63 |
| 36–37 weeks (n, %) | 4 (4.0) | 45 (11.1) | 4.60 | 0.036 | 4 (4.2) | 10 (7.5) | 1.01 | 0.3 |
| Macrosomia (n, %) | 36 (36.0) | 91 (22.4) | 7.90 | 0.005 | 16 (16.7) | 22 (16.5) | 0.001 | 0.98 |
| Caesarean section (n, %) | 51 (51.0) | 174 (50.1) | 0.02 | 0.88 | 47 (49.0) | 59 (44.4) | 0.47 | 0,49 |
| Planned (n, %) | 18 (18.0) | 63 (18.1) | 0.001 | 0.98 | 21 (21.9) | 21 (15.8) | 0.38 | 0.24 |
| Emergency (n, %) | 23 (23.0) | 100 (28.7) | 1.28 | 0.26 | 15 (15.6) | 18 (13.5) | 0.20 | 0.66 |
| Small for gestational age (n, %) * | 2 (2.0) | 39 (9.6) | 6.23 | 0.013 | 6 (6.3) | 36 (27.1) | 16.1 | <0.0001 |
| Type 1 DM (n = 506) | Type 2 DM (n = 229) | |||||||
|---|---|---|---|---|---|---|---|---|
| Complications | OR (95% CI) | p-Value | Adjusted OR (95% CI) * | p-Value | OR (95% CI) | p-Value | Adjusted OR (95% CI) ** | p-Value |
| Preeclampsia | 0.64 (0.39–1.02) | 0.06 | 0.92 (0.47–1.24) | 0.08 | 0.57 (0.46–0.71) | <0.01 | 0.65 (0.52–0.79) | 0.02 |
| Moderate | 0.90 (0.64–1.28) | 0.56 | 0.96 (0.72–1.38) | 0.72 | 0.64 (0.55–0.74) | <0.01 | 0.78 (0.57–0.91) | 0.04 |
| Severe | 0.87 (0.84–0.9) | <0.01 | 0.92 (0.87–0.99) | 0.05 | 0.99 (0.89–1.1) | 0.87 | 1.06 (0.86–1.14) | 0.92 |
| Premature birth | 0.27 (0.13–0.55) | <0.01 | 0.33 (0.15–0.62) | 0.03 | 0.51 (0.24–1.08) | 0.07 | 0.69 (0.2–1.16) | 0.16 |
| <30 weeks | 0.99 (0.97–1.00) | 0.22 | 1.21 (0.97–1.45) | 0.3 | 1.39 (0.19–1.77) | 0.74 | 1.53 (0.48–2.05) | 0.91 |
| 30–34 weeks | 0.96 (0.95–0.98) | 0.05 | 1.05 (0.97–1.12) | 0.09 | 0.96 (0.92–0.99) | 0.04 | 0.98 (0.95–1.13) | 0.06 |
| 34–36 weeks | 0.43 (0.17–1.12) | 0.07 | 0.57 (0.32–1.28) | 0.13 | 0.76 (0.25–2.34) | 0.63 | 0.84 (0.54–1.87) | 0.71 |
| 36–37 weeks | 0.33 (0.12–0.95) | 0.03 | 0.51 (0.39–0.84) | 0.05 | 0.54 (0.16–1.76) | 0.30 | 0.66 (0.43–1.52) | 0.36 |
| Macrosomia | 1.95 (1.22–3.12) | 0.005 | 1.82 (1.13–2.86) | 0.009 | 1.00 (0.50–2.00) | 0.98 | 1.15 (0.75–1.64) | 0.85 |
| Postpartum hemorrhage | – | – | – | – | – | – | – | – |
| Caesarean section | 1.04 (0.66–1.62) | 0.88 | 1.28 (0.87–1.75) | 1.12 | 1.20 (0.70–2.04) | 0.49 | 1.29 (0.86–1.72) | 0.53 |
| Planned | 0.99 (0.56–1.77) | 0.98 | 1.16 (0.95–1.44) | 0.99 | 1.49 (0.76–2.90) | 0.24 | 1.47 (0.81–2.70) | 0.47 |
| Emergency | 0.74 (0.44–1.25) | 0.26 | 0.93 (0.54–1.38) | 0.67 | 1.18 (0.56–2.48) | 0.66 | 1.31 (0.74–2.29) | 0.7 |
| Small for gestational age | 0.19 (0.05–0.81) | 0.013 | 0.35 (0.17–0.62) | 0.025 | 0.18 (0.07–0.45) | <0.001 | 0.31 (0.19–0.44) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapustin, R.V.; Tcybuk, E.M.; Korenevsky, A.V.; Kopteeva, E.V.; Alekseenkova, E.N.; Tiselko, A.V.; Arzhanova, O.N. Efficacy of Low Doses of Acetylsalicylic Acid in the Prevention of Preeclampsia in Women with Type 1 and 2 Diabetes Mellitus. Reprod. Med. 2021, 2, 144-154. https://doi.org/10.3390/reprodmed2040015
Kapustin RV, Tcybuk EM, Korenevsky AV, Kopteeva EV, Alekseenkova EN, Tiselko AV, Arzhanova ON. Efficacy of Low Doses of Acetylsalicylic Acid in the Prevention of Preeclampsia in Women with Type 1 and 2 Diabetes Mellitus. Reproductive Medicine. 2021; 2(4):144-154. https://doi.org/10.3390/reprodmed2040015
Chicago/Turabian StyleKapustin, Roman V., Elizaveta M. Tcybuk, Andrew V. Korenevsky, Ekaterina V. Kopteeva, Elena N. Alekseenkova, Alena V. Tiselko, and Olga N. Arzhanova. 2021. "Efficacy of Low Doses of Acetylsalicylic Acid in the Prevention of Preeclampsia in Women with Type 1 and 2 Diabetes Mellitus" Reproductive Medicine 2, no. 4: 144-154. https://doi.org/10.3390/reprodmed2040015
APA StyleKapustin, R. V., Tcybuk, E. M., Korenevsky, A. V., Kopteeva, E. V., Alekseenkova, E. N., Tiselko, A. V., & Arzhanova, O. N. (2021). Efficacy of Low Doses of Acetylsalicylic Acid in the Prevention of Preeclampsia in Women with Type 1 and 2 Diabetes Mellitus. Reproductive Medicine, 2(4), 144-154. https://doi.org/10.3390/reprodmed2040015






