Effects of Chemotherapy on Fertility Preservation in Patients with Tumors of the Hematopoietic and Lymphoid Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Schedule of Chemotherapy and COS Protocol
2.3. Statistical Analysis
3. Results
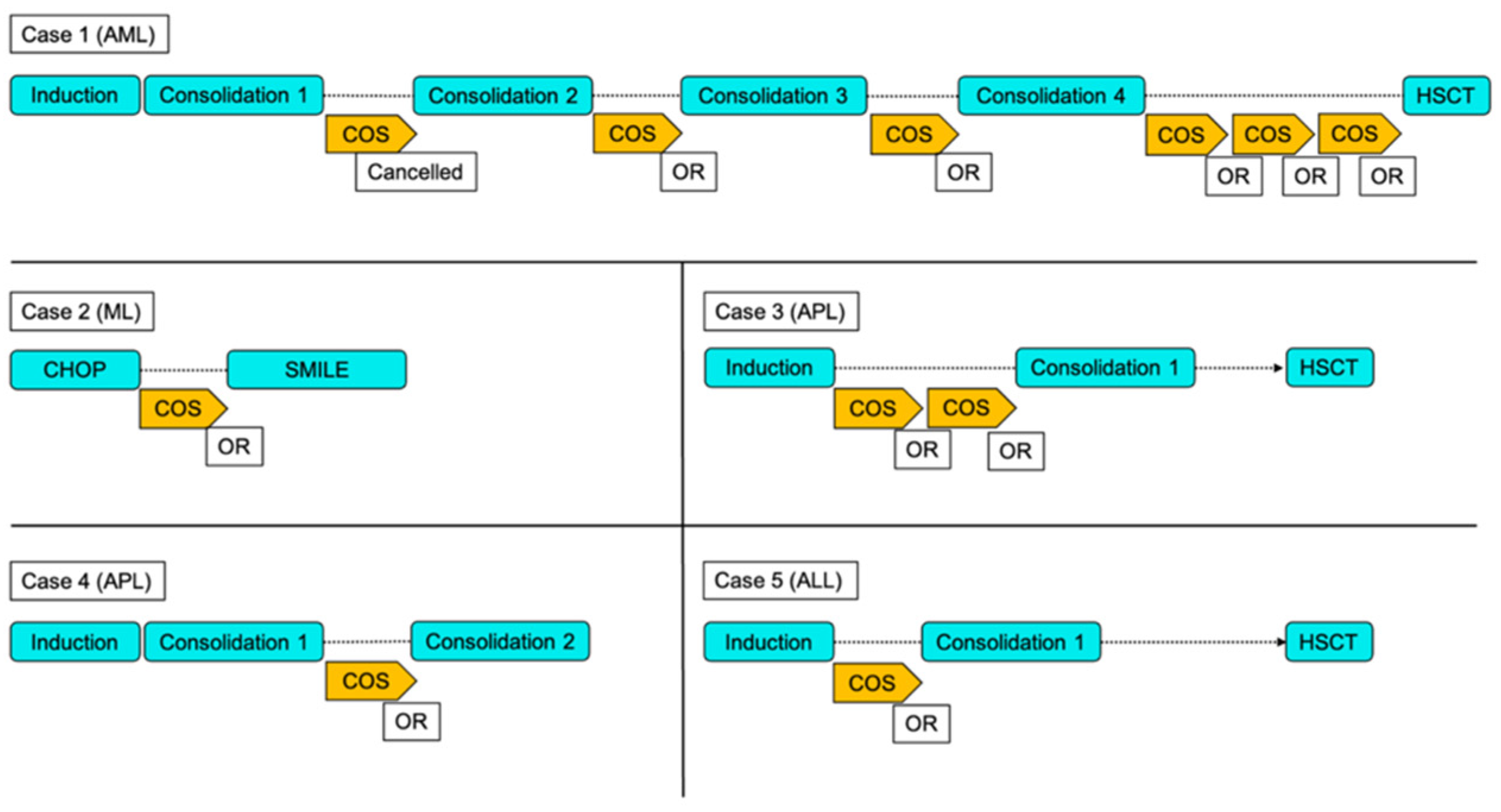
Individual Case Presentations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Dorp, W.; Mulder, R.L.; Kremer, L.C.M.; Hudson, M.M.; van den Heuvel-Eibrink, M.M.; van den Berg, M.H.; Levine, J.M.; van Dulmen-den Broeder, E.; di Iorgi, N.; Albanese, A.; et al. Recommendations for Premature Ovarian Insufficiency Surveillance for Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Report From the International Late Effects of Childhood Cancer Guideline Harmonization Group in Collaboration With the PanCareSurFup Consortium. J. Clin. Oncol. 2016, 34, 3440–3450. [Google Scholar] [PubMed]
- Barton, S.E.; Najita, J.S.; Ginsburg, E.S.; Leisenring, W.M.; Stovall, M.; E Weathers, R.; Sklar, C.A.; Robison, L.L.; Diller, L. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013, 14, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature Menopause in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018, 14, 381–385. [Google Scholar] [CrossRef]
- Harada, M.; Osuga, Y. Fertility preservation for female cancer patients. Int. J. Clin. Oncol. 2018, 24, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Marinescu, C.; Saussoy, P.; Van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Morimoto, Y.; Nakaoka, Y.; Yamasaki, T.; Suehiro, Y.; Sugimoto, H.; Yoshida, M.; Irahara, M. Age-specific serum anti-Müllerian hormone concentration in Japanese women and its usefulness as a predictor of the ovarian response. Reprod. Med. Biol. 2017, 16, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Kuwatsuka, Y.; Tomizawa, D.; Kihara, R.; Nagata, Y.; Shiba, N.; Iijima-Yamashita, Y.; Shimada, A.; Deguchi, T.; Miyachi, H.; Tawa, A.; et al. Prognostic value of genetic mutations in adolescent and young adults with acute myeloid leukemia. Int. J. Hematol. 2018, 107, 201–210. [Google Scholar] [CrossRef]
- Gürgen, S.G.; Erdoğan, D.; Elmas, Ç.; Kaplanoğlu, G.T.; Özer, Ç. Chemoprotective effect of ascorbic acid, α-tocopherol, and selenium on cyclophosphamide-induced toxicity in the rat ovarium. Nutrition 2013, 29, 777–784. [Google Scholar] [CrossRef]
- Familiari, G.; Caggiati, A.; Nottola, S.A.; Ermini, M.; di Benedetto, M.R.; Motta, P.M. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum. Reprod. 1993, 8, 2080–2087. [Google Scholar] [CrossRef]
- Albanese, R. Induction and transmission of chemically induced chromosome aberrations in female germ cells. Environ. Mol. Mutagen. 1987, 10, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Higdon, R.E.; Marchetti, F.; Mailhes, J.B.; Phillips, G.L. The effects of cisplatin on murine metaphase II oocytes. Gynecol. Oncol. 1992, 47, 348–352. [Google Scholar] [CrossRef]
- Mailhes, J.B.; Marchetti, F.; Young, D. Synergism between gonadotrophins and vinblastine relative to the frequencies of metaphase I, diploid and aneuploid mouse oocytes. Mutagenesis 1995, 10, 185–188. [Google Scholar] [CrossRef]
- Meirow, D.; Epstein, M.; Lewis, H.; Nugent, D.; Gosden, R. Administration of cyclophosphamide at different stages of follicular maturation in mice: Effects on reproductive performance and fetal malformations. Hum. Reprod. 2001, 16, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Levis, A. Detection of aneuploidy in male germ cells of mice by means of a meiotic micronucleus assay. Mutat. Res. Lett. 1992, 281, 187–191. [Google Scholar] [CrossRef]
- Sudman, P.; Rutledge, J.; Bishop, J.; Generoso, W. Bleomycin: Female-specific dominant lethal effects in mice. Mutat. Res. Genet. Toxicol. 1992, 296, 143–156. [Google Scholar] [CrossRef]
- Takami, A. Hematopoietic stem cell transplantation for acute myeloid leukemia. Int. J. Hematol. 2018, 107, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Schiff, E. Appraisal of chemotherapy effects on reproductive outcome according to animal studies and clinical data. J. Natl. Cancer Inst. Monogr. 2005, 2005, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793. [Google Scholar] [CrossRef] [Green Version]
- Ting, A.Y.; Petroff, B.K. Tamoxifen decreases ovarian follicular loss from experimental toxicant DMBA and chemotherapy agents cyclophosphamide and doxorubicin in the rat. J. Assist. Reprod. Genet. 2010, 27, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Kojima, M.; Kawanishi, S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005, 76, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, A.; Roscino, M.; Binetti, F.; Sciorsci, R. Roles of Reactive Oxygen Species in Female Reproduction. Reprod. Domest. Anim. 2012, 47, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Sugino, N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 2005, 4, 31–44. [Google Scholar] [PubMed]
- Kimball, A.P.; Wilson, M.J. Inhibition of DNA polymerase by beta-D-arabinosylcytosine and reversal of inhibition by deoxycytidine-5′-triphosphate. Proc. Soc. Exp. Biol. Med. 1968, 127, 429–432. [Google Scholar] [CrossRef]
- A Smets, L.; Homan-Blok, J. S1-phase cells of the leukemic cell cycle sensitive to 1-beta-D-arabinofuranosylcytosine at a high-dose level. Cancer Res. 1985, 45, 3113–3117. [Google Scholar]
- Chomienne, C.; Abita, J.P.; Balitrand, N.; Degos, L. Drug association including ara-C in myeloid leukemia cell differentiation: In vitro studies. Semin. Oncol. 1985, 12 (Suppl. S3), 60–64. [Google Scholar]
- Azem, F.; Amit, A.; Merimsky, O.; Lessing, J.B. Successful transfer of frozen-thawed embryos obtained after subtotal colectomy for colorectal cancer and before fluorouracil-based chemotherapy. Gynecol. Oncol. 2004, 93, 263–265. [Google Scholar] [CrossRef]
- Twelves, C.; Wong, A.; Nowacki, M.P.; Abt, M.; Burris, H.; Carrato, A.; Cassidy, J.; Cervantes, A.; Fagerberg, J.; Georgoulias, V.; et al. Capecitabine as Adjuvant Treatment for Stage III Colon Cancer. N. Engl. J. Med. 2005, 352, 2696–2704. [Google Scholar] [CrossRef]
- Burt, R.; Dey, A.; Aref, S.; Aguiar, M.; Akarca, A.; Bailey, K.; Day, W.; Hooper, S.; Kirkwood, A.; Kirschner, K.; et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019, 134, 1415–1429. [Google Scholar] [CrossRef]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefansdottir, A.; Johnston, Z.C.; Powles-Glover, N.; Anderson, R.A.; Adams, I.R.; Spears, N. Etoposide damages female germ cells in the developing ovary. BMC Cancer 2016, 16, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-L.; Yu, C.; Ji, S.-Y.; Li, X.-M.; Zhang, Y.-P.; Zhang, D.; Zhou, D.; Fan, H.-Y. TOP2β is essential for ovarian follicles that are hypersensitive to chemotherapeutic drugs. Mol. Endocrinol. 2013, 27, 1678–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar]
- Winship, A.; Carpenter, M.; Griffiths, M.; Hutt, K.J. Vincristine Chemotherapy Induces Atresia of Growing Ovarian Follicles in Mice. Toxicol. Sci. 2019, 169, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.V.; Missmer, S.; Correia, K.F.; Wadleigh, M.; Ginsburg, E.S. Ovarian Reserve in Women Treated for Acute Lymphocytic Leukemia or Acute Myeloid Leukemia with Chemotherapy, but Not Stem Cell Transplantation. ISRN Oncol. 2012, 2012, 956190. [Google Scholar] [CrossRef]
- Decanter, C.; Morschhauser, F.; Pigny, P.; Lefebvre, C.; Gallo, C.; Dewailly, D. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: Preliminary results. Reprod. Biomed. Online 2010, 20, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.L.; Johnson, L.N.C.; Efymow, B.L.; Sammel, M.D.; Gracia, C.R. Outcomes of ovarian stimulation after treatment with chemotherapy. J. Assist. Reprod. Genet. 2015, 32, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
| Case | Age (Years) | Gravida | Para | Married | Cancer Type | AMH (ng/mL) |
|---|---|---|---|---|---|---|
| 1 | 27 | 0 | 0 | None | AML | 0.26 |
| 2 | 30 | 0 | 0 | None | ML | 0.75 |
| 3 | 32 | 0 | 0 | None | APL | 0.1 |
| 4 | 28 | 0 | 0 | None | APL | 0.32 |
| 5 | 26 | 0 | 0 | None | ALL | 0.28 |
| Case | Chemotherapy | Gonadotoxic Risk | |
|---|---|---|---|
| 1 | Induction | Idarubicin/Cytarabine | Low |
| Consolidation1 | Cytarabine/Mitoxantrone Hydrochloride | Low | |
| Consolidation2 | Cytarabine/Daunorubicin | Low | |
| Consolidation3 | Cytarabine/Aclarubicin | Low | |
| Consolidation4 | Cytarabine/Etoposide/Vincristine/Vindesine | Very low or none | |
| 2 | CHOP | Cyclophosphamide/Dokisorubisin/Vincristine/Predonin | Low |
| 3 | Induction | Idarubicin/Cytarabine/Tretinoin | Low and unknown |
| 4 | Induction | Idarubicin/Cytarabine/Tretinoin | Low and unknown |
| Consolidation | Mitoxantrone Hydrochloride/Cytarabine | Low | |
| 5 | Induction | Daunorubicin/Cyclophosphamide/Vincristine/Predonin/L-Asparaginase | Low |
| Case | Cycle Number | COS Protocol | Number of Days Stimulated | Total Dose of FSH/hMG (IU) | Peak E2 (pg/mL) | Number of Retrieved Oocytes | Number of MII Oocytes | Maturation Rate (MII/Oocyte) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Random start | 14 | 3150 | 50 | Cancelled | ||
| 2 | Antagonist | 25 | 2925 | 627 | 2 | 1 | 50.0% | |
| 3 | Random start | 26 | 5475 | 1127 | 3 | 2 | 66.7% | |
| 4 | Random start | 12 | 2775 | 570 | 2 | 2 | 100.0% | |
| 5 | Random start | 16 | 3300 | 346 | 5 | 3 | 60.0% | |
| 6 | Random start | 9 | 1725 | 255 | 2 | 1 | 50.0% | |
| 2 | 1 | Random start | 31 | 8400 | 345 | 8 | 4 | 50.0% |
| 3 | 1 | Random start | 2 | 450 | 565 | 1 | 1 | 100.0% |
| 2 | Random start | 13 | 4350 | 343 | 2 | 1 | 50.0% | |
| 4 | 1 | Random start | 12 | 3300 | 330 | 5 | 4 | 80.0% |
| 5 | 1 | Random start | 13 | 4350 | 520 | 2 | 2 | 100.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akino, R.; Nishii, S.; Odawara, K.; Saito, M.; Sakamoto, M.; Kondo, T.; Sekizawa, A. Effects of Chemotherapy on Fertility Preservation in Patients with Tumors of the Hematopoietic and Lymphoid Tissues. Reprod. Med. 2022, 3, 141-149. https://doi.org/10.3390/reprodmed3020012
Akino R, Nishii S, Odawara K, Saito M, Sakamoto M, Kondo T, Sekizawa A. Effects of Chemotherapy on Fertility Preservation in Patients with Tumors of the Hematopoietic and Lymphoid Tissues. Reproductive Medicine. 2022; 3(2):141-149. https://doi.org/10.3390/reprodmed3020012
Chicago/Turabian StyleAkino, Ryosuke, Shogo Nishii, Kei Odawara, Megumi Saito, Miwa Sakamoto, Tetsuro Kondo, and Akihiko Sekizawa. 2022. "Effects of Chemotherapy on Fertility Preservation in Patients with Tumors of the Hematopoietic and Lymphoid Tissues" Reproductive Medicine 3, no. 2: 141-149. https://doi.org/10.3390/reprodmed3020012
APA StyleAkino, R., Nishii, S., Odawara, K., Saito, M., Sakamoto, M., Kondo, T., & Sekizawa, A. (2022). Effects of Chemotherapy on Fertility Preservation in Patients with Tumors of the Hematopoietic and Lymphoid Tissues. Reproductive Medicine, 3(2), 141-149. https://doi.org/10.3390/reprodmed3020012






