Early Identification of the Maternal, Placental and Fetal Dialog in Gestational Diabetes and Its Prevention
Abstract
:1. Introduction
2. Pathophysiology of GDM
3. Importance of GDM Prediction
4. Predicting GDM by Maternal Risk Factors
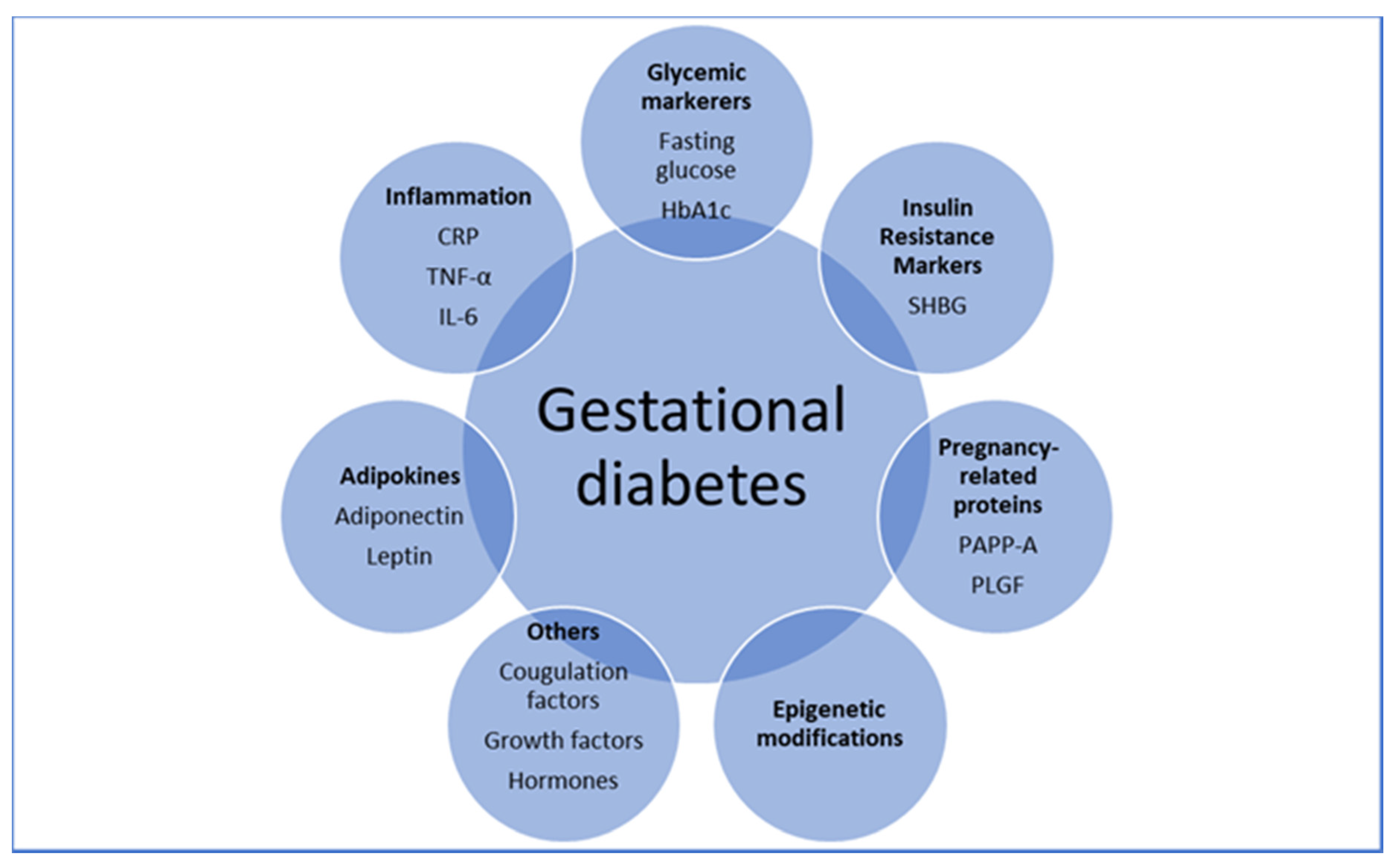
5. Predicting GDM Using Individual Biomarkers
6. Predicting GDM by Early Glycemic Markers
6.1. Fasting Glucose
6.2. HbA1c
7. Predicting GDM by Adipokines
7.1. Adiponectin
7.2. Leptin
8. Predicting GDM by Pregnancy-Related Proteins
8.1. Pregnancy-Associated Plasma Protein A (PAPP-A)
8.2. Placental Growth Factor (PLGF)
8.3. First-Trimester Combined Test (FTCT)
9. Predicting GDM by Inflammatory Markers
9.1. Tumor Necrosis Factor-α (TNF-α)
9.2. C-Reactive Protein (CRP)
9.3. Interleukin 6 (IL-6)
10. Predicting GDM by Insulin Resistance Markers
SHBG
11. Early Prediction to Improve Maternal, Placental and Fetal Dialog
12. Future Directions
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018, 131, e49–e64.
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef]
- Goedegebure, E.A.R.; Koning, S.H.; Hoogenberg, K.; Korteweg, F.J.; Lutgers, H.L.; Diekman, M.J.M.; Stekkinger, E.; van den Berg, P.P.; Zwart, J.J. Pregnancy outcomes in women with gestational diabetes mellitus diagnosed according to the WHO-2013 and WHO-1999 diagnostic criteria: A multicentre retrospective cohort study. BMC Pregnancy Childbirth 2018, 18, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, O.; Yogev, Y.; Most, O.; Xenakis, E.M.J. Gestational diabetes: The consequences of not treating. Am. J. Obstet. Gynecol. 2005, 192, 989–997. [Google Scholar] [CrossRef]
- Fetita, L.-S.; Sobngwi, E.; Serradas, P.; Calvo, F.; Gautier, J.-F. Consequences of Fetal Exposure to Maternal Diabetes in Offspring. J. Clin. Endocrinol. Metab. 2006, 91, 3718–3724. [Google Scholar] [CrossRef]
- Shah, B.R.; Retnakaran, R.; Booth, G.L. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008, 31, 1668–1669. [Google Scholar] [CrossRef] [Green Version]
- Vandorsten, J.P.; Dodson, W.C.; Espeland, M.A.; Grobman, W.A.; Guise, J.M.; Mercer, B.M.; Minkoff, H.L.; Poindexter, B.; Prosser, L.A.; Sawaya, G.F.; et al. NIH consensus development conference: Diagnosing gestational diabetes mellitus. NIH Consens. State Sci. Statements 2013, 29, 1–31. [Google Scholar] [PubMed]
- Huhn, E.A.; Rossi, S.W.; Hoesli, I.; Göbl, C.S. Controversies in Screening and Diagnostic Criteria for Gestational Diabetes in Early and Late Pregnancy. Front. Endocrinol. 2018, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Bogdanet, D.; O’Shea, P.; Lyons, C.; Shafat, A.; Dunne, F. The Oral Glucose Tolerance Test-Is It Time for a Change?—A Literature Review with an Emphasis on Pregnancy. J. Clin. Med. 2020, 9, 3451. [Google Scholar] [CrossRef]
- Catalano, P.M. Carbohydrate metabolism and gestational diabetes. Clin. Obstet. Gynecol. 1994, 37, 25–38. [Google Scholar] [CrossRef]
- Baz, B.; Riveline, J.-P.; Gautier, J.-F. Endocrinology of pregnancy: Gestational diabetes mellitus: Definition, aetiological and clinical aspects. Eur. J. Endocrinol. 2016, 174, R43–R51. [Google Scholar] [CrossRef] [Green Version]
- Yogev, Y.; Xenakis, E.M.J.; Langer, O. The association between preeclampsia and the severity of gestational diabetes: The impact of glycemic control. Am. J. Obstet. Gynecol. 2004, 191, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, S.; Piant, H.; Gaudineau, A.; Lefebvre, F.; Langer, B.; Koch, A. Risk factors for obstetric anal sphincter injuries (OASIS) and the role of episiotomy: A retrospective series of 496 cases. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, S.; Viljakainen, M.; Männistö, T.; Gissler, M.; Pouta, A.; Kaaja, R.; Eriksson, J.; Laivuori, H.; Kajantie, E.; Vääräsmäki, M. Pregnancy outcomes according to the definition of gestational diabetes. PLoS ONE 2020, 15, e0229496. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E. Long-term Outcomes in Mothers Diagnosed with Gestational Diabetes Mellitus and Their Offspring. Clin. Obstet. Gynecol. 2007, 50, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet Lond. Engl. 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Mitanchez, D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010, 36, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D.; Burguet, A.; Simeoni, U. Infants born to mothers with gestational diabetes mellitus: Mild neonatal effects, a long-term threat to global health. J. Pediatr. 2014, 164, 445–450. [Google Scholar] [CrossRef]
- Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; England, J.L.; Sharma, J.A.; Njoroge, T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: A systematic review. Exp. Diabetes Res. 2011, 2011, 541308. [Google Scholar] [CrossRef] [Green Version]
- Roeckner, J.T.; Sanchez-Ramos, L.; Jijon-Knupp, R.; Kaunitz, A.M. Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 215, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Berezowsky, A.; Raban, O.; Aviram, A.; Zafrir-Danieli, H.; Krispin, E.; Hadar, E. Glucose tolerance test with a single abnormal value in pregnancy and the risk of type-2 diabetes mellitus. Arch. Gynecol. Obstet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M.; et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Greco, E.; Foti, D.; Brunetti, A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr. Opin. Pharmacol. 2021, 60, 1–10. [Google Scholar] [CrossRef]
- Farrar, D.; Simmonds, M.; Bryant, M.; Lawlor, D.A.; Dunne, F.; Tuffnell, D.; Sheldon, T.A. Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: A systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS ONE 2017, 12, e0175288. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [CrossRef]
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [Green Version]
- Roos, N.; Kieler, H.; Sahlin, L.; Ekman-Ordeberg, G.; Falconer, H.; Stephansson, O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: Population based cohort study. BMJ 2011, 343, d6309. [Google Scholar] [CrossRef] [Green Version]
- Hiersch, L.; Berger, H.; Okby, R.; Ray, J.G.; Geary, M.; Mcdonald, S.D.; Murry-Davis, B.; Riddell, C.; Halperin, I.; Hasan, H.; et al. Incidence and risk factors for gestational diabetes mellitus in twin versus singleton pregnancies. Arch. Gynecol. Obstet. 2018, 298, 579–587. [Google Scholar] [CrossRef]
- Wang, Y.A.; Nikravan, R.; Smith, H.C.; Sullivan, E.A. Higher prevalence of gestational diabetes mellitus following assisted reproduction technology treatment. Hum. Reprod. Oxf. Engl. 2013, 28, 2554–2561. [Google Scholar] [CrossRef] [Green Version]
- Syngelaki, A.; Pastides, A.; Kotecha, R.; Wright, A.; Akolekar, R.; Nicolaides, K.H. First-Trimester Screening for Gestational Diabetes Mellitus Based on Maternal Characteristics and History. Fetal. Diagn. Ther. 2015, 38, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gabbay-Benziv, R.; Doyle, L.E.; Blitzer, M.; Baschat, A.A. First trimester prediction of maternal glycemic status. J. Perinat. Med. 2015, 43, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Diabetes in Pregnancy: Management of Diabetes and Its Complications from Pre-Conception to the Postnatal Period|Guidance|NICE [Internet]. NICE. Available online: https://www.nice.org.uk/guidance/CG63 (accessed on 19 October 2021).
- Coustan, D.R.; Nelson, C.; Carpenter, M.W.; Carr, S.R.; Rotondo, L.; Widness, J.A. Maternal age and screening for gestational diabetes: A population-based study. Obstet. Gynecol. 1989, 73, 557–561. [Google Scholar] [PubMed]
- Bogdanet, D.; Reddin, C.; Murphy, D.; Doheny, H.C.; Halperin, J.A.; Dunne, F.; O’Shea, P. Emerging Protein Biomarkers for the Diagnosis or Prediction of Gestational Diabetes—A Scoping Review. J. Clin. Med. 2021, 10, 1533. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 201: Pregestational Diabetes Mellitus. Obstet. Gynecol. 2018, 132, e228–e248. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [Green Version]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Hillier, T.A.; Pedula, K.L.; Ogasawara, K.K.; Vesco, K.K.; Oshiro, C.E.S.; Lubarsky, S.L.; Van Marter, J. A Pragmatic, Randomized Clinical Trial of Gestational Diabetes Screening. N. Engl. J. Med. 2021, 384, 895–904. [Google Scholar] [CrossRef]
- Riskin-Mashiah, S.; Younes, G.; Damti, A.; Auslender, R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care 2009, 32, 1639–1643. [Google Scholar] [CrossRef] [Green Version]
- Riskin-Mashiah, S.; Damti, A.; Younes, G.; Auslender, R. First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 152, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Yeral, M.I.; Ozgu-Erdinc, A.S.; Uygur, D.; Seckin, K.D.; Karsli, M.F.; Danisman, A.N. Prediction of gestational diabetes mellitus in the first trimester, comparison of fasting plasma glucose, two-step and one-step methods: A prospective randomized controlled trial. Endocrine 2014, 46, 512–518. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care 2016, 39 (Suppl. 1), S4–S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, A.; Serra, A.E.; Gabby, L.; Wing, D.A.; Berkowitz, K.M. Use of hemoglobin A1c as an early predictor of gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2014, 211, 641.e1–641.e7. [Google Scholar] [CrossRef]
- Osmundson, S.S.; Zhao, B.S.; Kunz, L.; Wang, E.; Popat, R.; Nimbal, V.C.; Palaniappan, L.P. First Trimester Hemoglobin A1c Prediction of Gestational Diabetes. Am. J. Perinatol. 2016, 33, 977–982. [Google Scholar] [CrossRef]
- Hughes, R.C.E.; Moore, M.P.; Gullam, J.E.; Mohamed, K.; Rowan, J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care 2014, 37, 2953–2959. [Google Scholar] [CrossRef] [Green Version]
- Hughes, R.C.E.; Rowan, J.; Florkowski, C.M. Is There a Role for HbA1c in Pregnancy? Curr. Diabetes Rep. 2016, 16, 5. [Google Scholar] [CrossRef]
- Osmundson, S.S.; Norton, M.E.; El-Sayed, Y.Y.; Carter, S.; Faig, J.C.; Kitzmiller, J.L. Early Screening and Treatment of Women with Prediabetes: A Randomized Controlled Trial. Am. J. Perinatol. 2016, 33, 172–179. [Google Scholar]
- Bao, W.; Baecker, A.; Song, Y.; Kiely, M.; Liu, S.; Zhang, C. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: A systematic review. Metabolism 2015, 64, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Fasshauer, M.; Blüher, M.; Stumvoll, M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014, 2, 488–499. [Google Scholar] [CrossRef]
- Nanda, S.; Poon, L.C.Y.; Muhaisen, M.; Acosta, I.C.; Nicolaides, K.H. Maternal serum resistin at 11 to 13 weeks’ gestation in normal and pathological pregnancies. Metabolism 2012, 61, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.F.A.; Rezende, J.C.; Vaikousi, E.; Akolekar, R.; Nicolaides, K.H. Maternal serum visfatin at 11-13 weeks of gestation in gestational diabetes mellitus. Clin. Chem. 2011, 57, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Liu, Q.; Huang, X.; Tan, H. Serum level and polymorphisms of retinol-binding protein-4 and risk for gestational diabetes mellitus: A meta-analysis. BMC Pregnancy Childbirth 2016, 16, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Tan, B.; Karteris, E.; Zervou, S.; Digby, J.; Hillhouse, E.W.; Vatish, M.; Randeva, H.S. Secretion of adiponectin by human placenta: Differential modulation of adiponectin and its receptors by cytokines. Diabetologia 2006, 49, 1292–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- Lain, K.Y.; Daftary, A.R.; Ness, R.B.; Roberts, J.M. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin. Endocrinol. 2008, 69, 407–411. [Google Scholar] [CrossRef]
- Lacroix, M.; Battista, M.-C.; Doyon, M.; Ménard, J.; Ardilouze, J.-L.; Perron, P.; Hivert, M.-F. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care 2013, 36, 1577–1583. [Google Scholar] [CrossRef] [Green Version]
- Iliodromiti, S.; Sassarini, J.; Kelsey, T.W.; Lindsay, R.S.; Sattar, N.; Nelson, S.M. Accuracy of circulating adiponectin for predicting gestational diabetes: A systematic review and meta-analysis. Diabetologia 2016, 59, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllou, G.A.; Paschou, S.A.; Mantzoros, C.S. Leptin and Hormones: Energy Homeostasis. Endocrinol. Metab. Clin. N. Am. 2016, 45, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Fattah, C.; Barry, S.; O’connor, N.; Farah, N.; Stuart, B.; Turner, M.J. Maternal leptin and body composition in the first trimester of pregnancy. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2011, 27, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Malamitsi-Puchner, A. Reviews: Adipocytokines in normal and complicated pregnancies. Reprod. Sci. Thousand Oaks Calif. 2009, 16, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Jenum, A.K.; Waage, C.W.; Mørkrid, K.; Sletner, L.; Birkeland, K.I. Ethnic differences in BMI, subcutaneous fat, and serum leptin levels during and after pregnancy and risk of gestational diabetes. Eur. J. Endocrinol. 2015, 172, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Maple-Brown, L.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Maternal pregravid weight is the primary determinant of serum leptin and its metabolic associations in pregnancy, irrespective of gestational glucose tolerance status. J. Clin. Endocrinol. Metab. 2012, 97, 4148–4155. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, H.M.; Lappas, M.; Georgiou, G.M.; Marita, A.; Bryant, V.J.; Hiscock, R.; Permezel, M.; Khalil, Z.; Rice, G.E. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008, 45, 157–165. [Google Scholar] [CrossRef]
- Boldt, H.B.; Conover, C.A. Pregnancy-associated plasma protein-A (PAPP-A): A local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm. IGF Res. 2007, 17, 10–18. [Google Scholar] [CrossRef]
- Dugoff, L.; Hobbins, J.C.; Malone, F.D.; Porter, T.F.; Luthy, D.; Comstock, C.H.; Hankins, G.; Berkowitz, R.L.; Merkatz, I.; Craigo, S.D.; et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (the FASTER Trial). Am. J. Obstet. Gynecol. 2004, 191, 1446–1451. [Google Scholar] [CrossRef]
- Ren, Z.; Zhe, D.; Li, Z.; Sun, X.-P.; Yang, K.; Lin, L. Study on the correlation and predictive value of serum pregnancy-associated plasma protein A, triglyceride and serum 25-hydroxyvitamin D levels with gestational diabetes mellitus. World J. Clin. Cases 2020, 8, 864–873. [Google Scholar] [CrossRef]
- Beneventi, F.; Simonetta, M.; Lovati, E.; Albonico, G.; Tinelli, C.; Locatelli, E.; Spinillo, A. First trimester pregnancy-associated plasma protein-A in pregnancies complicated by subsequent gestational diabetes. Prenat. Diagn. 2011, 31, 523–528. [Google Scholar] [CrossRef]
- Ramezani, S.; Doulabi, M.A.; Saqhafi, H.; Alipoor, M. Prediction of Gestational Diabetes by Measuring the Levels of Pregnancy Associated Plasma Protein-A (PAPP-A) During Gestation Weeks 11-14. J. Reprod. Infertil. 2020, 21, 130–137. [Google Scholar] [PubMed]
- Wells, G.; Bleicher, K.; Han, X.; McShane, M.; Chan, Y.F.; Bartlett, A.; White, C.; Lau, S.M. Maternal Diabetes, Large-for-Gestational-Age Births, and First Trimester Pregnancy-Associated Plasma Protein-A. J. Clin. Endocrinol. Metab. 2015, 100, 2372–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, B.M.; Nidey, N.L.; Jasper, E.A.; Robinson, J.G.; Bao, W.; Saftlas, A.F.; Ryckman, K.K. First trimester prenatal screening biomarkers and gestational diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0201319. [Google Scholar] [CrossRef] [PubMed]
- Husslein, H.; Lausegger, F.; Leipold, H.; Worda, C. Association between pregnancy-associated plasma protein-A and gestational diabetes requiring insulin treatment at 11-14 weeks of gestation. J. Matern.-Fetal Neonatal Med. 2012, 25, 2230–2233. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, M.D.; Syngelaki, A.; Muhaisen, M.; Emelyanenko, E.; Nicolaides, K.H. First trimester maternal serum free β-human chorionic gonadotropin and pregnancy-associated plasma protein A in pregnancies complicated by diabetes mellitus. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 410–416. [Google Scholar] [CrossRef]
- Syngelaki, A.; Kotecha, R.; Pastides, A.; Wright, A.; Nicolaides, K.H. First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism 2015, 64, 1485–1489. [Google Scholar] [CrossRef]
- Chau, K.; Hennessy, A.; Makris, A. Placental growth factor and pre-eclampsia. J. Hum. Hypertens. 2017, 31, 782–786. [Google Scholar] [CrossRef] [Green Version]
- O’Gorman, N.; Wright, D.; Syngelaki, A.; Akolekar, R.; Wright, A.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 2016, 214, 103.e1–103.e12. [Google Scholar] [CrossRef] [Green Version]
- Eleftheriades, M.; Papastefanou, I.; Lambrinoudaki, I.; Kappou, D.; Lavranos, D.; Akalestos, A.; Souka, A.P.; Pervanidou, P.; Hassiakos, D.; Chrousos, G.P. Elevated placental growth factor concentrations at 11–14 weeks of gestation to predict gestational diabetes mellitus. Metabolism 2014, 63, 1419–1425. [Google Scholar] [CrossRef]
- Gorkem, U.; Togrul, C.; Arslan, E. Relationship between elevated serum level of placental growth factor and status of gestational diabetes mellitus. J. Matern.-Fetal Neonatal Med. 2020, 33, 4159–4163. [Google Scholar] [CrossRef]
- Mosimann, B.; Amylidi, S.; Risch, L.; Wiedemann, U.; Surbek, D.; Baumann, M.; Stettler, C.; Raio, L. First-Trimester Placental Growth Factor in Screening for Gestational Diabetes. Fetal Diagn. Ther. 2016, 39, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maymon, R.; Meiri, H.; Svirski, R.; Weiner, E.; Cuckle, H. Maternal serum screening marker levels in twin pregnancies affected by gestational diabetes. Arch. Gynecol. Obstet. 2019, 299, 655–663. [Google Scholar] [CrossRef]
- Visconti, F.; Quaresima, P.; Chiefari, E.; Caroleo, P.; Arcidiacono, B.; Puccio, L.; Mirabelli, M.; Foti, D.P.; Di Carlo, C.; Vero, R.; et al. First Trimester Combined Test (FTCT) as a Predictor of Gestational Diabetes Mellitus. Int. J. Environ. Res. Public Health 2019, 16, 3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tul, N.; Pusenjak, S.; Osredkar, J.; Spencer, K.; Novak-Antolic, Z. Predicting complications of pregnancy with first-trimester maternal serum free-betahCG, PAPP-A and inhibin-A. Prenat. Diagn. 2003, 23, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Hauguel-de Mouzon, S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 2007, 30 (Suppl. 2), S120–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhao, Y.H.; Chen, Y.P.; Yuan, X.L.; Wang, J.; Zhu, H.; Lu, C.M. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: A systematic review and meta-analysis. Sci. World J. 2014, 2014, 926932. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Yang, H.; Zhao, Y. Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin. Med. J. 2008, 121, 701–705. [Google Scholar] [CrossRef]
- Guillemette, L.; Lacroix, M.; Battista, M.-C.; Doyon, M.; Moreau, J.; Ménard, J.; Ardilouze, J.-L.; Perron, P.; Hivert, M.-F. TNFα dynamics during the oral glucose tolerance test vary according to the level of insulin resistance in pregnant women. J. Clin. Endocrinol. Metab. 2014, 99, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Syngelaki, A.; Visser, G.H.A.; Krithinakis, K.; Wright, A.; Nicolaides, K.H. First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism 2016, 65, 131–137. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Maged, A.M.; Moety, G.A.F.; Mostafa, W.A.; Hamed, D.A. Comparative study between different biomarkers for early prediction of gestational diabetes mellitus. J. Matern.-Fetal Neonatal Med. 2014, 27, 1108–1112. [Google Scholar] [CrossRef]
- Ozgu-Erdinc, A.S.; Yilmaz, S.; Yeral, M.I.; Seckin, K.D.; Erkaya, S.; Danisman, A.N. Prediction of gestational diabetes mellitus in the first trimester: Comparison of C-reactive protein, fasting plasma glucose, insulin and insulin sensitivity indices. J. Matern.-Fetal Neonatal Med. 2015, 28, 1957–1962. [Google Scholar] [CrossRef]
- Alamolhoda, S.H.; Yazdkhasti, M.; Namdari, M.; Zakariayi, S.J.; Mirabi, P. Association between C-reactive protein and gestational diabetes: A prospective study. J. Obstet. Gynaecol. 2020, 40, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Alyas, S.; Roohi, N.; Ashraf, S.; Ilyas, S.; Ilyas, A. Early pregnancy biochemical markers of placentation for screening of gestational diabetes mellitus (GDM). Diabetes Metab. Syndr. 2019, 13, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.M.; Achamallah, N.; Loughlin, J.O.; Stafford, P.; Dicker, P.; Malone, F.D.; Breathnach, F. First trimester serum biomarkers to predict gestational diabetes in a high-risk cohort: Striving for clinically useful thresholds. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 222, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Sandler, L.; Hsu, K.; Vossen-Smirnakis, K.; Ecker, J.L.; Thadhani, R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 2003, 26, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Retnakaran, R.; Hanley, A.J.G.; Raif, N.; Connelly, P.W.; Sermer, M.; Zinman, B. C-reactive protein and gestational diabetes: The central role of maternal obesity. J. Clin. Endocrinol. Metab. 2003, 88, 3507–3512. [Google Scholar] [CrossRef] [Green Version]
- Berggren, E.K.; Roeder, H.A.; Boggess, K.A.; Moss, K.; Offenbacher, S.; Campbell, E.; Grotegut, C.A. First-trimester maternal serum C-reactive protein as a predictor of third-trimester impaired glucose tolerance. Reprod. Sci. Thousand Oaks Calif. 2015, 22, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Amirian, A.; Rahnemaei, F.A.; Abdi, F. Role of C-reactive Protein(CRP) or high-sensitivity CRP in predicting gestational diabetes Mellitus: Systematic review. Diabetes Metab. Syndr. 2020, 14, 229–236. [Google Scholar] [CrossRef]
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–278. [Google Scholar] [CrossRef]
- Jordan, S.C.; Choi, J.; Kim, I.; Wu, G.; Toyoda, M.; Shin, B.; Vo, A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 2017, 101, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Hoene, M.; Weigert, C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2008, 9, 20–29. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.-Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.-L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Morisset, A.-S.; Dubé, M.-C.; Côté, J.A.; Robitaille, J.; Weisnagel, S.J.; Tchernof, A. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2011, 90, 524–530. [Google Scholar] [CrossRef]
- Siddiqui, S.; Waghdhare, S.; Goel, C.; Panda, M.; Soneja, H.; Sundar, J.; Banerjee, M.; Jha, S.; Dubey, S. Augmentation of IL-6 production contributes to development of gestational diabetes mellitus: An Indian study. Diabetes Metab. Syndr. 2019, 13, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Abell, S.K.; Shorakae, S.; Harrison, C.L.; Hiam, D.; Moreno-Asso, A.; Stepto, N.K.; De Courten, B.; Teede, H.J. The association between dysregulated adipocytokines in early pregnancy and development of gestational diabetes. Diabetes Metab. Res. Rev. 2017, 33, e2926. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.O.; Negrato, C.A.; Matta, M.D.F.B.D.; Carneiro, J.R.I.; Gomes, M.B. Relationship between inflammatory markers, glycated hemoglobin and placental weight on fetal outcomes in women with gestational diabetes. Arch. Endocrinol. Metab. 2019, 63, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šimják, P.; Cinkajzlová, A.; Anderlová, K.; Kloučková, J.; Kratochvílová, H.; Lacinová, Z.; Kaválková, P.; Krejčí, H.; Mráz, M.; Pařízek, A.; et al. Changes in plasma concentrations and mRNA expression of hepatokines fetuin A, fetuin B and FGF21 in physiological pregnancy and gestational diabetes mellitus. Physiol. Res. 2018, 67, S531–S542. [Google Scholar] [CrossRef] [PubMed]
- Hassiakos, D.; Eleftheriades, M.; Papastefanou, I.; Lambrinoudaki, I.; Kappou, D.; Lavranos, D.; Akalestos, A.; Aravantinos, L.; Pervanidou, P.; Chrousos, G. Increased Maternal Serum Interleukin-6 Concentrations at 11 to 14 Weeks of Gestation in Low Risk Pregnancies Complicated with Gestational Diabetes Mellitus: Development of a Prediction Model. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2016, 48, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Amirian, A.; Mahani, M.B.; Abdi, F. Role of interleukin-6 (IL-6) in predicting gestational diabetes mellitus. Obstet. Gynecol. Sci. 2020, 63, 407–416. [Google Scholar] [CrossRef]
- Powe, C.E.; Allard, C.; Battista, M.-C.; Doyon, M.; Bouchard, L.; Ecker, J.L.; Perron, P.; Florez, J.C.; Thadhani, R.; Hivert, M.-F. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 1052–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnakis, K.V.; Martinez, A.; Blatman, K.H.; Wolf, M.; Ecker, J.L.; Thadhani, R. Early pregnancy insulin resistance and subsequent gestational diabetes mellitus. Diabetes Care 2005, 28, 1207–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, E.; Kansara, S.; Kachhawa, G.; Ammini, A.C.; Kriplani, A.; Aggarwal, N.; Gupta, N.; Khadgawat, R. Prediction of gestational diabetes mellitus at 24 to 28 weeks of gestation by using first-trimester insulin sensitivity indices in Asian Indian subjects. Metabolism 2012, 61, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Bitó, T.; Földesi, I.; Nyári, T.; Pál, A. Prediction of gestational diabetes mellitus in a high-risk group by insulin measurement in early pregnancy. Diabet. Med. J. Br. Diabet. Assoc. 2005, 22, 1434–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, I.R.; McKinley, M.C.; Bell, P.M.; Hunter, S.J. Sex hormone binding globulin and insulin resistance. Clin. Endocrinol. 2013, 78, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedderson, M.M.; Xu, F.; Darbinian, J.A.; Quesenberry, C.P.; Sridhar, S.; Kim, C.; Gunderson, E.P.; Ferrara, A. Prepregnancy SHBG concentrations and risk for subsequently developing gestational diabetes mellitus. Diabetes Care 2014, 37, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- Veltman-Verhulst, S.M.; van Haeften, T.W.; Eijkemans, M.J.C.; de Valk, H.W.; Fauser, B.C.J.M.; Goverde, A.J. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum. Reprod. Oxf. Engl. 2010, 25, 3123–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caglar, G.S.; Ozdemir, E.D.U.; Cengiz, S.D.; Demirtaş, S. Sex-hormone-binding globulin early in pregnancy for the prediction of severe gestational diabetes mellitus and related complications. J. Obstet. Gynaecol. Res. 2012, 38, 1286–1293. [Google Scholar] [CrossRef]
- Smirnakis, K.V.; Plati, A.; Wolf, M.; Thadhani, R.; Ecker, J.L. Predicting gestational diabetes: Choosing the optimal early serum marker. Am. J. Obstet. Gynecol. 2007, 196, 410.e1-6. [Google Scholar] [CrossRef]
- Nanda, S.; Savvidou, M.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat. Diagn. 2011, 31, 135–141. [Google Scholar] [CrossRef]
- McElduff, A.; Hitchman, R.; McElduff, P. Is sex hormone-binding globulin associated with glucose tolerance? Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Gabbay-Benziv, R.; Baschat, A.A. Gestational diabetes as one of the “great obstetrical syndromes”—The maternal, placental, and fetal dialog. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Madazli, R.; Tuten, A.; Calay, Z.; Uzun, H.; Uludag, S.; Ocak, V. The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol. Obstet. Investig. 2008, 65, 227–232. [Google Scholar] [CrossRef]
- Daskalakis, G.; Marinopoulos, S.; Krielesi, V.; Papapanagiotou, A.; Papantoniou, N.; Mesogitis, S.; Antsaklis, A. Placental pathology in women with gestational diabetes. Acta Obstet. Gynecol. Scand. 2008, 87, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Gauster, M.; Desoye, G.; Tötsch, M.; Hiden, U. The placenta and gestational diabetes mellitus. Curr. Diab. Rep. 2012, 12, 16–23. [Google Scholar] [CrossRef]
- Jones, C.J.; Fox, H. Placental changes in gestational diabetes. An ultrastructural study. Obstet. Gynecol. 1976, 48, 274–280. [Google Scholar]
- Ravnsborg, T.; Andersen, L.L.T.; Trabjerg, N.D.; Rasmussen, L.M.; Jensen, D.M.; Overgaard, M. First-trimester multimarker prediction of gestational diabetes mellitus using targeted mass spectrometry. Diabetologia 2016, 59, 970–979. [Google Scholar] [CrossRef] [Green Version]
- Thériault, S.; Giguère, Y.; Massé, J.; Girouard, J.; Forest, J.-C. Early prediction of gestational diabetes: A practical model combining clinical and biochemical markers. Clin. Chem. Lab. Med. 2016, 54, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Chen, X.; Chen, C.; Zhang, H.; Law, K.P. Metabolomics in gestational diabetes. Clin. Chim. Acta Int. J. Clin. Chem. 2017, 475, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gao, H.; Zeng, W.; Chen, S.; Feng, L.; Deng, D.; Qiao, F.; Liao, L.; McCormick, K.; Ning, Q.; et al. Placental DNA methylation of peroxisome-proliferator-activated receptor-γ co-activator-1α promoter is associated with maternal gestational glucose level. Clin. Sci. Lond. Engl. 1979 2015, 129, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Farrell, W.E.; Haworth, K.E.; Emes, R.D.; Kitchen, M.O.; Glossop, J.R.; Hanna, F.W.; Fryer, A.A. Maternal genome-wide DNA methylation profiling in gestational diabetes shows distinctive disease-associated changes relative to matched healthy pregnancies. Epigenetics 2018, 13, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008, 41, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Suter, M.A.; Chen, A.; Burdine, M.S.; Choudhury, M.; Harris, R.A.; Lane, R.H.; Friedman, J.E.; Grove, K.L.; Tackett, A.J.; Aagaard, K.M. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 5106–5114. [Google Scholar] [CrossRef] [Green Version]
- Hjort, L.; Martino, D.; Grunnet, L.G.; Naeem, H.; Maksimovic, J.; Olsson, A.H.; Zhang, C.; Ling, C.; Olsen, S.F.; Saffery, R.; et al. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight 2018, 3, 122572. [Google Scholar] [CrossRef]
- Haertle, L.; El Hajj, N.; Dittrich, M.; Müller, T.; Nanda, I.; Lehnen, H.; Haaf, T. Epigenetic signatures of gestational diabetes mellitus on cord blood methylation. Clin. Epigenet. 2017, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Weng, X.; Liu, F.; Zhang, H.; Kan, M.; Wang, T.; Dong, M.; Liu, Y. Genome-wide DNA methylation profiling in infants born to gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 142, 10–18. [Google Scholar] [CrossRef]
- Elliott, H.R.; Sharp, G.C.; Relton, C.L.; Lawlor, D.A. Epigenetics and gestational diabetes: A review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia 2019, 62, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
| Risk Factor | Odds Ratio |
|---|---|
| 1. Ethnicity: Asian, Middle Eastern, Hispanic, Latino, African American, and Indigenous | 2.32 [26] |
| 2. Maternal age ≥35 years | 3.54 [27] |
| 3. Pre-pregnancy BMI >25 kg/m2 | 2.14 [28] |
| 4. Polycystic ovary syndrome | 2.32 [29] |
| 5. GDM in a previous pregnancy | 5.9 [26] |
| 6. Previous delivery of macrocosmic baby (birth weight >4000 gr or >90th centile) | 1.54 [26] |
| 7. Family history of diabetes (1st degree relative) | 1.36 [26] |
| 8. Multiple pregnancy | 1.13 [30] |
| 9. Assisted reproductive technology | 1.26 [31] |
| Biomarker | Function | Suggested Involvement in GDM Pathophysiology |
|---|---|---|
| Adiponectin | Modulation of glucose and fatty acid metabolism. Involvement in inflammation, apoptosis, and angiogenesis. | Low levels associated with decreased insulin sensitivity and GDM |
| Leptin | Regulation of energy balance and expenditure. Role in hormone regulation and immunity. | High leptin levels cause hyperinsulinemia and increase insulin resistance |
| PAPP-A | Increase bioavailability of IGF-1 and promotes somatic growth. Involvement in wound healing and bone remodeling. | Decreased levels contribute to an increase in insulin resistance |
| PLGF | Vascular endothelial growth factor-like protein. Role in angiogenesis and placentation. | High PLGF levels promote the abnormal vascular network in placentas of GDM pregnancies |
| TNF-α | Inflammatory cytokine involved in the regulation of immune cells, inflammation, and autoimmune diseases. | Increased levels impair insulin signaling and beta-cell function, leading to insulin resistance and GDM |
| CRP | Acute-phase reactant. Role in tissue injury, inflammation, and infection. | High levels associated with insulin resistance and systemic inflammation |
| IL-6 | Circulating inflammatory cytokine. Role in immune response regulation, inflammation, and hematopoiesis. | Increased secretion by adipocytes and placental cells, leading to a chronic inflammatory process and insulin resistance |
| SHBG | Glycoprotein that binds androgen and estrogen. | Decrease SHBG levels associated with hyperinsulinemia and GDM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeh, A.; Maor-Sagie, E.; Hallak, M.; Gabbay-Benziv, R. Early Identification of the Maternal, Placental and Fetal Dialog in Gestational Diabetes and Its Prevention. Reprod. Med. 2022, 3, 1-14. https://doi.org/10.3390/reprodmed3010001
Naeh A, Maor-Sagie E, Hallak M, Gabbay-Benziv R. Early Identification of the Maternal, Placental and Fetal Dialog in Gestational Diabetes and Its Prevention. Reproductive Medicine. 2022; 3(1):1-14. https://doi.org/10.3390/reprodmed3010001
Chicago/Turabian StyleNaeh, Amir, Esther Maor-Sagie, Mordechai Hallak, and Rinat Gabbay-Benziv. 2022. "Early Identification of the Maternal, Placental and Fetal Dialog in Gestational Diabetes and Its Prevention" Reproductive Medicine 3, no. 1: 1-14. https://doi.org/10.3390/reprodmed3010001
APA StyleNaeh, A., Maor-Sagie, E., Hallak, M., & Gabbay-Benziv, R. (2022). Early Identification of the Maternal, Placental and Fetal Dialog in Gestational Diabetes and Its Prevention. Reproductive Medicine, 3(1), 1-14. https://doi.org/10.3390/reprodmed3010001







