Abstract
We assessed the prevalence of neonatal abstinence syndrome (NAS) and fetal growth outcomes in neonates exposed to methadone compared to buprenorphine in utero. Three authors assessed the titles and abstracts of all potentially eligible studies. The selection criteria were randomized controlled trials and observational cohort studies from January 2000 to January 2020 which indexed and reported original data for occurrence of NAS and fetal growth outcomes in pregnant people who received methadone vs. buprenorphine treatment. The quality and possible bias of each study was assessed using the Cochrane-risk-of-bias tool. Data were pooled to compare the occurrence of NAS and fetal growth restriction among women who received methadone vs. buprenorphine treatment. Of the 106 articles screened, 1 randomized controlled trial and 5 observational cohort studies including 2041 pregnancies fulfilled the inclusion criteria. Buprenorphine is associated with less NAS and improved growth outcomes compared to methadone. (OR = 0.515; p-value < 0.001). Compared to methadone, buprenorphine is associated with less adverse neonatal outcomes in terms of gestational age at birth, birthweight, and head circumference. With the prevalence of NAS continuing to rise, this study adds to the expanding academic research aimed at creating safer treatment protocols.
1. Introduction
In the United States, opioid use disorder (OUD) in pregnant women quadrupled between 1999 and 2014 and now affects 6.5 per 1000 women delivering in hospitals [1]. Opioid use by pregnant women has detrimental effects on both the mother and the neonate. Notably, infants born to women exposed to opioids during pregnancy can develop neonatal opioid withdrawal syndrome (NOWS) or neonatal abstinence syndrome (NAS) which is a more general term applied to multiple substance exposures [2,3]; however, other complications are also common. With the increasing prevalence of NAS and NOWS (from here on collectively referred to as NAS), the need for medications for addiction treatment (MAT) is imperative. MAT has been shown to improve neonatal outcomes, decrease the risks associated with high-risk behaviors, and reduce the risk of relapse to illicit substances [4].
Newborns exposed to opioids in utero are more likely to be born preterm, have lower birth weights for gestational age, and have smaller head circumferences compared to newborns not exposed to opioids in utero [5]. These infants are likely to have longer hospital stays and are more likely than non-opioid exposed neonates to require care in the NICU. Furthermore, if pregnant women with opioid dependence are not treated through medication-assisted treatment (MAT), their neonates have significantly lower birth weight, increased fetal mortality, increased preterm birth, and increased risk of pre-eclampsia [6]. Collectively, existing studies highlight that buprenorphine-exposed infants are less likely to have symptoms of NAS than untreated infants and are generally healthier neonates.
The treatment of OUD in pregnancy is similar to the treatment of non-pregnant patients and includes opioid agonist or partial agonist therapies. The most broadly known are buprenorphine, buprenorphine-naloxone, and methadone. Detoxification without the assistance of MAT is not encouraged due to the risk of relapse. In addition, acute withdrawal has been associated with abruption and preterm labor [4]. Withdrawal causes stress on the body and the fetus due to autonomic instability, which in turn increases the chances of miscarriage and preterm delivery [7].
Many studies comparing the two medications’ efficacy and safety exist. Methadone is more commonly used to treat OUD, but the use of buprenorphine is increasing. Methadone has been the traditional choice given its use since the 1970s. It is subject to extensive federal regulation and use is limited by the need for daily dosing clinics. Buprenorphine was shown in a large, randomized control trial to have improved neonatal outcomes [8]. It can be prescribed by general practitioners and obtained at a local pharmacy. In addition, the risk of overdose is considerably less given its partial antagonism of the opioid receptor. Given the less stringent oversight, there is an increased risk of diversion. Our goal was to compare the existing literature on MAT in pregnancy, namely, to assess the risk of NAS and fetal growth outcomes. Our hypothesis was that women who were prescribed buprenorphine will have a lower percentage of neonates that show overall symptoms of NAS, and their infants will have higher birth weight compared to women who were prescribed methadone.
2. Materials and Methods
2.1. Data Sources
HC and AS followed the PRISMA guidelines to identify the relevancy and strength of each study [9]. Relevant trials were identified through a search of PubMed, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Google Scholar. Our search combined terms in four areas: (1) “Methadone or Buprenorphine Treatment”, (2) “Pregnancy”, (3) “Pregnancy Outcomes”, and (4) “Newborn Outcomes”. Accordingly, the following eight search terms were used as keywords to find studies, where [MeSH] was added to the end of each keyword and indicated “Medical Subject Heading”: Pregnancy, Newborn, Buprenorphine, Pregnancy Complication, Methadone/therapeutic use, Treatment Outcome, Pregnancy Outcome, and Infant. Terms from different variable areas were combined using the Boolean operator “AND”. Terms from the same area were combined using the Boolean operator “OR”.
2.2. Study Selection
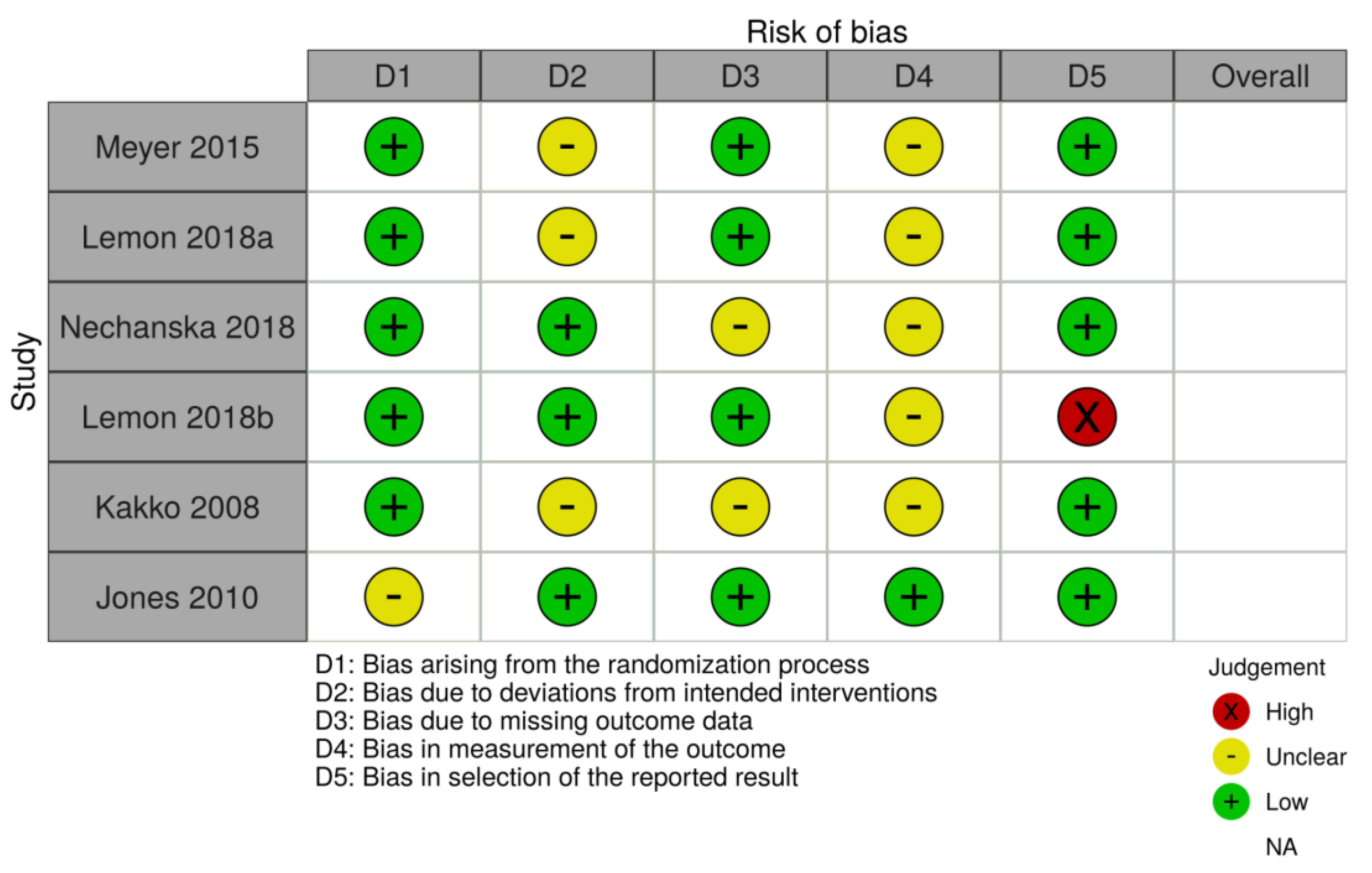
HC and AS assessed the abstracts of these titles and discarded those which did not hold pertinent information. From the relevant titles, full-length texts were analyzed for original data that compared the defined treatment groups and focused on NAS cases and fetal growth outcomes as an outcome. Studies using the wrong medication, unoriginal research such as reviews or expert opinions, and those with ineligible study designs were excluded. Any differences were resolved by consultation with a third author (JS). The quality of each study was assessed by HC, AS, and JS using the PRISMA checklist. Additionally, HC and AS assessed the bias of each study using the Cochrane risk-of-bias tool (Figure 1) [10]. Extracted data included the presence of NAS, gestational age at delivery, birth weight, and head circumference.
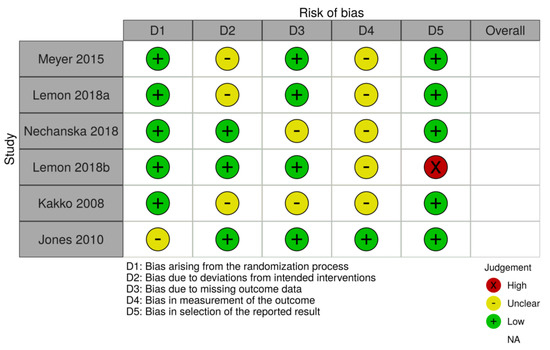
Figure 1.
Cochrane risk-of-bias tool.
2.3. Statistical Analysis
Forest plots were analyzed to compare the treatment outcomes of buprenorphine versus methadone exposed neonates. To create these forest plots, the effect size and confidence interval for each study was calculated to see whether the chosen studies significantly agreed with each other. We then generated the pooled OR by pooling the events of NAS occurring in each treatment group and the number of non-events occurring in each treatment group. A chi-square test was used to generate the heterogeneity statistic (I-squared) for the studies pertaining to the presence of NAS. When analyzing studies individually, a p-value of less than 0.05 was needed for the outcome to be deemed significant.
3. Results
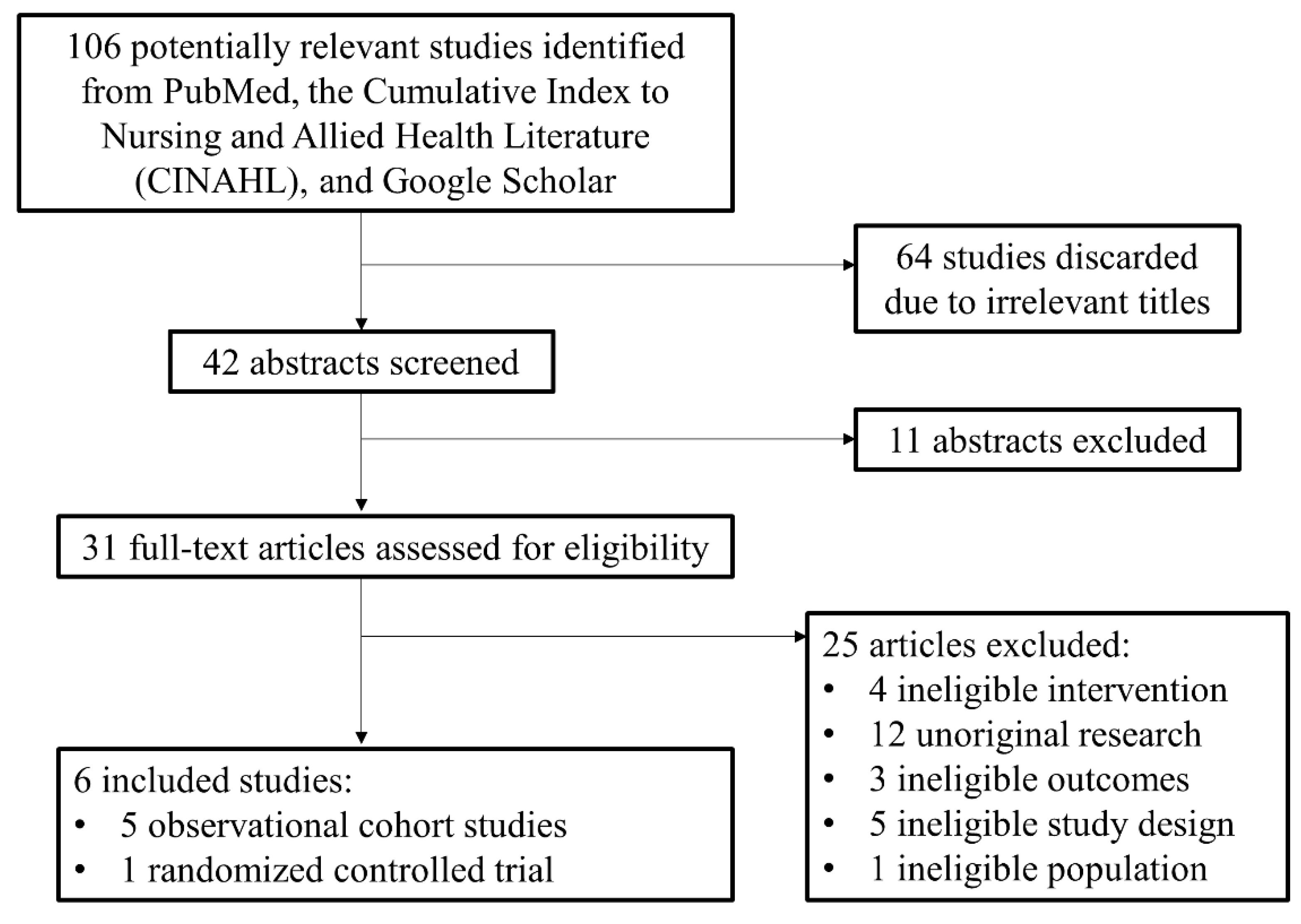
The initial search identified 106 potential studies. Of these, 64 were discarded due to irrelevant titles. The abstracts of 42 studies were analyzed and 11 were excluded due to irrelevant study design and outcomes. The 31 remaining publications were assessed using the metrics defined in Table 1. Of these, 25 studies were excluded due to ineligible intervention or comparison (i.e., wrong medication), unoriginal research (i.e., reviews, or physician guidelines), and ineligible study design (i.e., focused on metrics other than those defined in Table 1) [8,11,12,13,14,15]. Six studies [8,11,12,13,14,15] were included in our analysis, one of which had two cohorts from two separate countries (Figure 2). These included five observational cohort studies (OCS) (n = 1910) and one randomized controlled trial (RCT) (n = 131). The sample sizes for treatment groups ranged from 26 to 407 women and the pooled results for percentage of NAS cases included 1737 women. The range of gestational age at study enrollment ranged from 6.1 weeks to 38 weeks. The mother’s average dose at delivery ranged from 9 to 21.8 mg in the buprenorphine group and 37.5 to 137.3 mg in the methadone group. The outcome definitions among the chosen studies were largely consistent with one study being published as two papers by the same authors with the same cohort of women [12,14].

Table 1.
Overview of included studies.
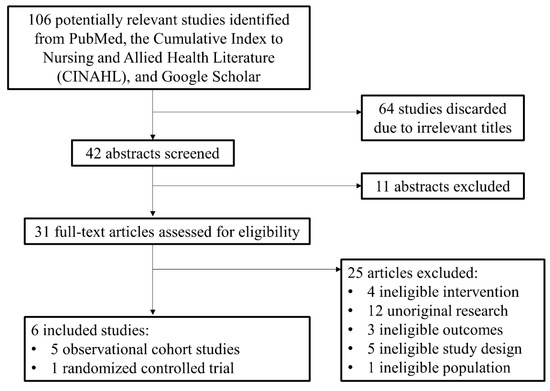
Figure 2.
PRISMA diagram.
3.1. Presence of Neonatal Abstinence Syndrome
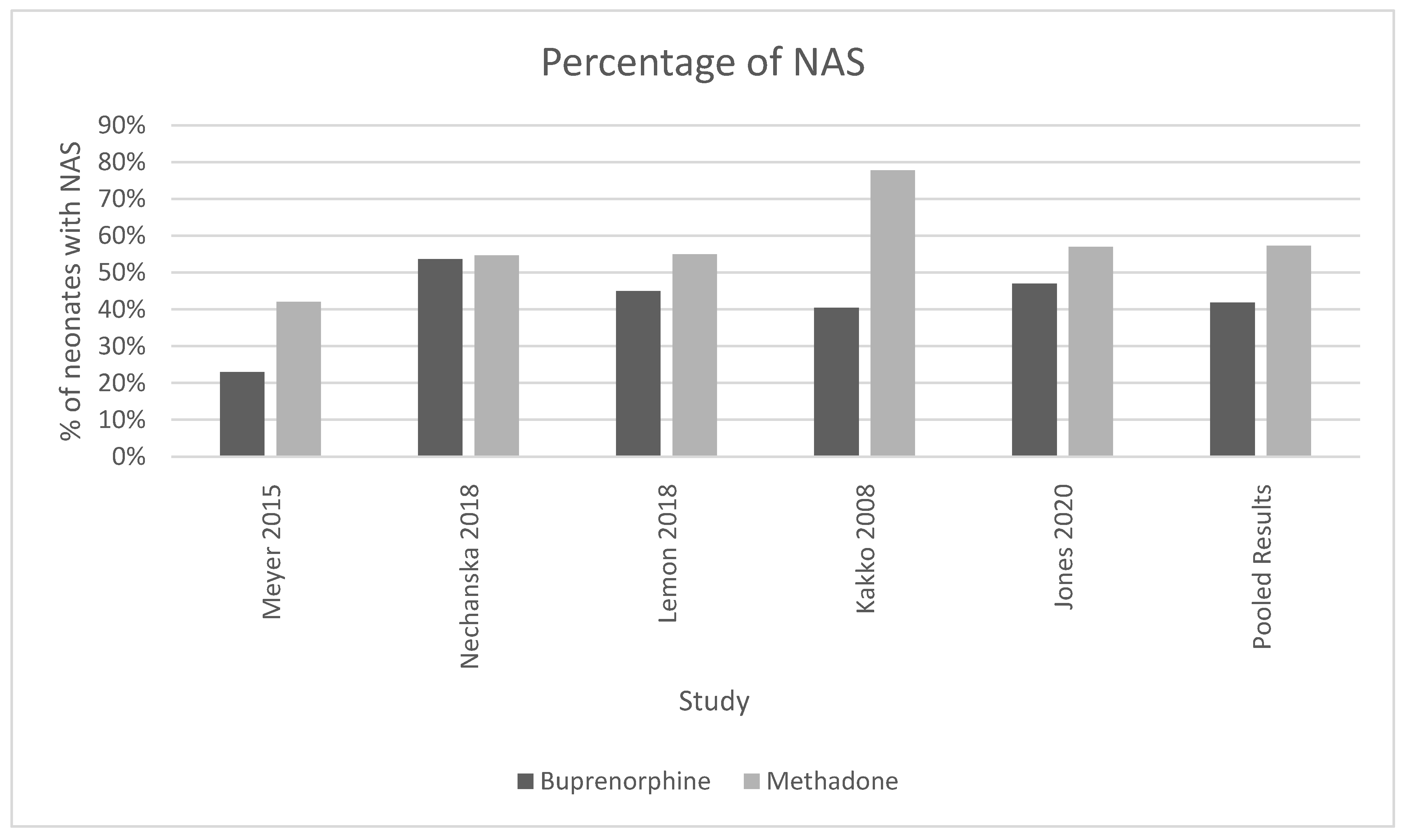
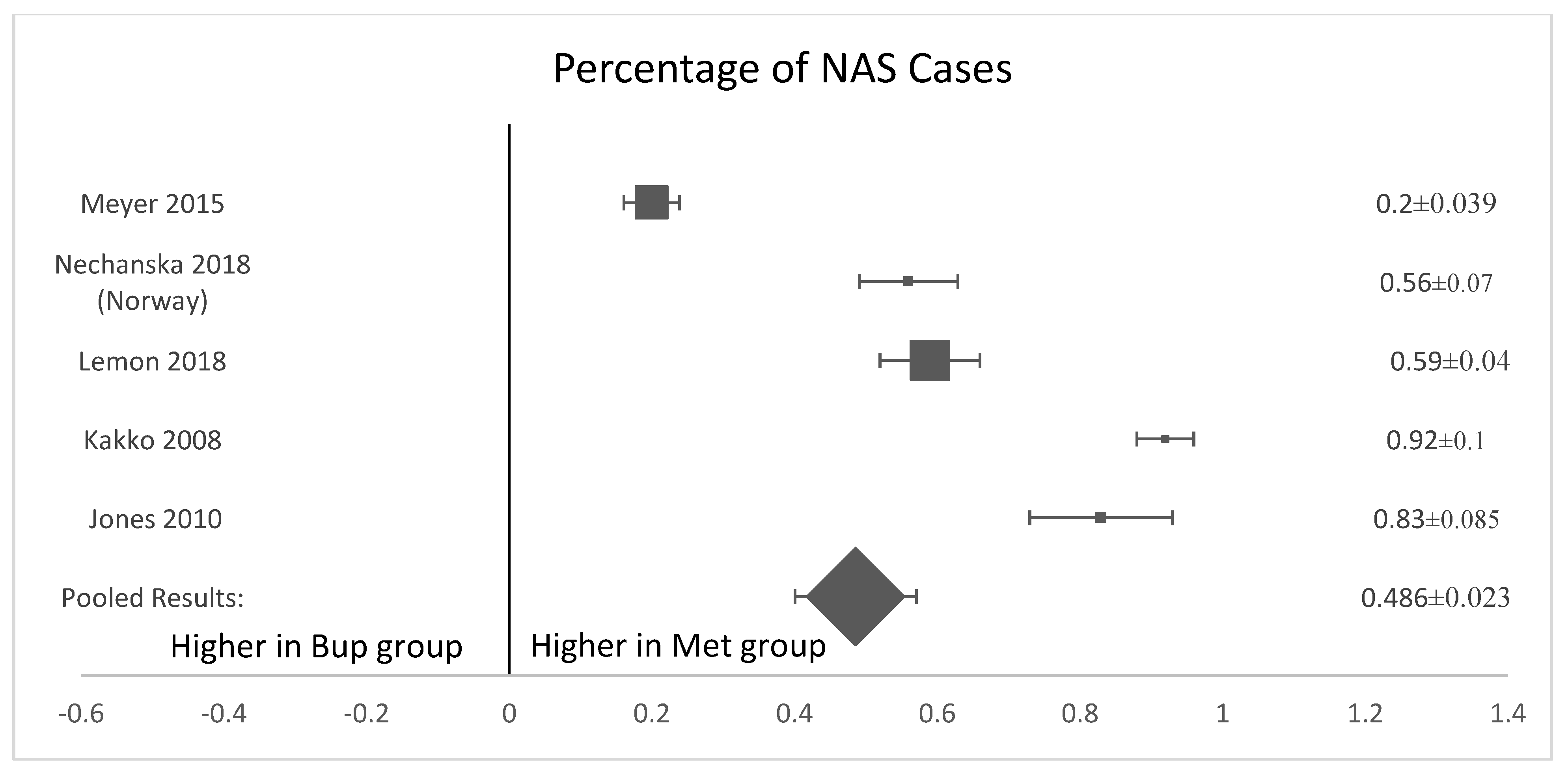
Five of the six analyzed studies [8,11,12,13,15] found that neonates exposed to buprenorphine in utero were significantly less likely to present with NAS after birth (Figure 3). The pooled results of all women who were treated with methadone had a higher mean percentage of neonates born with the symptoms or presence of NAS (57.3%, SD 0.135) compared to neonates exposed to buprenorphine (41.85%, standard deviation 0.131, p-value = 0.035). (Figure 3). The OR was calculated to be 0.52, with a confidence interval of 95% and a p-value < 0.001. The I-squared value was 63.91. The forest plot generated for this outcome shows that the number of NAS cases was significantly higher in the methadone compared to the buprenorphine group (Figure 4).
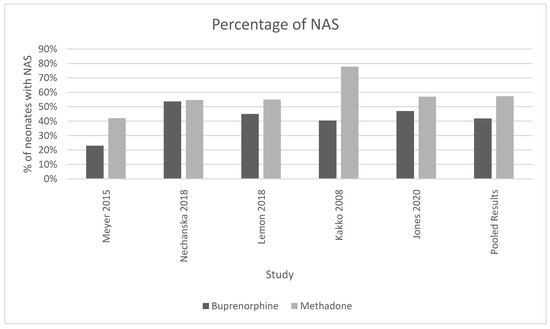
Figure 3.
This histogram showcases the percentage of neonates with NAS found in methadone and buprenorphine treatment groups for each study and the cumulative of all the studies. For the pooled results, 57.3% of neonates born to mothers on methadone had NAS (standard deviation 0.135) and 41.85% of neonates born to mothers on buprenorphine had NAS (standard deviation 0.131), chi-squared p-value = 0.035. I-squared for these studies was 63.91.
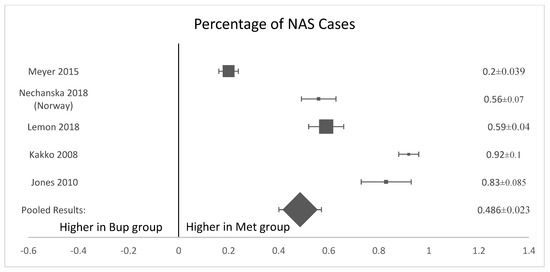
Figure 4.
Forest plot of the difference in percentage of NAS cases between buprenorphine and methadone. (p-value < 0.001).
3.2. Birth and Growth Outcomes
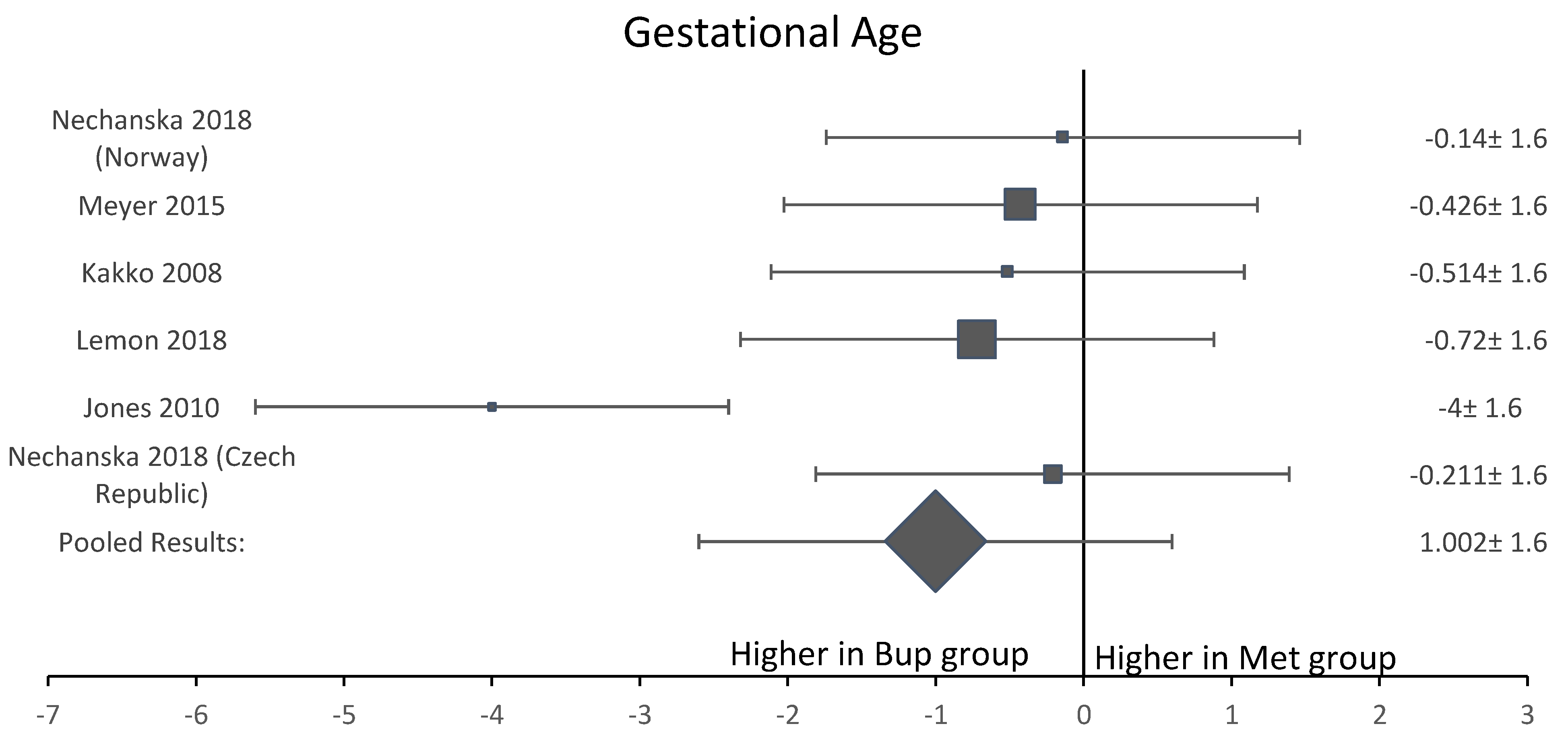
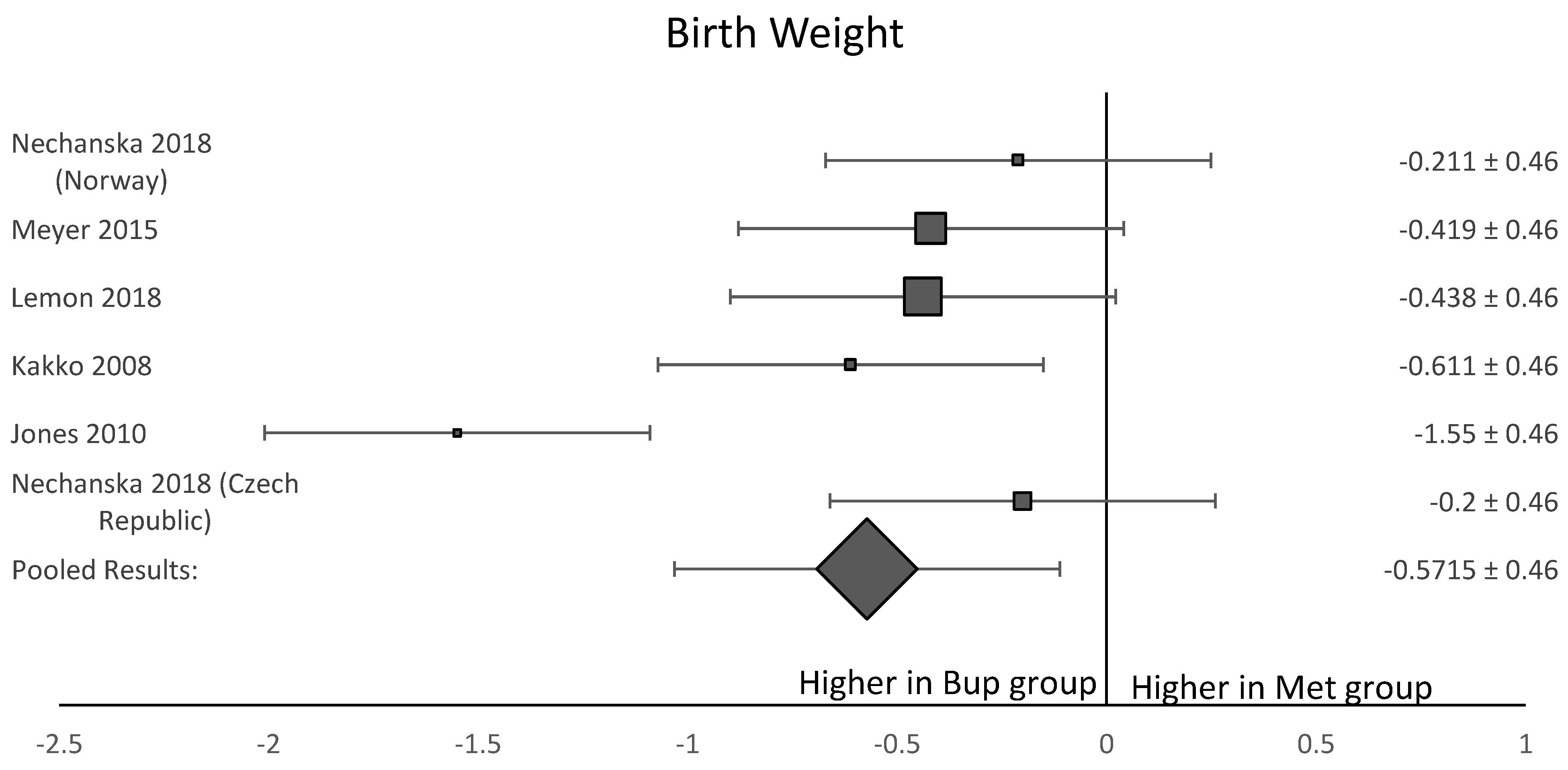
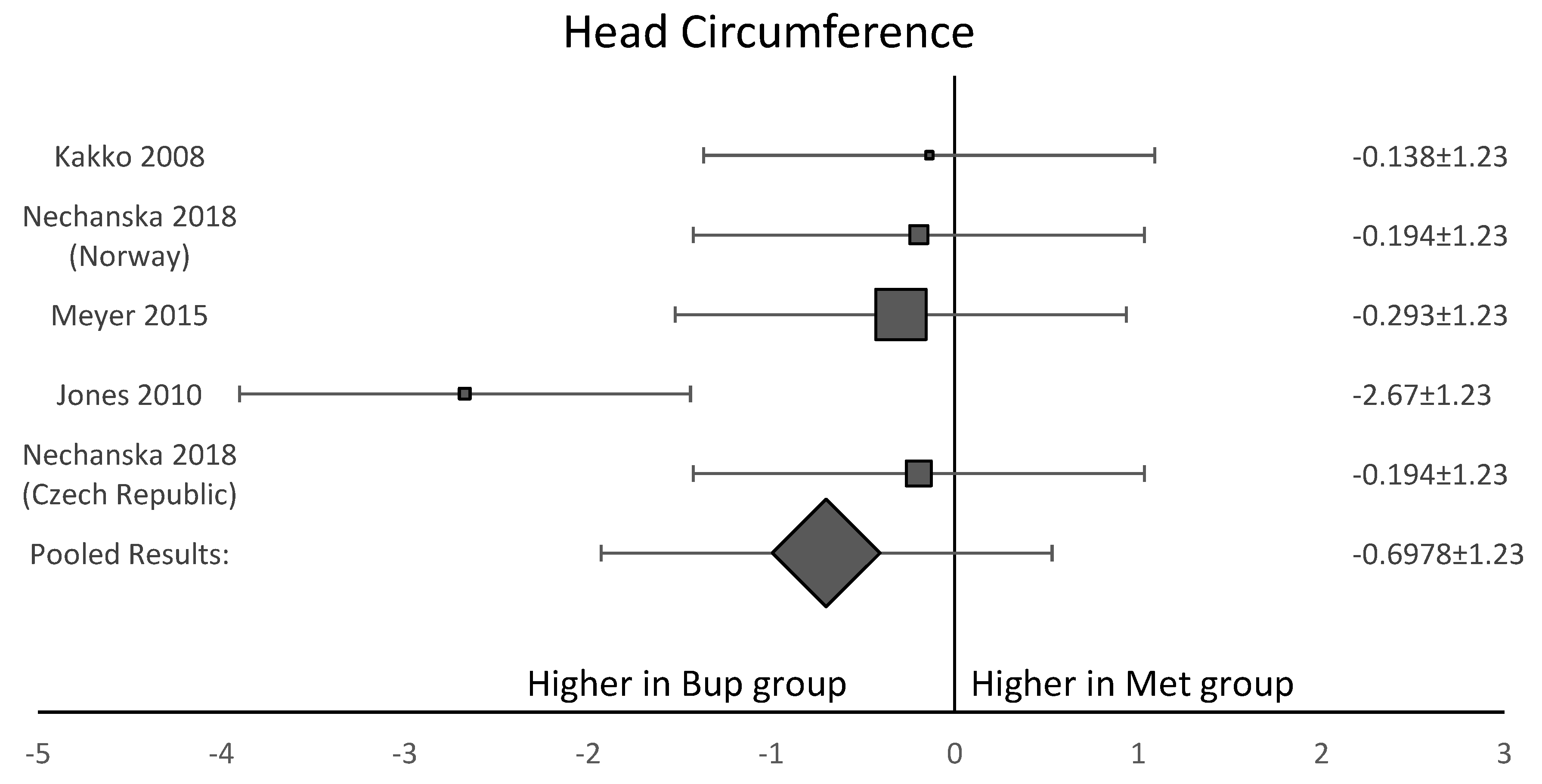
Among the six studies included in this analysis, five OCS [8,11,12,13,15] assessed gestational age at delivery. A forest plot of the difference in gestational age at delivery is shown in Figure 5. This forest plot showed that gestational age was not significantly longer in the buprenorphine than in the methadone group. When assessing birth weight, five of the six studies [8,11,12,13,15] were included. Neonates born to women in the buprenorphine treatment group consistently had a higher birth weight than neonates born to women in the methadone group (Figure 6). The average difference in birth weight between these groups was 229.3 g. For the assessment of neonatal head circumference, four OCS were included [8,11,13,15]. The neonates exposed to buprenorphine had significantly larger head circumferences than neonates exposed to methadone, but the difference was not large enough to be considered significant (Figure 7). In the four OCS, mean head circumference was 0.40 cm bigger for the buprenorphine group compared to the methadone group.
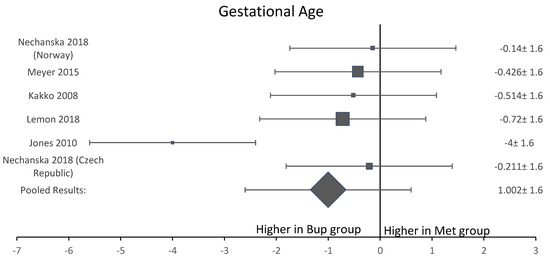
Figure 5.
Forest plot of the difference in gestational age at delivery between buprenorphine and methadone.
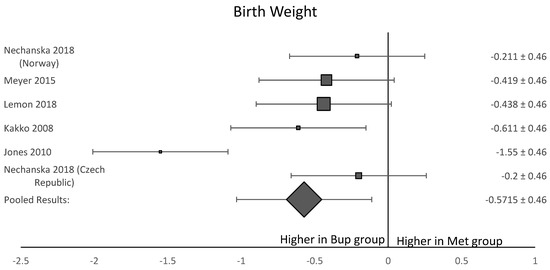
Figure 6.
Forest plot of the difference in birthweight in grams between neonates born to women who received buprenorphine versus methadone during pregnancy (p-value < 0.05).
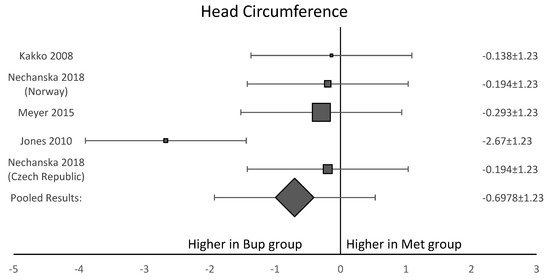
Figure 7.
Forest plot showing that three of the four included studies agreed with each other. This shows the average head circumference for neonates exposed to buprenorphine was larger than neonates exposed to methadone, but the result was not significant (p-value > 0.05).
4. Discussion
Buprenorphine for treatment of OUD during pregnancy is associated with a lower rate of NAS and higher birth weight. The I-squared for these studies on NAS was 63.91, which qualifies these studies as substantially heterogeneous. Our results suggest that buprenorphine may lead to better outcomes for infants, and thus may be preferred during pregnancy.
Our findings are consistent with the findings of prior authors. Tran et al. reviewed research conducted in the last twenty years to provide an overview of the risks and benefits of each medication [16]. In the largest study (n = 609), they found that infants exposed to buprenorphine in utero were less likely to be treated for NAS (p-value < 0.001) than those exposed to methadone [16]. This review also states that in most of the studies it examined, neonates exposed to buprenorphine had a higher birth weight than those exposed to methadone, which agrees with what we found through this meta-analysis [16]. Since the origin of their data arose from smaller studies, these researchers concluded that although buprenorphine shows promising results, more research is needed to definitively assert this. Our meta-analysis has included a larger sample size with 2041 pregnancies.
Our findings are also in accordance with Wilder et al. Comparing methadone and buprenorphine as treatment therapies, they affirm the use of buprenorphine as a first-line drug treatment for opioid-dependent mothers, largely due to the reduced rates of NAS associated with its use. In general, researchers found that exposure to buprenorphine is associated with a greater gestational age at delivery, larger head circumference, and reduced rate of preterm birth [7]. Collectively, this study asserts the necessity of both methadone and buprenorphine in opioid-exposed pregnancies, while also arguing for the continuation of research on these medications’ benefits and risks associated with both maternal and fetal outcomes.
This analysis has limitations. In many of the studies, it was noted that some women would switch from buprenorphine to methadone, which was accounted for in our Cochrane-risk-of-bias tool assessment [10]. Women may switch from buprenorphine to methadone for a variety of reasons, including the side effect profile, lack of a provider to prescribe buprenorphine, or lack of a provider who is comfortable prescribing buprenorphine during pregnancy. Other reasons women may switch include a change in custodial status as prisons may not provide both treatment options. Finally, it may be that women switch from one to another due to compliance, which may result in the methadone group most likely having a higher saturation of highly addicted women than the buprenorphine group did. Indeed, many clinical protocols recommend methadone for women who previously relapsed on buprenorphine. Another limitation of this study is the varying number of participants between the analyzed studies. Two studies were large and had around 300 women in each treatment group, one had about 250 women per treatment group, and the remaining studies had less than 75 women per treatment group. While RCTs provide less biased estimates of treatment effects, the RCT included here was small.
Additionally, the studies used in this analysis had data that mainly came from large administrative databases, which often conveyed inadequate details on OUD histories and prescribed treatment plans. This lack of information may have contributed to confounding variables in our analysis. For example, the included studies did not mention co-administration of SSRIs, and variably reported on smoking, or benzodiazepines, or gabapentin which have known associations with NAS. Finally, the included studies only evaluated the medications that were prescribed without the ability to confirm compliance. In this study, we focused primarily on NAS presence as an outcome, which could have narrowed our focus to more severe manifestations of the condition [8]. Lastly, this population is highly vulnerable and challenging to study. Each individual mother may have a plethora of differing social circumstances which makes achieving high quality research challenging [5]. These issues could vary from difficulty maintaining continuous care, implicit bias among medical professionals lowering the quality of care, as well as mental and physical health issues [6]. Moreover, it is hard to compare the outcomes of exposed neonates to a control group.
The findings of this analysis are nevertheless significant. We surveyed an array of outcomes—presence of NAS, gestational age, birth weight, and head circumference—to provide a more holistic view of how buprenorphine and methadone differentially affect neonates in utero. Our findings expand upon existing literature aiming to quantify these differences in outcomes, which is increasingly necessary given the severity of the opioid epidemic.
As research continues in this area of OUD and pregnancy, studies should not only focus on the presence of NAS at birth but should also follow these exposed infants in the long term. A longitudinal approach would allow us to understand the possible long-term effects of opioid exposure, which warrants consideration in the safety concerns of these treatment options. Furthermore, it is also important to review the maternal adverse effects that may be caused by either of these medications, both during and following pregnancy. The persistent nature of the opioid epidemic in the United States emphasizes the need to expand research on mitigating adverse effects for pregnant women and their children. As NAS becomes increasingly prevalent, this study adds to the growing scholarship aimed at creating safer treatment protocols that may decrease its presence.
Author Contributions
Conceptualization, H.L.C., A.A.S., J.E.G.S., J.H.A. and K.M.A.; methodology, H.L.C., A.A.S., J.E.G.S., K.K.H. and K.M.A.; software, J.E.G.S.; validation, H.L.C., A.A.S., J.E.G.S., K.K.H., and K.M.A.; formal analysis, H.L.C., A.A.S., and J.E.G.S.; investigation, H.L.C., A.A.S., and J.E.G.S.; resources, J.H.A., K.K.H., and K.M.A.; data curation, H.L.C., A.A.S.; writing—original draft preparation, H.L.C., A.A.S., and J.E.G.S.; writing—review and editing, H.L.C., A.A.S., J.E.G.S., J.H.A., K.K.H., and K.M.A.; visualization, J.E.G.S. and H.L.C.; supervision, K.M.A., K.K.H., and J.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This was a meta-analysis which did not directly involve humans. All data used to perform this secondary analysis was previously published.
Acknowledgments
This project was supported by the University of Wisconsin-Madison Department of Biology and the University of Wisconsin Department of Obstetrics & Gynecology. We thank the University of Wisconsin Department of Biology for supporting undergraduate research projects in the medical sciences. We also thank Robert Koehler, medical librarian, for his assistance with the literature search.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mullins, N.; Galvin, S.; Ramage, M.; Gannon, M.; Lorenz, K.; Sager, B.; Coulson, C.C. Buprenorphine and Naloxone Versus Buprenorphine for Opioid Use Disorder in Pregnancy: A Cohort Study. J. Addict. Med. 2019, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants ii Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Available online: http://store.samhsa.gov (accessed on 28 February 2020).
- Patrick, S.W.; Schumacher, R.E.; Benneyworth, B.D.; Krans, E.E.; McAllister, J.M.; Davis, M.M. Neonatal Abstinence Syndrome and Associated Health Care Expenditures. JAMA 2012, 307, 1934–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ACOG. Opioid Use and Opioid Use Disorder in Pregnancy. Available online: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/08/opioid-use-and-opioid-use-disorder-in-pregnancy (accessed on 19 July 2021).
- Laslo, J.; Brunner, J.-M.; Burns, D.; Butler, E.; Cunningham, A.; Killpack, R.; Pyeritz, C.; Rinard, K.; Childers, J.; Horzempa, J. An overview of available drugs for management of opioid abuse during pregnancy. Matern. Health Neonatol. Perinatol. 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. How Do Medications toTreat Opioid Use Disorder Work?|National Institute on Drug Abuse (NIDA). National Institute on Drug Abuse Research Reports. Available online: https://www.drugabuse.gov/publications/research-reports/medications-to-treat-opioid-addiction/how-do-medications-to-treat-opioid-addiction-work (accessed on 28 February 2020).
- Wilder, C.M.; Winhusen, T. Pharmacological Management of Opioid Use Disorder in Pregnant Women. CNS Drugs 2015, 29, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.E.; Kaltenbach, K.; Heil, S.H.; Stine, S.M.; Coyle, M.G.; Arria, A.M.; O’Grady, K.E.; Selby, P.; Martin, P.R.; Fischer, G. Neonatal Abstinence Syndrome After Methadone or Buprenorphine Exposure. N. Engl. J. Med. 2010, 363, 2320–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses. 2021. Available online: http://www.prisma-statement.org/ (accessed on 31 January 2020).
- Sterne, J.A.C.; Savović, J.; Page, M.; Elbers, R.G.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakko, J.; Heilig, M.; Sarman, I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: Comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008, 96, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.C.; Johnston, A.M.; Crocker, A.M.; Heil, S.H. Methadone and Buprenorphine for Opioid Dependence during Pregnancy. J. Addict. Med. 2015, 9, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemon, L.S.; Caritis, S.N.; Venkataramanan, R.; Platt, R.W.; Bodnar, L.M. Methadone Versus Buprenorphine for Opioid Use Dependence and Risk of Neonatal Abstinence Syndrome. Epidemiology 2018, 29, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lemon, L.S.; Naimi, A.; Caritis, S.N.; Platt, R.W.; Venkataramanan, R.; Bodnar, L.M. The Role of Preterm Birth in the Association between Opioid Maintenance Therapy and Neonatal Abstinence Syndrome. Paediatr. Périnat. Epidemiol. 2018, 32, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Nechanská, B.; Mravcik, V.; Skurtveit, S.; Lund, I.O.; Gabrhelik, R.; Engeland, A.; Handal, M. Neonatal outcomes after fetal exposure to methadone and buprenorphine: National registry studies from the Czech Republic and Norway. Addiction 2018, 113, 1286–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.H.; Griffin, B.L.; Stone, R.H.; Vest, K.M.; Todd, T.J. Methadone, Buprenorphine, and Naltrexone for the Treatment of Opioid Use Disorder in Pregnant Women. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 824–839. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).