Examining the Impact of the 2019 Novel Coronavirus and Pandemic-Related Hardship on Adverse Pregnancy and Infant Outcomes: Design and Launch of the HOPE COVID-19 Study
Abstract
1. Introduction
Background and Rationale
2. Materials and Methods
2.1. Aims
2.2. Study Design
2.3. Selection and Enrollment of Participants
2.3.1. Inclusion Criteria
- 18 years of age or older.
- Comfortable reading and writing in English (Spanish to be added in early 2021).
- 3.
- Live in target geography for phase (phase I–2: San Francisco, Marin, Sonoma, Napa, Solano, Contra Costa, Alameda, Santa Clara, San Mateo Yolo, Sacramento, Los Angeles, Orange, and San Diego counties in California; phase 3–4: all of these counties plus Seattle and Tacoma, Washington and Portland and Eugene, Oregon, if funding is secured).
- 4.
- Willingness to have their hospital records reviewed for study purposes.
- 5.
- Willingness to contribute nasal swab and blood specimens at all study time points (enrollment, subsequent trimesters, and 6–8 weeks after birth).
- Able to contribute blood spots from an infant heel prick.
2.3.2. Exclusion Criteria
- Not able or willing to fill out surveys on phone or computer.
- Not able or willing to download and use mobile phone applications.
- Infants will be excluded if in the care of the NICU or of uncertain viability.
2.4. Recruitment
Study Enrollment and Consent
2.5. Participant Management
Participant Follow-Up
2.6. Specimen and Data Collection
2.6.1. Detail: Survey Measures
2.6.2. Molecular Measures
2.7. Medical Chart Review and Biomonitoring
2.8. Qualitative Interviews
2.9. Results Reporting
2.10. Duration of Follow-Up and Criteria for Premature Study Withdrawal
3. Statistical Considerations
3.1. Analyses
3.1.1. Quantitative Analyses
3.1.2. Qualitative Analyses
3.2. Sample Size and Power
4. Human Subjects
4.1. Subject Selection Criteria
4.2. Reimbursement of Subjects
5. Institutional Review Board (IRB) Review and Informed Consent
6. Study Launch
7. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 22 June 2020).
- Sutton, D.; Fuchs, K.; D’Alton, M.; Goffman, D. Universal screening for SARS-CoV-2 in women admitted for delivery. N. Engl. J. Med. 2020, 382, 2163–2164. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, L.; Wu, X.; Zheng, D.; Wang, J.; Yang, L.; Zheng, C. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: A preliminary analysis. AJR Am. J. Roentgenol. 2020, 215, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Kalafat, E.; Benlioglu, C.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Heath, P.; Ladhani, S.; et al. SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020, 100446. [Google Scholar] [CrossRef]
- Smith, V.; Seo, D.; Warty, R.; Payne, O.; Salih, M.; Chin, K.L.; Ofori-Asenso, R.; Krishnan, S.; da Silva Costa, F.; Vollenhoven, B.; et al. Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review. PLoS ONE 2020, 15, e0234187. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. MFM 2020, 2, 100118. [Google Scholar] [CrossRef]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020, 369, m2107. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Tang, K.; Guo, Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020. [Google Scholar] [CrossRef]
- Peyronnet, V.; Sibiude, J.; Deruelle, P.; Huissoud, C.; Lescure, X.; Lucet, J.C.; Mandelbrot, L.; Nisand, I.; Vayssière, C.; Yazpandanah, Y.; et al. SARS-CoV-2 infection during pregnancy. Information and proposal of management care. CNGOF. Gynecol. Obstet. Fertil. Senol. 2020, 48, 436–443. [Google Scholar] [CrossRef]
- Brodin, P. Why is COVID-19 so mild in children? Acta Paediatr. 2020, 109, 1082–1083. [Google Scholar] [CrossRef]
- Rebmann, T. Severe acute respiratory syndrome: Implications for perinatal and neonatal nurses. J. Perinat. Neonatal Nurs. 2005, 19, 332–345. [Google Scholar] [CrossRef]
- Parri, N.; Magistà, A.M.; Marchetti, F.; Cantoni, B.; Arrighini, A.; Romanengo, M.; Felici, E.; Urbino, A.; Da Dalt, L.; Verdoni, L.; et al. Characteristic of COVID-19 infection in pediatric patients: Early findings from two Italian pediatric research networks. Eur. J. Pediatr. 2020, 179, 1315–1323. [Google Scholar] [CrossRef]
- Anderson, J.G.; Rogers, E.E.; Baer, R.J.; Oltman, S.P.; Paynter, R.; Partridge, J.C.; Rand, L.; Jelliffe-Pawlowski, L.L.; Steurer, M.A. Racial and ethnic disparities in preterm infant mortality and severe morbidity: A population-based study. Neonatology 2018, 113, 44–54. [Google Scholar] [CrossRef]
- Ross, K.M.; Dunkel Schetter, C.; McLemore, M.R.; Chambers, B.D.; Paynter, R.A.; Baer, R.; Feuer, S.K.; Flowers, E.; Karasek, D.; Pantell, M.; et al. Socioeconomic status, preeclampsia risk and gestational length in black and white women. J. Racial Ethn. Health Disparities 2019, 6, 1182–1191. [Google Scholar] [CrossRef]
- Goldfarb, I.T.; Clapp, M.A.; Soffer, M.D.; Shook, L.L.; Rushfirth, K.; Edlow, A.G.; Boatin, A.A.; Kaimal, A.J.; Barth, W.H., Jr.; Bryant, A.S. Prevalence and severity of coronavirus disease 2019 (COVID-19) Illness in symptomatic pregnant and postpartum women stratified by hispanic ethnicity. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef]
- Travers, C.P.; Carlo, W.A.; McDonald, S.A.; Das, A.; Ambalavanan, N.; Bell, E.F.; Sánchez, P.J.; Stoll, B.J.; Wyckoff, M.H.; Laptook, A.R.; et al. Racial/ethnic disparities among extremely preterm infants in the United States from 2002 to 2016. JAMA Netw. Open 2020, 3, e206757. [Google Scholar] [CrossRef] [PubMed]
- Howell, E.A.; Janevic, T.; Blum, J.; Zeitlin, J.; Egorova, N.N.; Balbierz, A.; Hebert, P.L. Double disadvantage in delivery hospital for black and hispanic women and high-risk infants. Matern. Child Health J. 2020, 24, 687–693. [Google Scholar] [CrossRef]
- Chambers, B.D.; Baer, R.J.; McLemore, M.R.; Jelliffe-Pawlowski, L.L. Using index of concentration at the extremes as indicators of structural racism to evaluate the association with preterm birth and infant mortality-California, 2011–2012. J. Urban Health 2019, 96, 159–170. [Google Scholar] [CrossRef]
- Cook, N.; Ayers, S.; Horsch, A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: A systematic review. J. Affect. Disord. 2018, 225, 18–31. [Google Scholar] [CrossRef]
- Szegda, K.; Markenson, G.; Bertone-Johnson, E.R.; Chasan-Taber, L. Depression during pregnancy: A risk factor for adverse neonatal outcomes? A critical review of the literature. J. Matern. Fetal Neonatal Med. 2014, 27, 960–967. [Google Scholar] [CrossRef]
- Hoffman, S.; Hatch, M.C. Stress, social support and pregnancy outcome: A reassessment based on recent research. Paediatr. Perinat. Epidemiol. 1996, 10, 380–405. [Google Scholar] [CrossRef]
- Hamad, R.; Collin, D.F.; Baer, R.J.; Jelliffe-Pawlowski, L.L. Association of revised WIC food package with perinatal and birth outcomes: A quasi-experimental study. JAMA Pediatr. 2019, 173, 845–852. [Google Scholar] [CrossRef]
- Howell, E.A.; Brown, H.; Brumley, J.; Bryant, A.S.; Caughey, A.B.; Cornell, A.M.; Grant, J.H.; Gregory, K.D.; Gullo, S.M.; Kozhimannil, K.B.; et al. Reduction of peripartum racial and ethnic disparities: A conceptual framework and maternal safety consensus bundle. J. Obstet. Gynecol. Neonatal Nurs. 2018, 47, 275–289. [Google Scholar] [CrossRef]
- Laurencin, C.T.; McClinton, A. The COVID-19 pandemic: A call to action to identify and address racial and ethnic disparities. J. Racial Ethn. Health Disparities 2020, 7, 398–402. [Google Scholar] [CrossRef]
- Scott, K.A.; Britton, L.; McLemore, M.R. The ethics of perinatal care for black women: Dismantling the structural racism in “mother blame” narratives. J. Perinat. Neonatal Nurs. 2019, 33, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chambers, B.D.; Arabia, S.E.; Arega, H.A.; Altman, M.R.; Berkowitz, R.; Feuer, S.K.; Franck, L.S.; Gomez, A.M.; Kober, K.; Pacheco-Werner, T.; et al. Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health 2020, 36, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Raman, S. COVID-19 Amplifies Racial Disparities in Maternal Health. Available online: https://www.rollcall.com/2020/05/14/covid-19-amplifies-racial-disparities-in-maternal-health (accessed on 18 June 2020).
- Daniel, A. For Maternal Health and Covid-19, a Racial Divide. Available online: http://blog.lareviewofbooks.org/essays/maternal-health-covid-19-racial-divide/ (accessed on 18 June 2020).
- Citizen Science. Covid-19. Available online: covid19.eurekaplatform.org (accessed on 18 June 2020).
- MotherToBaby. Home Page. Available online: https://mothertobaby.org/ (accessed on 18 June 2020).
- Azumio. Instant Heart Rate. Available online: https://azumio.com/s/instantheartrate/index.html (accessed on 22 June 2020).
- ACES Aware. Adverse Childhood Experiences Revised Questionnaire. Available online: https://acesaware.org/screen/screening-tools (accessed on 22 June 2020).
- The Philadelphia ACE Project. Philadelphia ACE Survey. Available online: https://philadelphiaaces.org/philadelphia-ace-survey (accessed on 22 June 2020).
- Williams, D.R.; Yan, Y.; Jackson, J.S.; Anderson, N.B. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J. Health Psychol. 1997, 2, 335–351. [Google Scholar] [CrossRef]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The Satisfaction With Life Scale. J. Personal. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Huizink, A.C.; Mulder, E.J.; Robles de Medina, P.G.; Visser, G.H.; Buitelaar, J.K. Is pregnancy anxiety a distinctive syndrome? Early Hum. Dev. 2004, 79, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.; Peplau, L.A.; Ferguson, M.L. Developing a measure of loneliness. J. Personal. Assess. 1978, 42, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Memelstein, R.; Kamarck, T.; Hoberman, H. Measuring the functional components of social support. In Social Support: Theory, Research and Application; Sarason, I.G.S.B., Ed.; Martinus Nijhoff: The Hague, The Netherlands, 1985; pp. 73–94. [Google Scholar]
- Smith, B.W.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Moyer, C. Experiences with Pregnancy During COVID-19 Survey; University of Michigan: Ann Arbor, MI, USA, 2020; Ongoing work. [Google Scholar]
- Connor, K.M.; Davidson, J.R. Development of a new resilience scale: The connor-davidson resilience scale (CD-RISC). Depress. Anxiety 2003, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Council on Community Pediatrics; Committee on Nutrition. Promoting food security for all children. Pediatrics 2015, 136, e1431–e1438. [Google Scholar] [CrossRef]
- Hagen, I.H.; Svindseth, M.F.; Nesset, E.; Orner, R.; Iversen, V.C. Validation of the neonatal satisfaction survey (NSS-8) in six Norwegian neonatal intensive care units: A quantitative cross-sectional study. BMC Health Serv. Res. 2018, 18, 222. [Google Scholar] [CrossRef]
- Schonhaut, L.; Armijo, I.; Schönstedt, M.; Alvarez, J.; Cordero, M. Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics 2013, 131, e1468–e1474. [Google Scholar] [CrossRef]
- Ahluwalia, I.B.; Helms, K.; Morrow, B. Assessing the validity and reliability of three indicators self-reported on the pregnancy risk assessment monitoring system survey. Public Health Rep. 2013, 128, 527–536. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item edinburgh postnatal depression scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Research Use Only 2019-Novel Coronavirus (2019-nCoV) Real- Time RT-PCR Primer and Probe Information. Available online: https://cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf (accessed on 24 April 2020).
- Providence Health & Services. Coronavirus (COVID-19) PCR. Available online: https://www.testmenu.com/providence/Tests/1035005 (accessed on 18 June 2020).
- Moran, A.; Beavis, K.G.; Matushek, S.M.; Ciaglia, C.; Francois, N.; Tesic, V.; Love, N. The detection of SARS-CoV-2 using the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 Assays. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Wilmet, A.; Beukinga, I.; Dogné, J.M.; Douxfils, J.; Blairon, L. Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- ThermoFisher Scientific. Cytokine/Chemokine/Growth Factor 45-Plex Human ProcartaPlex™ Pnael 1. Available online: https://thermofisher.com/order/catalog/product/EPX450-12171-901#/EPX450-12171-901 (accessed on 28 April 2020).
- Providence Health & Services. Respiratory Pathogen Panel, NAAT. Available online: https://www.testmenu.com/providence/Tests/620487 (accessed on 18 June 2020).
- University of California Diego. Mother-Milk-Infant Center of Research Excellence. Available online: https://medschool.ucsd.edu/research/coe/momicore/pages/default.aspx (accessed on 28 April 2020).
- Siemens Healthineers. Multistix® 10 SG Reagent Strips. Available online: https://siemens-healthineers.com/urinalysis-products/urinalysis-reagents/multistix-10-sg-reagent-strips (accessed on 28 April 2020).
- Pipitprapat, W.; Harnchoowong, S.; Suchonwanit, P.; Sriphrapradang, C. The validation of smartphone applications for heart rate measurement. Ann. Med. 2018, 50, 721–727. [Google Scholar] [CrossRef]
- Healthsmart. Healthsmart® Digiscan® Forehead and Ear Thermometer. Available online: https://Livehealthsmart.com/HealthSmart-DigiScan-Forehead-and-Ear-Thermometer (accessed on 26 April 2020).
- OMRON. 5-Series Upper Arm Blood Pressure Monitor. Available online: https://omronhealthcare.com/products/5-series-upper-arm-blood-pressure-monitor-bp7200/ (accessed on 24 June 2020).
- Pearl, J. Causal Inference in statistics: An overview. Stat. Surv. 2009, 3, 96–146. [Google Scholar] [CrossRef]
- Omics Data Automation, Inc. (ODA). Available online: https://omicsautomation.com (accessed on 24 June 2020).
- Dedoose. Home Page. Available online: https://dedoose.com (accessed on 26 April 2020).
- California Office of Statewide Health Planning and Development. Research Data Request Information. Available online: http://oshpd.ca.gov/data-and-reports/research-data-request-information (accessed on 24 April 2020).
- County of San Francisco. San Francisco COVID-19 Data Tracker. Available online: https://Data.sfgov.org/stories/s/San-Francisco-COVID-19-Data-Tracker/fjki-2fab (accessed on 16 April 2020).
- County of San Diego. County of San Diego Coronavirus Disease (COVID-19) Dashboard. Available online: www.sandiegocounty.gov/content/sdc/hhsa/programs/phs/community_epidemiology/dc/2019-nCoV/status.html (accessed on 16 April 2020).
- County of San Diego. COVID-19 Tests by Date Reported. Available online: https://Sandiegocounty.gov/content/dam/sdc/hhsa/programs/phs/Epidemiology/COVID-19%20Tests%20in%20San%20Diego%20County%20by%20Date%20Reported.pdf (accessed on 16 April 2020).
- McClure, E.S.; Vasudevan, P.; Bailey, Z.; Patel, S.; Robinson, W.R. Racial Capitalism within Public Health: How Occupational Settings Drive COVID-19 Disparities. Am J Epidemiol. 2020. [Google Scholar] [CrossRef]
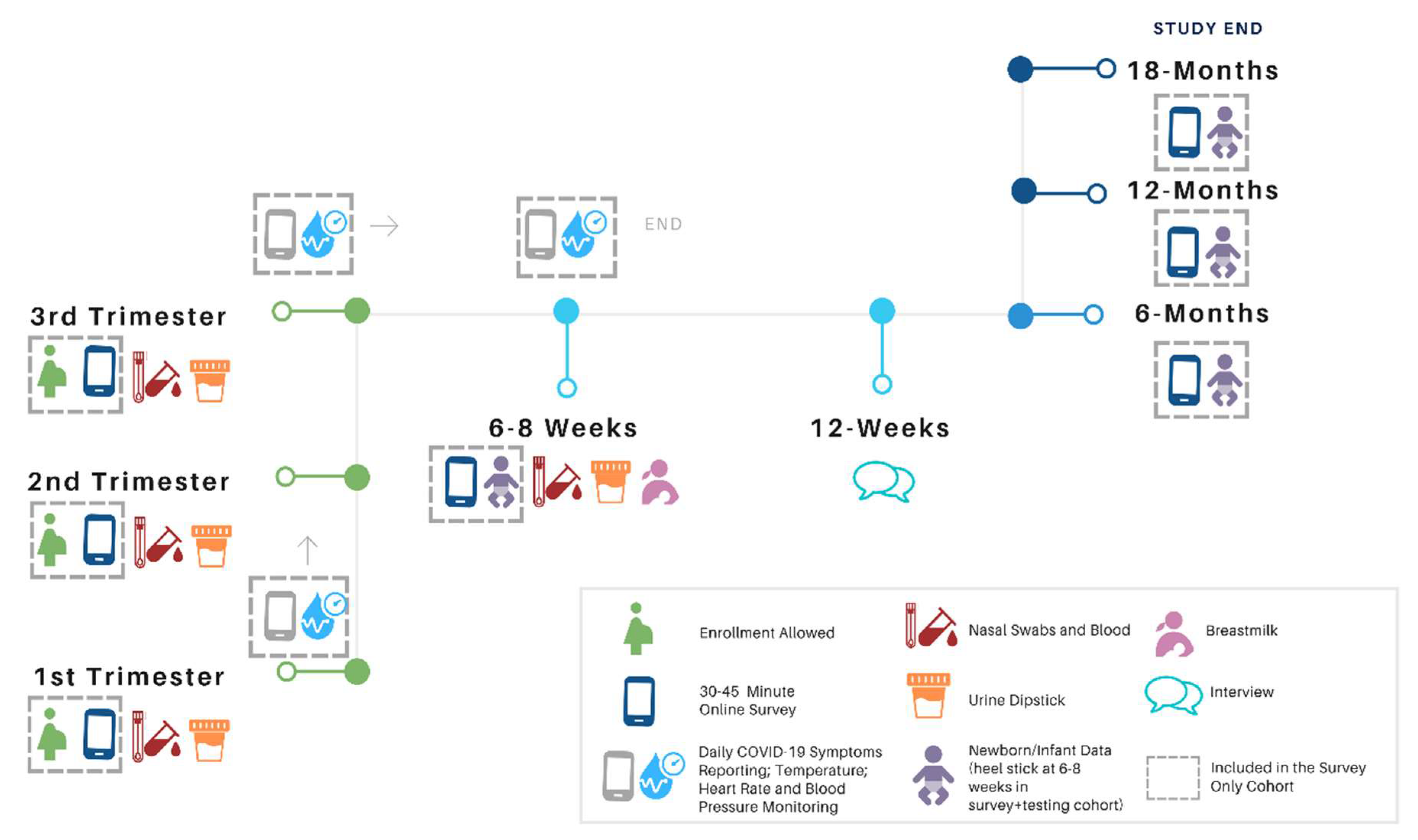
| Prenatal | 6–8 Week Postpartum | Infant Health at 6, 8 and 12, and 18 Months |
|---|---|---|
| “E” = Enrollment Survey “S” = Subsequent Trimester Surveys Contact/Demographics 17 Questions/sets (all E, some S) Psychosocial Health before Pregnancy 2 Questions/sets (all E only) Including (in part):Physical Health before Pregnancy 8 Questions/sets (all E only) Health Care 2 Questions (all E, some S) Physical Health Current Pregnancy 19 Questions/sets (all E, S) Psychosocial Health Current Pregnancy 25 Questions/sets (all E, S) Including (in part):
23 Questions/sets (all E, some S) Including (in part)
| New Baby Information 14 Questions/sets
5 Questions/sets Including:
11 Questions/sets Including (in part):
4 Questions/sets Including (in part):
21 Questions/sets Psychosocial Health (since last survey to current) 21 Questions/sets Including (in part):
18 Questions/sets Including (in part):
| Infant Health 7 questions/sets Infant Development 5 questions/sets Including (in part)
3 Questions/sets Including (in part):
24 Questions/sets Including (in part):
18 Questions/sets Including (in part):
|
| Rate of SARS-COV-2 in Sample | |||
|---|---|---|---|
| 5% | 10% | 15% | |
| DD, RR | DD, RR | DD, RR | |
| Sample size/unadjusted models/adverse pregnancy outcomes * | |||
| 600 | 0.25, 1.76 | 0.18, 1.56 | 0.15, 1.48 |
| 1200 | 0.18, 1.53 | 0.13, 1.39 | 0.11, 1.33 |
| 2400 | 0.13, 1.37 | 0.09, 1.27 | 0.08, 1.23 |
| 4800 | 0.09, 1.26 | 0.06, 1.19 | 0.05, 1.16 |
| 9600 | 0.06, 1.18 | 0.05, 1.13 | 0.04, 1.11 |
| Sample size/adjusted models **/adverse pregnancy outcomes * | |||
| 600 | 0.29, 1.88 | 0.21, 1.64 | 0.18, 1,55 |
| 1200 | 0.21, 1.61 | 0.15, 1.45 | 0.13, 1.38 |
| 2400 | 0.15, 1.42 | 0.11, 1.31 | 0,09, 1.26 |
| 4800 | 0.10, 1.30 | 0.07, 1.22 | 0.06, 1.18 |
| 9600 | 0.07, 1.21 | 0.05, 1.15 | 0.04, 1.13 |
| Sample size/unadjusted models/adverse infant outcomes * | |||
| 600 | 0.20, 2.22 | 0.15, 1.90 | 0.12, 1.77 |
| 1200 | 0.14, 1.84 | 0.11, 1.62 | 0.09, 1.53 |
| 2400 | 0.10, 1.58 | 0.07, 1.43 | 0.06, 1.36 |
| 4800 | 0.07, 1.41 | 0.05, 1.30 | 0.04, 1.25 |
| 9600 | 0.05, 1.29 | 0.04, 1.21 | 0.03, 1.18 |
| Sample size/adjusted models **/adverse infant outcomes * | |||
| 600 | 0.24, 2.42 | 0.17, 2.05 | 0.14, 1.91 |
| 1200 | 0.17, 1.97 | 0.12, 1.72 | 0.10, 1.62 |
| 2400 | 0.12, 1.68 | 0.08, 1.50 | 0.07, 1.42 |
| 4800 | 0.08, 1.47 | 0.06, 1.35 | 0.05, 1.24 |
| 9600 | 0.06, 1.33 | 0.04, 1.24 | 0.04, 1.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelliffe-Pawlowski, L.L.; Oltman, S.P.; Rand, L.; Scott, K.A.; Kuppermann, M.; Baer, R.; Bell, A.; Bandoli, G.; Costello, J.; Diamond-Smith, N.; et al. Examining the Impact of the 2019 Novel Coronavirus and Pandemic-Related Hardship on Adverse Pregnancy and Infant Outcomes: Design and Launch of the HOPE COVID-19 Study. Reprod. Med. 2020, 1, 91-107. https://doi.org/10.3390/reprodmed1020007
Jelliffe-Pawlowski LL, Oltman SP, Rand L, Scott KA, Kuppermann M, Baer R, Bell A, Bandoli G, Costello J, Diamond-Smith N, et al. Examining the Impact of the 2019 Novel Coronavirus and Pandemic-Related Hardship on Adverse Pregnancy and Infant Outcomes: Design and Launch of the HOPE COVID-19 Study. Reproductive Medicine. 2020; 1(2):91-107. https://doi.org/10.3390/reprodmed1020007
Chicago/Turabian StyleJelliffe-Pawlowski, Laura L., Scott P. Oltman, Larry Rand, Karen A. Scott, Miriam Kuppermann, Rebecca Baer, April Bell, Gretchen Bandoli, Jean Costello, Nadia Diamond-Smith, and et al. 2020. "Examining the Impact of the 2019 Novel Coronavirus and Pandemic-Related Hardship on Adverse Pregnancy and Infant Outcomes: Design and Launch of the HOPE COVID-19 Study" Reproductive Medicine 1, no. 2: 91-107. https://doi.org/10.3390/reprodmed1020007
APA StyleJelliffe-Pawlowski, L. L., Oltman, S. P., Rand, L., Scott, K. A., Kuppermann, M., Baer, R., Bell, A., Bandoli, G., Costello, J., Diamond-Smith, N., Epel, E., Jackson, R., Jiang, F., Karasek, D. A., Lindan, C., O’Leary, A., Olgin, J., Pantell, M., Paquette, A., ... Chambers, C. (2020). Examining the Impact of the 2019 Novel Coronavirus and Pandemic-Related Hardship on Adverse Pregnancy and Infant Outcomes: Design and Launch of the HOPE COVID-19 Study. Reproductive Medicine, 1(2), 91-107. https://doi.org/10.3390/reprodmed1020007








