Real-Life Measurement of Vasoregulation in Patients with Cyanotic Congenital Heart Disease: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Device
2.2. Calibration Protocol
2.3. Data Handling
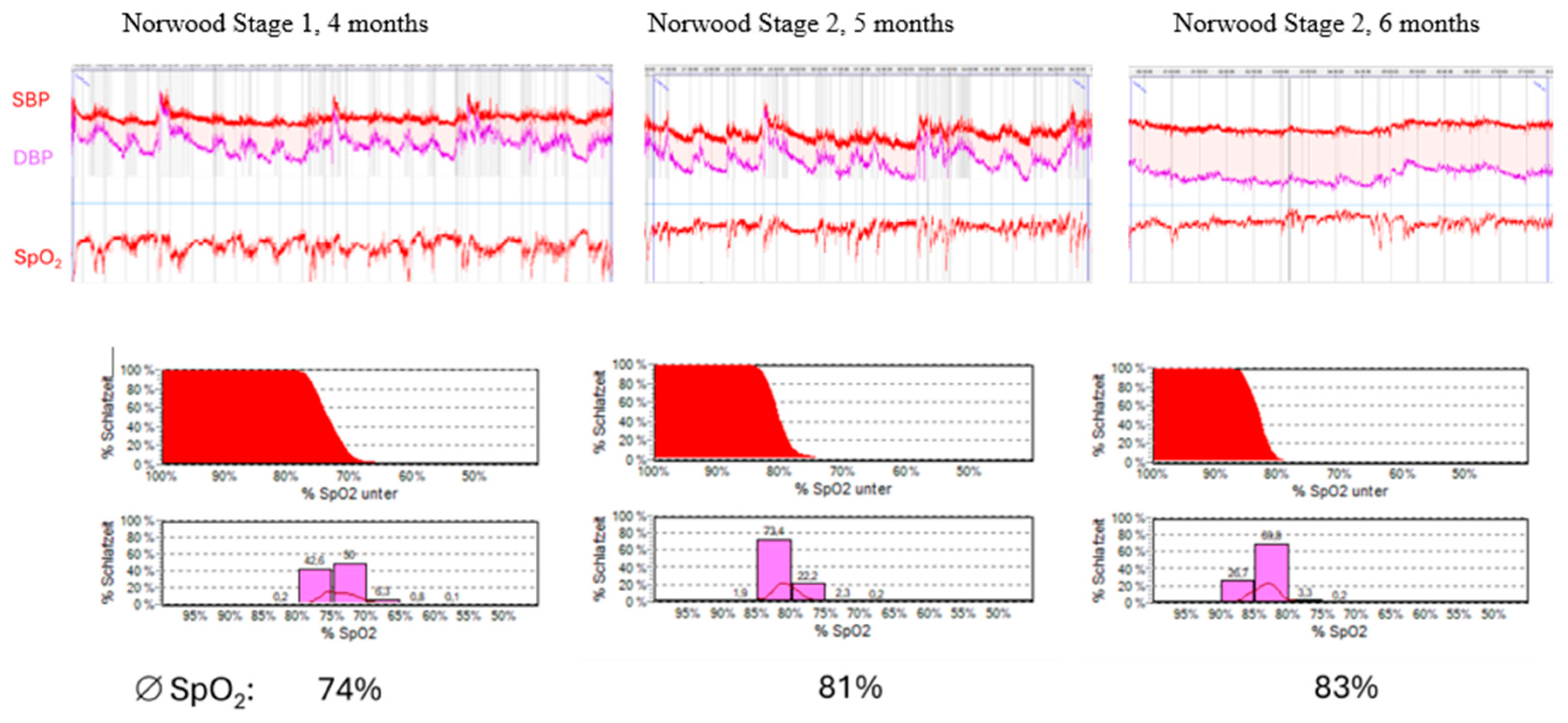
2.4. Case 1
2.5. Case 2
2.6. Case 3
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ccTGA | congenital corrected transposition of the great arteries |
| HPV | hypoxic pulmonary vasoconstriction |
| HIF | hypoxia-inducible factor |
| DPB | diastolic blood pressure |
| SBP | systolic blood pressure |
| BP | blood pressure |
| PTT | pulse transit time |
| VEGF | vascular endothelial growth factor |
References
- Breeyear, J.H.; Keaton, J.M.; Torstenson, E.S.; Smith, A.H.; Klarin, D.; Damrauer, S.M.; Natarajan, P.; Van Driest, S.L.; Weiner, J.G.; Kannankeril, P.J.; et al. Diastolic Blood Pressure Alleles Improve Congenital Heart Defect Repair Outcomes. Circ. Res. 2022, 130, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Buchhorn, R.; Bartmus, D.; Buhre, W.; Bursch, J. Pathogenetic mechanisms of venous congestion after the Fontan procedure. Cardiol. Young 2001, 11, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Nur, S.M.; Xiaochen, Y. Association between obstructive sleep apnea and resistant hypertension: Systematic review and meta-analysis. Front. Med. 2023, 10, 1200952. [Google Scholar] [CrossRef] [PubMed]
- Bokov, P.; Dudoignon, B.; Delclaux, C. Hypoxic burden as a cause of cardiovascular morbidity in childhood obstructive sleep apnea. Pediatr. Res. 2025, 1–6. [Google Scholar] [CrossRef]
- Waypa, G.B.; Schumacker, P.T. Hypoxia-induced changes in pulmonary and systemic vascular resistance: Where is the O2 sensor? Respir. Physiol. Neurobiol. 2010, 174, 201–211. [Google Scholar] [CrossRef]
- Abdul-Khaliq, H.; Gomes, D.; Meyer, S.; von Kries, R.; Wagenpfeil, S.; Pfeifer, J.; Poryo, M. Trends of mortality rate in patients with congenital heart defects in Germany-analysis of nationwide data of the Federal Statistical Office of Germany. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2024, 113, 750–760. [Google Scholar] [CrossRef]
- Forestieri, N.E.; Olshan, A.F.; Oster, M.E.; Ailes, E.C.; Fundora, M.P.; Fisher, S.C.; Shumate, C.; Romitti, P.A.; Rebecca, F.L.; Nembhard, W.N.; et al. Survival of Children with Critical Congenital Heart Defects in the National Birth Defects Prevention Study. Birth Defects Res. 2024, 116, e2394. [Google Scholar] [CrossRef]
- Gesche, H.; Grosskurth, D.; Küchler, G.; Patzak, A. Continuous blood pressure measurement by using the pulse transit time: Comparison to a cuff-based method. Eur. J. Appl. Physiol. 2012, 112, 309–315. [Google Scholar] [CrossRef]
- Bilo, G.; Zorzi, C.; Ochoa Munera, J.E.; Torlasco, C.; Giuli, V.; Parati, G. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015, 20, 291–294. [Google Scholar] [CrossRef]
- Hunter, R.B.; Saruwatari, M.S.; Bliss, N.D.; Kim, S.; Turcu, R.; Krbec, B.A.; Yusuf, K.; Rao, A.; Quan, X.; Soghier, L. Accuracy and Safety of a Continuous Noninvasive Blood Pressure Monitor in Neonates. Neonatology 2025, 122, 595–605. [Google Scholar] [CrossRef]
- Aronsson, A.; Sigurdsson, T.S.; Lindberg, L. The effect of oxygen on hemodynamic variables in neonates with single ventricle after the Norwood procedure. medRxiv 2025. [Google Scholar] [CrossRef]
- Wunderle, M.; Bachler, M.; Stadler, D.; Hametner, B.; Orter, S.; Küchle, C.; Krenn, S.; Heemann, U.; Wassertheurer, S.; Schmaderer, C.; et al. Continuous measurement of pulse arrival time for identification of blood pressure changes in patients undergoing hemodialysis-a feasibility study. Clin. Kidney J. 2025, 18, sfaf255. [Google Scholar] [CrossRef] [PubMed]
- Pielmus, A.G.; Mühlstef, J.; Bresch, E.; Glos, M.; Jungen, C.; Mieke, S.; Orglmeister, R.; Schulze, A.; Stender, B.; Voigt, V.; et al. Surrogate based continuous noninvasive blood pressure measurement. Biomed. Tech. 2021, 66, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Claessens, P.J.; Peeters, R.; Claessens, L.; Claessens, C.; Claessens, J.; Claessens, P.M. Pulse wave analysis measurements: Important, underestimated and undervalued parameters in cardiovascular health problems. Front. Cardiovasc. Med. 2023, 10, 1266258. [Google Scholar] [CrossRef]
- Baker, S.; Yogavijayan, T.; Kandasamy, Y. Towards Non-Invasive and Continuous Blood Pressure Monitoring in Neonatal Intensive Care Using Artificial Intelligence: A Narrative Review. Healthcare 2023, 11, 3107. [Google Scholar] [CrossRef]
- Dissanayake, H.U.; Bin, Y.S.; Ucak, S.; de Chazal, P.; Sutherland, K.; Cistulli, P.A. Association between autonomic function and obstructive sleep apnea: A systematic review. Sleep Med. Rev. 2021, 57, 101470. [Google Scholar] [CrossRef]
- Sommer, N.; Dietrich, A.; Schermuly, R.T.; Ghofrani, H.A.; Gudermann, T.; Schulz, R.; Seeger, W.; Grimminger, F.; Weissmann, N. Regulation of hypoxic pulmonary vasoconstriction: Basic mechanisms. Eur. Respir. J. 2008, 32, 1639–1651. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Mamazhakypov, A.; Weissmann, N.; Seeger, W.; Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Investig. 2020, 130, 5638–5651. [Google Scholar] [CrossRef]
- Wan, J.J.; Yi, J.; Wang, F.Y.; Zhang, C.; Dai, A.G. Expression and regulation of HIF-1a in hypoxic pulmonary hypertension: Focus on pathological mechanism and Pharmacological Treatment. Int. J. Med. Sci. 2024, 21, 45–60. [Google Scholar] [CrossRef]
- Farhat, N.; Vazquez-Jimenez, J.; Heying, R.; Seghaye, M.C. Myocardial mRNA expression of interleukin-6 and hypoxia inducible factor-1α in neonates with congenital cardiac defects. Mol. Cell. Pediatr. 2024, 11, 14. [Google Scholar] [CrossRef]
- Yin, H.L.; Luo, C.W.; Dai, Z.K.; Shaw, K.P.; Chai, C.Y.; Wu, C.C. Hypoxia-inducible factor-1α, vascular endothelial growth factor, inducible nitric oxide synthase, and endothelin-1 expression correlates with angiogenesis in congenital heart disease. Kaohsiung J. Med. Sci. 2016, 32, 348–355. [Google Scholar] [CrossRef]
- Frank, B.S.; Khailova, L.; Silveira, L.; Mitchell, M.B.; Morgan, G.J.; DiMaria, M.V.; Davidson, J.A. Increased Circulating Endothelin 1 Is Associated with Postoperative Hypoxemia in Infants with Single-Ventricle Heart Disease Undergoing Superior Cavopulmonary Anastomosis. J. Am. Heart Assoc. 2022, 11, e024007. [Google Scholar] [CrossRef]
- Frank, B.S.; Niemiec, S.; Khailova, L.; Mancuso, C.A.; Mitchell, M.B.; Morgan, G.J.; Twite, M.; DiMaria, M.V.; Sucharov, C.C.; Davidson, J.A. Increased Endothelin-1 Is Associated with Morbidity in Single Ventricle Heart Disease in Children Undergoing Fontan Palliation. JACC Adv. 2025, 4, 101672. [Google Scholar] [CrossRef]
- Buchhorn, R.; Bartmus, D.; Siekmeyer, W.; Hulpke-Wette, M.; Schulz, R.; Bursch, J. Beta-blocker therapy of severe congestive heart failure in infants with left to right shunts. Am. J. Cardiol. 1998, 81, 1366–1368. [Google Scholar] [CrossRef]
- Buchhorn, R.; Hulpke-Wette, M.; Hilgers, R.; Bartmus, D.; Wessel, A.; Bursch, J. Propranolol treatment of congestive heart failure in infants with congenital heart disease: The CHF-PRO-INFANT Trial. Congestive heart failure in infants treated with propanol. Int. J. Cardiol. 2001, 79, 167–173. [Google Scholar] [CrossRef]
- Cheong, H.I.; Asosingh, K.; Stephens, O.R.; Queisser, K.A.; Xu, W.; Willard, B.; Hu, B.; Dermawan, J.K.T.; Stark, G.R.; Naga Prasad, S.V.; et al. Hypoxia sensing through β-adrenergic receptors. JCI Insight 2016, 1, e90240. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchhorn, R.; Hofmann, E. Real-Life Measurement of Vasoregulation in Patients with Cyanotic Congenital Heart Disease: A Feasibility Study. Hearts 2025, 6, 33. https://doi.org/10.3390/hearts6040033
Buchhorn R, Hofmann E. Real-Life Measurement of Vasoregulation in Patients with Cyanotic Congenital Heart Disease: A Feasibility Study. Hearts. 2025; 6(4):33. https://doi.org/10.3390/hearts6040033
Chicago/Turabian StyleBuchhorn, Reiner, and Elisabeth Hofmann. 2025. "Real-Life Measurement of Vasoregulation in Patients with Cyanotic Congenital Heart Disease: A Feasibility Study" Hearts 6, no. 4: 33. https://doi.org/10.3390/hearts6040033
APA StyleBuchhorn, R., & Hofmann, E. (2025). Real-Life Measurement of Vasoregulation in Patients with Cyanotic Congenital Heart Disease: A Feasibility Study. Hearts, 6(4), 33. https://doi.org/10.3390/hearts6040033





