1. Introduction
Dysnatremias and increased sodium variability portend worse outcomes in hospitalized children [
1,
2]. Hyponatremia is associated with increased hospital morbidity and mortality and occurs in 17% to 45% of hospitalized children [
3,
4,
5,
6]. Dysnatremia, defined as serum sodium outside of 135–145 mmol/L, has been seen to occur in 60% of infants within 48 h of surgery for congenital heart disease and is associated with longer stays in intensive care units [
4,
7]. The consequences of hyponatremia include vomiting, weakness, cerebral edema, and seizures [
3,
8]. Beyond dysnatremias alone, studies have found associations between worse outcomes in patients and increased sodium variability even when sodium remains within the normal range [
1,
9]. Neonates and infants undergoing cardiac surgery are independently at risk for neurologic injury and seizures, highlighting the importance of sodium regulation in the postoperative period [
10].
Vasopressin is increasingly used to treat hypotensive shock following cardiac surgery due to its ability to increase vasomotor tone without the inotropic effects of catecholamines that increase myocardial oxygen consumption [
11,
12]. Postoperative vasopressin use in children following cardiac surgery is correlated with lower heart rates and higher blood pressure as well as decreased inotropic scores, central venous pressures, fluid balance, and lactate levels [
11,
13,
14]. Vasopressin may be particularly beneficial in states of vasoplegia, which has been described in up to 45% of cardiac surgery cases and is a well-documented risk of cardiopulmonary bypass (CPB) [
15,
16]. At higher doses, vasopressin increases systemic vascular resistance by activating V1 receptors in smooth muscle, whereas lower doses increase free water retention by activating V2 receptors in the renal collecting ducts [
17,
18,
19]. Consequently, use of vasopressin for blood pressure management may inadvertently cause excess free water uptake. Vasopressin use has been associated with increased incidence of hyponatremia in critically ill children and in children undergoing cardiac surgery, possibly related to this mechanism [
3,
17,
20].
Our study aimed to assess whether neonates and infants up to 90 days of age who receive vasopressin post-cardiac surgery are more likely to have hyponatremia and sodium variability than those who do not receive vasopressin. We also hoped to identify additional risk factors for hyponatremia in neonates and infants who receive vasopressin in the immediate postoperative period.
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
A total of 75 consecutive neonates and infants under 90 days of age who underwent their first cardiac surgery, most involving CPB, at a single center between 2018 and 2020 were included in this retrospective analysis. This study had IRB approval (IRB #20-001274, approval date 15 July 2020). Patients were identified from the Society of Thoracic Surgeons (STS) internal database. Consent for study inclusion was waived per the IRB. The control group was defined as patients who did not receive any vasopressin during the study period, defined as the first 5 postoperative days, and the vasopressin group included patients who had any vasopressin exposure during the study period. We excluded patients with known end-stage kidney disease as well as those on extracorporeal membrane oxygenation (ECMO) postoperatively.
2.2. Operative and Postoperative Management
Two surgeons were involved in the operations and care of these patients and had a consistent approach to CPB and temperature management. Patients were also managed per the anesthesia and critical care teams, and vasopressin was initiated and discontinued per the discretion of the treatment team. In this CICU, vasopressin is used relatively early when adding vasopressor adjuncts and not limited to patients who have failed other vasoactive medications.
2.3. Data Collection
The maximum and minimum serum sodium levels were collected for the first 5 postoperative days as well as daily vasopressin dose ranges and the daily average vasopressin dose. Vasopressin dose was measured in U/kg/h. Additional data points collected included patient age, preoperative weight, total sodium intake, urine output, net daily fluid balance, diuretic dose, peak vasoactive inotrope score (VIS), and surgical variables including the use of modified ultrafiltration. VIS was the highest calculated score that occurred at any time during the 5-day study period. Urine sodium and osmolality are not routinely collected at this institution and as such were not captured in this retrospective study. We assessed patient outcomes of serum sodium levels, incidence of clinical or subclinical seizures within the postoperative period, ventilator duration, and mortality during the hospitalization. Per institutional protocol, patients were monitored via continuous electroencephalogram (cEEG) following CPB for a minimum of two days to screen for postoperative seizures.
Sodium intake was calculated via the product of fluid intake and sodium concentration of the fluid content. This included saline boluses, flushes, administration of sodium bicarbonate, 5% albumin, blood products, and total parenteral nutrition (TPN). Standard initial maintenance fluids in this patient population were 10% dextrose with 0.45% sodium chloride at half maintenance using the 4:2:1 rule rate until patients were transitioned to TPN or enteral feeds.
Measurement of diuretic dosage entailed individually measuring the milligrams of bumetanide, furosemide, and chlorothiazide given and converting to mg/kg. Diuretic use was determined by the treatment team as clinically indicated.
3. Statistical Analysis
Descriptive statistics were created for the vasopressin and control groups where categories were represented in number (%) and continuous variables were reported in mean ± SD or median with 25th and 75th percentiles. Statistical significance was defined as a less than 5% chance of incorrectly rejecting the null hypothesis; p < 0.05. Bivariate comparisons of the vasopressin to the control group were conducted using chi-square (or Fisher’s exact) for categorical variables and Welch’s t-test (or Wilcoxon rank sum) for continuous variables. Comparisons across postoperative days within and between patients who received vasopressin were examined using mixed-effects linear regression with a patient random intercept and fixed effects for group (vasopressin versus control), days from surgery (modeled as a factor), and their interaction. We tracked the maximum and minimum serum sodium levels for each postoperative day for each patient. Daily sodium variability was measured via the subtraction of serum sodium minimum from serum sodium maximum within a single day for each patient. Daily mean sodium level was determined by averaging the daily serum sodium maximum and minimum values. We then analyzed the change in sodium average or sodium variability across days from surgery. This change was assessed by comparing the difference in values both between consecutive days and between each postoperative day and the baseline (postoperative day 0). All analyses were conducted in Stata version 18.0, Stata Corp LLC (College Station, TX, USA).
4. Results
4.1. Demographics
A total of 36 of the 75 patients received vasopressin during the study. The median chronological age was 11 days in the control group and 6 days in the vasopressin group (
p = 0.052) (
Table 1). Groups were statistically equivalent in terms of gestational age, weight, supplemental sodium load, total diuretic dose, volume of modified ultrafiltration, seizure incidence, and mortality. The total diuretic dose was analyzed as a weight-based dose of furosemide, chlorothiazide, and bumetanide, accounting for both individual and aggregate amounts. The groups differed in that the vasopressin group had a younger median corrected age by 5 days, an increased total fluid intake (over 5 days) by 81 mL/kg, an increased urine output, and a net negative fluid balance by the end of the study when accounting for additional non-urinary fluid losses (
p < 0.05) (
Table 1). The vasopressin group had an overall longer duration of mechanical ventilation as well as a higher congenital heart surgery mortality risk stratification, as defined by the Society of Thoracic Surgeons–European Association for Cardio-Thoracic Surgery (STAT) category, with 53% in STAT category 4 or higher, compared to 36% of patients in the control group (
Table 1) [
21,
22]. The group receiving vasopressin received an average dose of 0.014 U/kg/h (
p < 0.001) when averaged across the 5-day study period, though notably, many patients in the vasopressin group did not receive vasopressin for all five of these days.
4.2. Absolute Sodium Levels
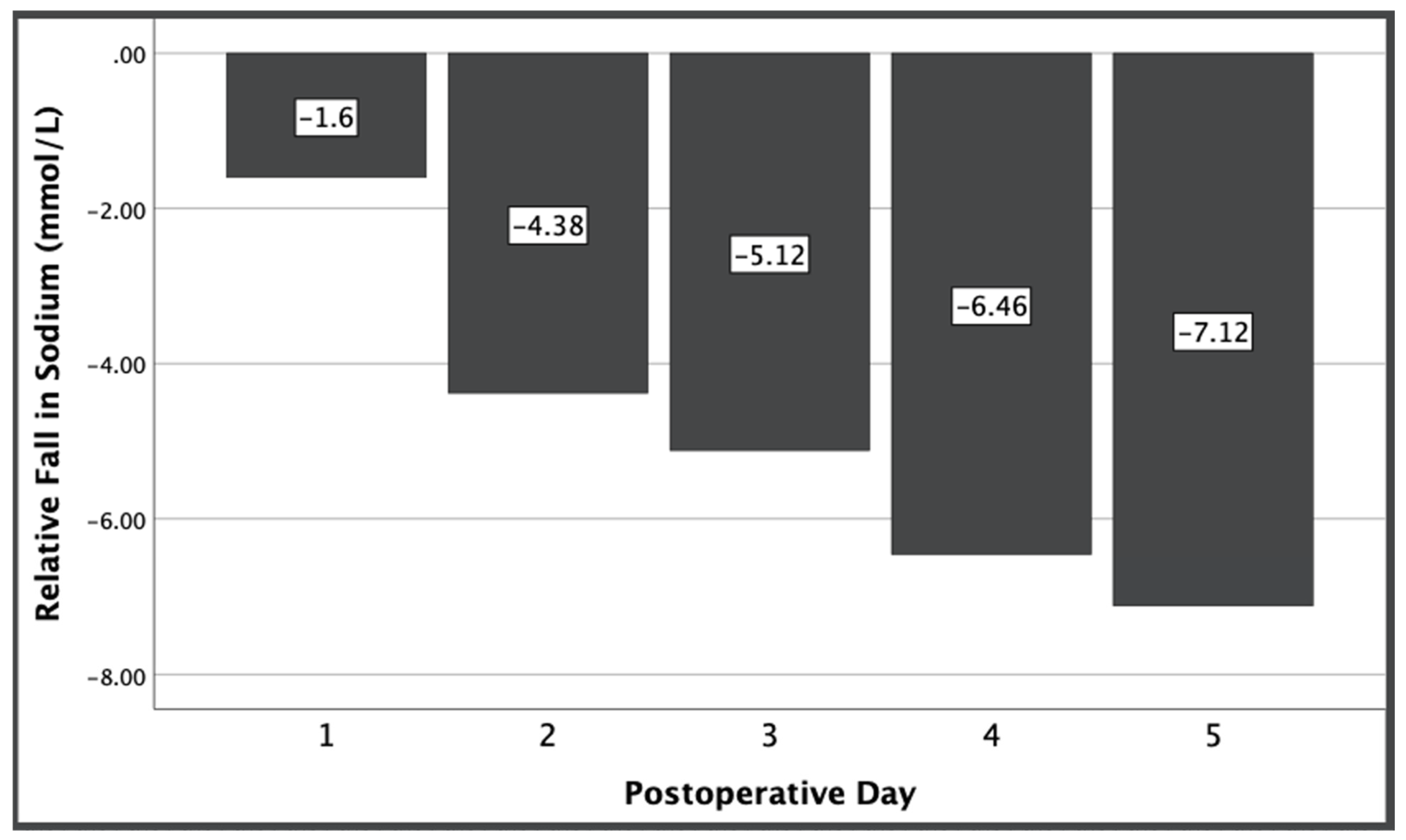
There was an overall decrease in serum sodium at day 5 among all patients relative to baseline serum sodium levels, ranging from a 5.6–9.7 mmol/L decrease (
p < 0.001). Despite each group receiving statistically equivalent sodium loads, the vasopressin group had an increased fall in sodium from preoperative levels compared to the fall in sodium in the control group that was significant beyond the first postoperative day and increased over time (
p < 0.001) (
Figure 1).
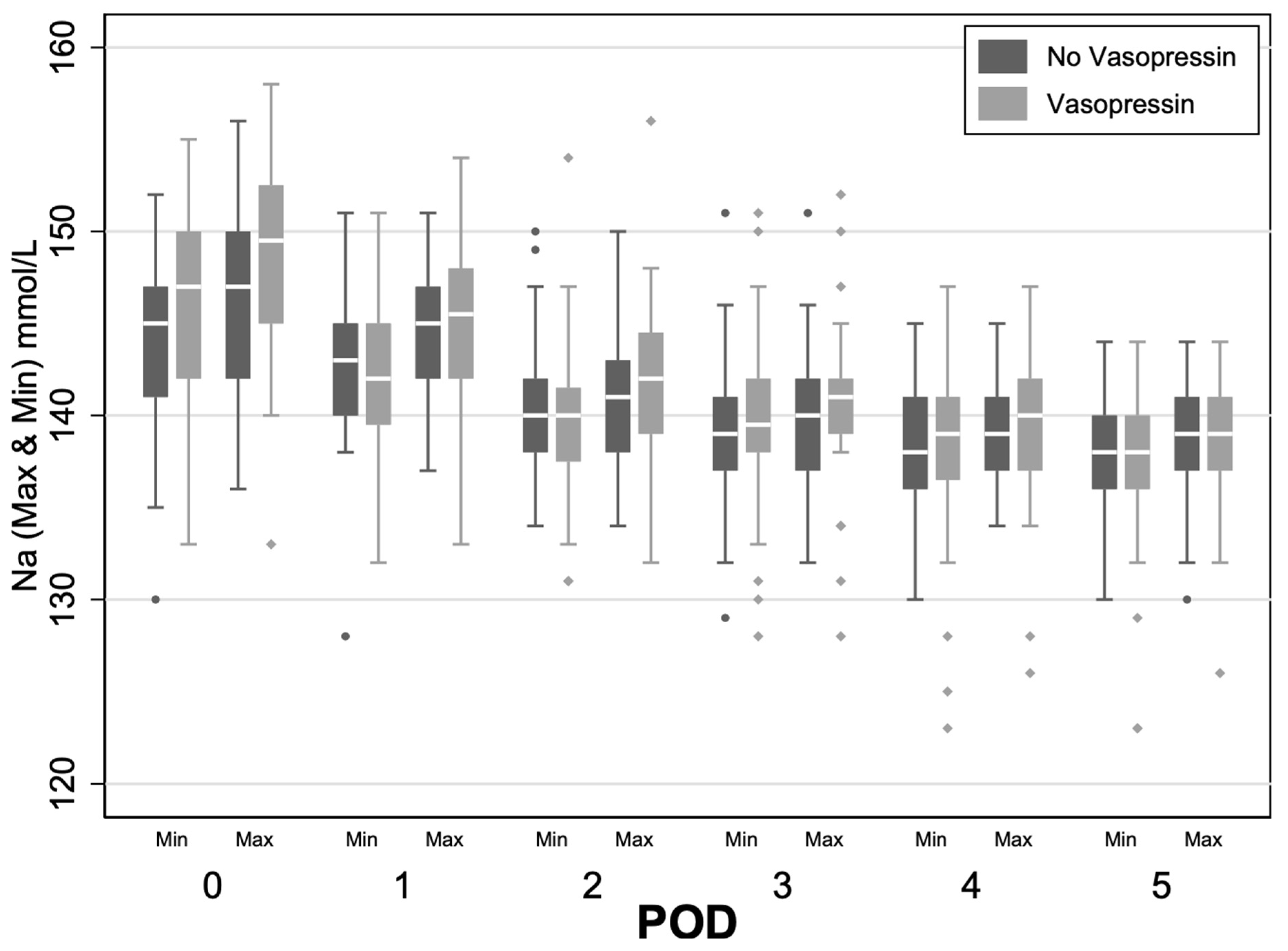
4.3. Sodium Variability
The vasopressin group showed increased variability between daily average sodium minimum and maximum levels on postoperative days 1 and 2 (POD1 1.31 mmol/L and POD2 1.23 mmol/L,
p < 0.05) relative to the control (
Figure 2). On day 3, both within- and between-group sodium variability compared to baseline began to significantly decrease (
p < 0.05). These findings indicate greater sodium fluctuations in the vasopressin group in the early postoperative period.
4.4. Risk Factors
Each study group was analyzed independently for risk factors for decreases in sodium or sodium variability. Chronological, corrected, and gestational age as well as volume of modified ultrafiltration and total diuretic dose were not found to be risk factors in either group for these effects. In both the vasopressin and control groups, total fluid intake was a risk for lower mean sodium values. Per each dL increase in intake, we found a change in sodium by −0.8 mmol/L (95% CI −1.3, −0.3) in the vasopressin group and by −1.6 mmol/L (95% CI −2, −1.2) in the control group; p < 0.001. Within the vasopressin group alone, cumulative vasopressin dose was found to be a risk factor for both hyponatremia and sodium variability. The mean cumulative vasopressin dose over the five study days was 0.04 U/kg (SD ± 0.03). Per every 0.01 U/kg increase in cumulative vasopressin dose that patients were exposed to over the study period, the average sodium decreased by 0.54 mmol/L and sodium variability increased by 0.17 mmol/L (p < 0.001).
4.5. Events
Differences in events between groups were not statistically significant at this sample size, though a larger population may find otherwise. Clinically, each group had two seizure occurrences (p = 0.99), and there was one more death in the vasopressin group (p = 0.60). Though not reaching significance, there were three incidences of moderate to severe hyponatremia (sodium < 130 mmol/L) in the vasopressin group and two in the control group, and of these, severe hyponatremia (sodium < 125 mmol/L) was only seen in the vasopressin group.
5. Discussion
This study showed that, while all patients had a fall in postoperative serum sodium, vasopressin exposure was associated with more pronounced decreases in absolute sodium as well as increased sodium variability. Furthermore, cumulative vasopressin dose was significantly correlated as a risk factor for these effects within the vasopressin group alone. This study is the first to explore within-day sodium variability in this population and the dose-dependent association of vasopressin, with an analysis of contributing risk factors. As all patients received maintenance hypotonic fluids with 0.45% saline per protocol, and there is significant practice variability in maintenance fluid management across institutions, perhaps protocols recommending early postoperative isotonic saline should be considered to mitigate risk for both groups [
23]. Sodium variability was most significant early postoperatively and decreased over time, suggesting progression to equilibrium.
In comparing the two groups, we noted equivalent chronological and gestational age, preoperative weight, weight-based sodium intake, modified ultrafiltration volume, and weight-based diuretic use. The vasopressin group had a younger corrected age by 5 days, which is statistically but not likely clinically significant. The vasopressin group also had higher STAT categories and a higher peak VIS, which may indicate a higher baseline illness severity, which is expected since exposure to vasoactives is the main study parameter [
24,
25,
26,
27,
28]. On further analysis, the STAT category did not independently enhance the outcomes of vasopressin exposure on serum sodium, and sodium outcomes within each group did not trend proportionally to a higher STAT category (
Appendix A). A higher baseline illness severity was consistent with findings of increased fluid intake, likely resulting from volume resuscitation and longer ventilator duration in this group. Despite fluid intake differences, both groups had equivalent sodium intake and weight-based diuretic doses. While positive pressure ventilation can increase the risk for SIADH, a known cause of hyponatremia, this is inconsistent with the increased urine output seen in the vasopressin group [
29]. It is unknown if the increased urination in the vasopressin group resulted from improved renal perfusion from blood pressure management, or from inappropriate autodiuresis in the setting of transient relative intrinsic vasopressin deficiency, which has been documented following surgery involving CPB in both adults and children [
15,
19,
30]. Overall, the vasopressin group had a higher risk of hyponatremia and sodium variability despite equivalent sodium loads in the setting of likely higher illness severity.
In our assessment of potential risk factors, total fluid intake in both groups was associated with falls in sodium. Within the vasopressin group, cumulative vasopressin dose was a risk factor for hyponatremia and sodium variability and the effect was proportional to the dose exposure. Other factors such as age, volume of modified ultrafiltration, and total diuretic dose were not risk factors for either effect in either group.
We found that the overall survival and incidence of seizures were equivalent between both groups in this study, though a larger study population may find otherwise. This does not exclude long-term cognitive implications of hyponatremia. Those exposed to vasopressin tended to have a more prolonged duration on invasive mechanical ventilation, which may have implications for hospitalization duration and other morbidities not captured in this study.
Limitations
Some limitations of this study included its retrospective nature in which findings of association cannot determine causality. The vasopressin group may represent a more critically ill population as inferred in prior studies [
28]. Similarly, there was no standard protocol regarding the criteria for vasopressin initiation to better compare the illness severity between the groups; however, sodium intake was equivalent between the groups despite potential differences in resuscitation needs. Management of hyponatremia and titration of vasopressin were not controlled for and were up to the clinical team. We did not collect all operative variables, though the STAT category is a surrogate marker of surgical complexity and patients were operated on by two surgeons with similar approaches. Our patient population primarily consisted of very young, neonatal-age patients, and it is unknown if any postnatal diuresis effects may have occurred or if our findings can be extrapolated to older patients. Regarding our analysis, while we measured daily sodium variability using the difference in daily serum sodium maximum and minimums, this does not factor in the true rate of rise and fall that these patients experienced—many of them likely experienced these fluctuations in much less than 24 h. We felt that analysis using daily sodium maximum and minimum differences was more intuitive in describing the clinical relevance of our findings, though using daily sodium standard deviation may have better captured aspects such as non-linear trends in daily sodium fluctuations. Finally, some data lacked power to be significant and may warrant further study.
6. Conclusions
Patients are at risk for hyponatremia following congenital heart surgery, and vasopressin is associated with both falls in sodium and early sodium variability in the immediate postoperative period in our study. Despite general concerns for sodium retention in neonates with congenital heart disease, both groups experienced significant decreases in sodium postoperatively, and this age group may benefit from full isotonic fluid during this period and the earlier weaning off of vasopressin. When encountering sodium disequilibrium syndrome in postoperative patients, clinicians should be aware of the correlation with vasopressin, especially those with a higher cumulative vasopressin dose and larger volumes of fluid resuscitation, and may consider alternative medications until sodium is corrected. One may consider prolonged postoperative neurological monitoring in patients exposed to vasopressin even in the absence of overt hyponatremia due to the wider sodium fluctuations seen in this population. Further research may provide insight into how to best mitigate sodium fluctuations in patients exposed to vasopressin following congenital heart surgery.
Author Contributions
Conceptualization, M.D.F., J.A.J., L.L.H. and N.J.J.; Data Curation, J.A.J. and M.D.F.; Formal Analysis, J.A.J., L.L.H., N.J.J. and M.D.F.; Investigation, J.A.J., M.J.H. and M.D.F.; Methodology, N.J.J., L.L.H., M.D.F. and J.A.J.; Supervision, M.D.F. and L.L.H.; Software, N.J.J.; Writing—Original Draft Preparation, J.A.J., M.D.F., L.L.H., M.J.H. and N.J.J.; Writing—Review and Editing, J.A.J., M.D.F., N.J.J. and L.L.H. All authors have read and agreed to the published version of the manuscript.
Funding
Statistical analysis funding for this research was provided by NIH/National Center for Advancing Translational Science (NCATS) University of California Los Angeles Clinical and Translational Science Institute (UCLA CTSI) Grant Number UL1TR001881.
Institutional Review Board Statement
This study received IRB approval (IRB #20-001274, approval date 15 July 2020) at the University of California Los Angeles. All procedures were followed in accordance with the ethical standards of the central IRB and with the Helsinki Declaration of 1975.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data were obtained from the Society of Thoracis Surgeons (STS)’s internal database.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Appendix A
Below are STAT category tables comparing the vasopressin group to the control group by STAT category.
Table A1.
Change in serum sodium from POD0 to POD5 (mmol/L).
Table A1.
Change in serum sodium from POD0 to POD5 (mmol/L).
| | Control
AvgNAPOD5 − AvgNAPOD0
Mean Change (95% CI) | Vasopressin
AvgNAPOD5 − AvgNAPOD0
Mean Change (95% CI) | p |
|---|
| STAT 1 | −4.93 (−8.93, −0.94) | −4.375 (−10.25, 1.50) | 0.877 |
| STAT 2 | −7.25 (−11.41, −3.09) | −10.25 (−14.48, −6.02) | 0.321 |
| STAT 3 | −8.70 (−13.26, −4.15) | −10.60 (−15.86, −5.34) | 0.593 |
| STAT 4 | −6.07 (−9.73, −2.41) | −11.10 (−16.36, −5.84) | 0.124 |
| STAT 5 | −10.00 (−16.79, −3.21) | −8.54 (−11.68, −5.39) | 0.701 |
Table A2.
Change in within-day sodium variability by POD5 relative to POD0 (mmol/L).
Table A2.
Change in within-day sodium variability by POD5 relative to POD0 (mmol/L).
| | Control
ΔNAPOD5 − ΔNAPOD0
Mean Change (95% CI) | Vasopressin
ΔNAPOD5 − ΔNAPOD0
Mean Change (95% CI) | p |
|---|
| STAT 1 | −2.69 (−4.61, −0.76) | −2.25 (−5.06, 0.56) | 0.801 |
| STAT 2 | −2.25 (−4.24, −0.26) | 0.47 (−1.57, 2.52) | 0.060 |
| STAT 3 | −1.58 (−3.78, 0.61) | −0.40 (−2.91, 2.11) | 0.487 |
| STAT 4 | −1.97 (−3.74, −0.20) | −1.40 (−3.91, 1.11) | 0.715 |
| STAT 5 | −2.00 (−5.24, 1.24) | −2.07 (−3.57, −0.57) | 0.968 |
References
- Lin, J.; Zhang, Y.; Chen, M.; Dai, J.; Song, A.; Chen, J.; Tao, X. The Association Between Variability in Electrolytes and the In-Hospital Mortality in Critically Ill Children in Pediatric Intensive Care Units. Front. Pediatr. 2021, 9, 692894. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Hochman, J.; Kurbatova, E.; Mullins, R. Study of outcomes associated with hyponatremia and hypernatremia in children. Pediatr. Dev. Pathol. 2011, 14, 117–123. [Google Scholar] [CrossRef]
- Davalos, M.C.; Barrett, R.; Seshadri, S.; Walters, H.L., 3rd; Delius, R.E.; Zidan, M.; Mastropietro, C.W. Hyponatremia during arginine vasopressin therapy in children following cardiac surgery. Pediatr. Crit. Care Med. 2013, 14, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, J.R.; Lindhardt, R.B.; Vejlstrup, N.; Holst, L.M.; Juul, K.; Smerup, M.H.; Gjedsted, J.; Ravn, H.B. Postoperative dysnatremia in infants after open-heart surgery occurs frequently and is associated with prolonged intensive care length of stay. Acta Anaesthesiol. Scand. 2022, 66, 337–344. [Google Scholar] [CrossRef]
- Ontaneda, A.M.; Coss-Bu, J.A.; Kennedy, C.; Akcan-Arikan, A.; Fernandez, E.; Lasa, J.J.; Price, J.F.; Shekerdemian, L.S. Post-operative dysnatremia is associated with adverse early outcomes after surgery for congenital heart disease. Pediatr. Res. 2023, 94, 611–617. [Google Scholar] [CrossRef]
- Mehta, A.; Fatima, M.; Yadav, A.K. Prevalence and risk factors of hyponatremia in hospitalized critically ill children: An observational study. Sudan. J. Paediatr. 2024, 24, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Phadke, D.; Tong, S.; Eshelman, J.; Newman, S.; Ruzas, C.; da Cruz, E.M.; Osorio, S. Clinical Associations of Early Dysnatremias in Critically Ill Neonates and Infants Undergoing Cardiac Surgery. Pediatr. Cardiol. 2017, 38, 149–154. [Google Scholar] [CrossRef]
- Moritz, M.L.; Ayus, J.C. Preventing neurological complications from dysnatremias in children. Pediatr. Nephrol. 2005, 20, 1687–1700. [Google Scholar] [CrossRef]
- Marshall, D.C.; Salciccioli, J.D.; Goodson, R.J.; Pimentel, M.A.; Sun, K.Y.; Celi, L.A.; Shalhoub, J. The association between sodium fluctuations and mortality in surgical patients requiring intensive care. J. Crit. Care 2017, 40, 63–68. [Google Scholar] [CrossRef]
- Marino, B.S.; Lipkin, P.H.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; Mussatto, K.A.; Uzark, K.; Goldberg, C.S.; Johnson, W.H., Jr.; et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation 2012, 126, 1143–1172. [Google Scholar] [CrossRef]
- Farias, J.S.; Villarreal, E.G.; Flores, S.; Mastropietro, C.W.; Vogel, M.; Schulz, K.; Culichia, C.; Iliopoulos, I.D.; Bronicki, R.A.; Loomba, R.S. Effects of Vasopressin Infusion After Pediatric Cardiac Surgery: A Meta-analysis. Pediatr. Cardiol. 2021, 42, 225–233. [Google Scholar] [CrossRef]
- Loomba, R.S.; Flores, S. Use of vasoactive agents in postoperative pediatric cardiac patients: Insights from a national database. Congenit. Heart Dis. 2019, 14, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ghotra, G.S.; Raj, S.; Tiwari, N.; Ramamurthy, H.R. Low-Dose vasopressin and renal perfusion in pediatric cardiac surgery. Ann. Card. Anaesth. 2023, 26, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, X.; Yang, J.; Li, S.; Yan, J. Vasopressin in Vasodilatory Shock for Both Left and Right Heart Anomalous Pediatric Patients After Cardiac Surgery. Shock 2018, 50, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.; Dharmadjati, B.B.; Mulia, E.P.B.; Rachmi, D.A. Vasoplegia: Mechanism and Management Following Cardiopulmonary Bypass. Eurasian J. Med. 2022, 54, 92–99. [Google Scholar] [CrossRef]
- Shaefi, S.; Mittel, A.; Klick, J.; Evans, A.; Ivascu, N.S.; Gutsche, J.; Augoustides, J.G.T. Vasoplegia After Cardiovascular Procedures-Pathophysiology and Targeted Therapy. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1013–1022. [Google Scholar] [CrossRef]
- Alakeel, Y.S.; Alkahtani, M.M.; Hijazi, O.M.; Algahtani, M.M. Vasopressin associated hyponatremia in critically ill children: A cross-sectional study. Saudi Pharm. J. 2022, 30, 1107–1112. [Google Scholar] [CrossRef]
- Bradford, C.V.; Miller, J.L.; Ranallo, C.D.; Neely, S.B.; Johnson, P.N. Vasopressin-Induced Hyponatremia in Infants Following Cardiovascular Surgery. Ann. Pharmacother. 2023, 57, 259–266. [Google Scholar] [CrossRef]
- Choong, K. Vasopressin in Pediatric Critical Care. J. Pediatr. Intensive Care 2016, 5, 182–188. [Google Scholar] [CrossRef]
- Patel, K.; Thomson, S.; Vijayan, M.; Makoni, M.; Johnson, P.N.; Stephens, K.; Neely, S.B.; Miller, J.L. Vasopressin induced hyponatremia in infants <3 months of age in the neonatal intensive care unit. Front. Pediatr. 2024, 12, 1465785. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Clarke, D.R.; Jacobs, J.P.; Jacobs, M.L.; Lacour-Gayet, F.G.; Pizarro, C.; Welke, K.F.; Maruszewski, B.; Tobota, Z.; Miller, W.J.; et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 1139–1153. [Google Scholar] [CrossRef]
- Jacobs, M.L.; Jacobs, J.P.; Thibault, D.; Hill, K.D.; Anderson, B.R.; Eghtesady, P.; Karamlou, T.; Kumar, S.R.; Mayer, J.E.; Mery, C.M.; et al. Updating an Empirically Based Tool for Analyzing Congenital Heart Surgery Mortality. World J. Pediatr. Congenit. Heart Surg. 2021, 12, 246–281. [Google Scholar] [CrossRef] [PubMed]
- Hanot, J.; Dingankar, A.R.; Sivarajan, V.B.; Sheppard, C.; Cave, D.; Garcia Guerra, G. Fluid Management Practices After Surgery for Congenital Heart Disease: A Worldwide Survey. Pediatr. Crit. Care Med. 2019, 20, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Gaies, M.G.; Gurney, J.G.; Yen, A.H.; Napoli, M.L.; Gajarski, R.J.; Ohye, R.G.; Charpie, J.R.; Hirsch, J.C. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr. Crit. Care Med. 2010, 11, 234–238. [Google Scholar] [CrossRef]
- Koponen, T.; Karttunen, J.; Musialowicz, T.; Pietiläinen, L.; Uusaro, A.; Lahtinen, P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br. J. Anaesth. 2019, 122, 428–436. [Google Scholar] [CrossRef]
- DeWitt, A.G.; Rossano, J.W.; Bailly, D.K.; Bhat, P.N.; Chanani, N.K.; Kirkland, B.W.; Moga, M.A.; Owens, G.E.; Retzloff, L.B.; Zhang, W.; et al. Predicting and Surviving Prolonged Critical Illness After Congenital Heart Surgery. Crit. Care Med. 2020, 48, e557–e564. [Google Scholar] [CrossRef] [PubMed]
- Kharrat, A.; Ripstein, G.; Baczynski, M.; Zhu, F.; Ye, X.Y.; Joye, S.; Jain, A. Validity of the vasoactive-inotropic score in preterm neonates receiving cardioactive therapies. Early Hum. Dev. 2022, 173, 105657. [Google Scholar] [CrossRef]
- Crow, S.S.; Robinson, J.A.; Burkhart, H.M.; Dearani, J.A.; Golden, A.W. Duration and magnitude of vasopressor support predicts poor outcome after infant cardiac operations. Ann. Thorac. Surg. 2014, 98, 655–661. [Google Scholar] [CrossRef]
- Ranadive, S.A.; Rosenthal, S.M. Pediatric disorders of water balance. Pediatr. Clin. N. Am. 2011, 58, 1271–1280, xi–xii. [Google Scholar] [CrossRef]
- Singh, V.K.; Sharma, R.; Agrawal, A.; Varma, A. Vasopressin in the pediatric cardiac intensive care unit: Myth or reality. Ann. Pediatr. Cardiol. 2009, 2, 65–73. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).