Surgical Outcomes of Three Repair Techniques for Partial Anomalous Pulmonary Venous Connection in Adult Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
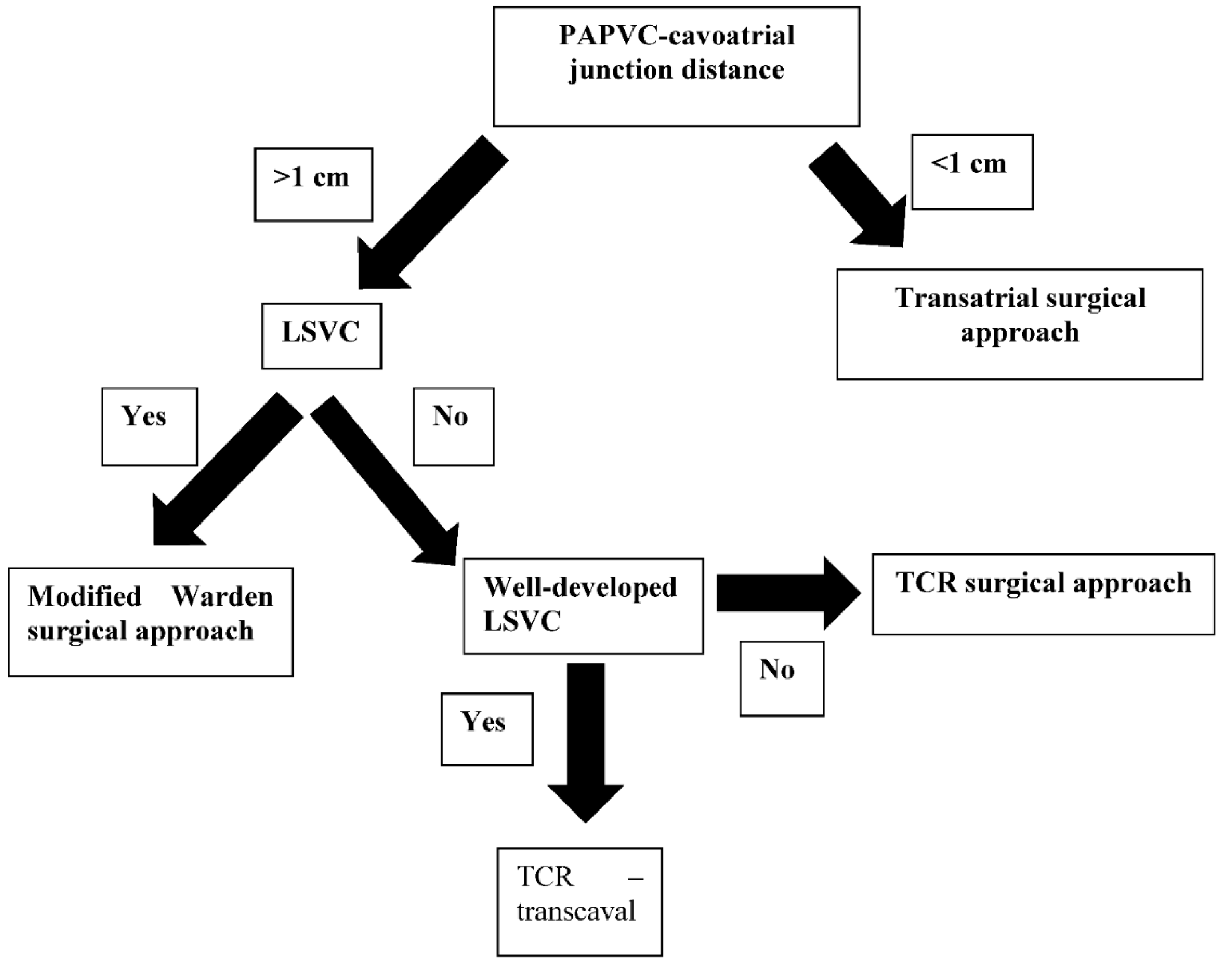
2.2. Surgical Techniques
2.3. Primary and Secondary Outcomes
2.4. Statistical Analysis
2.5. Clinical Data
2.6. Preoperative Evaluation
2.7. Postoperative Evaluation
2.8. Definitions
3. Results
3.1. In-Hospital Outcomes
3.2. Follow-Up
3.2.1. Primary Outcomes
3.2.2. Secondary Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Scientific Meeting Presentation
Abbreviations
| partial anomalous venous connection | PAPVC |
| superior vena cava | SVC |
| transcaval repair | TCR |
| atrial septal defect | ASD |
| pacemaker | PM |
| intensive care unit | ICU |
| adult congenital heart defect | ACHD |
References
- Gustafson, R.A.; Warden, H.E.; Murray, G.F. Partial anomalous pulmonary venous connection to the superior vena cava. Ann. Thorac. Surg. 1995, 60 (Suppl. S6), S614–S617. [Google Scholar] [CrossRef]
- William, W.G.; Rowe, R.D. Late results following repair of partial anomalous pulmonary venous connection with sinus venosus atrial septal defect. J. Thorac. Cardiovasc. Surg. 1980, 79, 776–781. [Google Scholar]
- Warden, H.E.; Gustafson, R.A.; Tarnay, T.J.; Neal, W.A. An alternative method for repair of partial anomalous pulmonary venous connection to the superior vena cava. Ann. Thorac. Surg. 1984, 38, 601–605. [Google Scholar] [CrossRef]
- Chartrand, C.; Payot, M.; Davignon, A.; Guerin, R.; Stanley, P.; Bruneau, J. A new surgical approach for correction of partial anomalous pulmonary venous drainage into the superior vena cava. J. Thorac. Cardiovasc. Surg. 1976, 71, 29–34. [Google Scholar] [CrossRef]
- Friedli, B.; Guerin, R.; Davignon, A.; Fouron, J.C.; Stanley, P. Surgical treatment of partial anomalous pulmonary venous drainage: A long-term follow-up study. Circulation 1972, 45, 159–170. [Google Scholar] [CrossRef]
- Nassar, M.; Fouilloux, V.; Macé, L.; Kreitmann, B.; Metras, D. Transcaval correction of partial anomalous pulmonary venous drainage into the superior vena cava. Ann. Thorac. Surg. 2012, 93, 193–196. [Google Scholar] [CrossRef]
- Perri, G.; Graziani, F.; Bruno, P.; Grandinetti, M.; Lanzillo, C.; Marziali, M.; Amodeo, A.; Massetti, M. Modified Warden procedure in adult with partial anomalous pulmonary venous connection after previous atrial septal defect repair. Cor Vasa 2016, 58, e501–e504. [Google Scholar] [CrossRef]
- Park, C.S.; Kwak, J.G.; Lee, C.; Lee, C.-H.; Lee, S.Y.; Choi, E.Y.; Song, J.Y.; Kim, S.-J. Partial anomalous pulmonary venous connection to the superior vena cava; the outcome after the warden procedure. Eur. J. Cardiothorac Surg. 2012, 41, 261–265. [Google Scholar] [CrossRef]
- Stewart, R.D.; Bailliard, F.; Kelle, A.M.; Backer, C.L.; Young, L.; Mavroudis, C. Evolving surgical strategy for sinus venosus atrial septal defect: Effect on sinus node function and late venous obstruction. Ann. Thorac. Surg. 2007, 84, 1651–1655. [Google Scholar] [CrossRef]
- Kottayil, B.P.; Dharan, B.S.; Menon, S.; Bijulal, S.; Neema, P.K.; Gopalakrishnan, S.K.; Jayakumar, K. Anomalous pulmonary venous connection to superior vena cava: Warden technique. Eur. J. Cardiothorac Surg. 2011, 39, 388–391. [Google Scholar] [CrossRef]
- Baron, O.; Roussel, J.C.; Videcoq, M.; Guerin, P.; Gournay, V.; Lefevre, M. Partial anomalous pulmonary venous connection; correction by intra-atrial baffle and cavoatrial anastomosis. J. Card. Surg. 2002, 17, 166–169. [Google Scholar] [CrossRef] [PubMed]
- DiBardino, D.J.; McKenzie, E.D.; Heinle, J.S.; Su, J.T.; Fraser, C.D. The Warden procedure for partially anomalous pulmonary venous connection to the superior caval vein. Cardiol. Young 2004, 14, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Dearani, J.A.; Connolly, H.M.; Martinez, R.; Fontanet, H.; Webb, G.D. Caring for adults with congenital cardiac disease: Successes and challenges for 2007 and beyond. Cardiol. Young 2007, 17 (Suppl. S2), 87–96. [Google Scholar] [CrossRef] [PubMed]
- Said, S.M.; Burkhart, H.M.; Dearani, J.A.; Eidem, B.; Stensrud, P.; Phillips, S.D.; Schaff, H.V. Outcomes of caval division for partial anomalous pulmonary venous connections to the superior vena cava. Ann. Thorac. Surg. 2011, 92, 980–984, discussion 985. [Google Scholar] [CrossRef]
- Nicholson, I.A.; Chard, R.B.; Nunn, G.R.; Cartmill, T.B. Transcaval repair of the sinus venosus syndrome. J. Thorac. Cardiovasc. Surg. 2000, 119, 741–744. [Google Scholar] [CrossRef]
- Baumgartner, H.; de Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD). Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [PubMed]
- Okonta, K.E.; Agarwal, V.; Abubakar, U. Superior repair: A useful approach for some anatomic variants of total anomalous pulmonary venous connection. Afr. J. Paediatr. Surg. 2013, 10, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Pan, W.; Lin, K.; Shi, Y.; Zhu, P.; Guo, Y.; Gan, C.; An, Q. Modified cavoatrial anastomosis in Warden procedure. Ann. Thorac. Surg. 2010, 89, 2047–2048. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Jneid, F.H.; Sundt III, T.M.; Thompson, A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Collage Cardiol. 2017, 70, 252–289. [Google Scholar]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- McLaughin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; Rosenson, R.S.; et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J. Am. Collega Cardiol. 2009, 53, 1573–1619. [Google Scholar]
- Marang-van de Mheen, P.J.; Stadlander, M.C.; Kievit, J. Adverse outcomes in surgical patients. Implementation of a nationwide routine reporting system. Qual. Saf. Health Care 2006, 15, 320–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e698–e800. [Google Scholar] [PubMed]
- Napoleone, C.P.; Mariucci, E.; Angeli, E.; Oppido, G.; Gargiulo, G.D. Sinus node dysfunction after partial anomalous pulmonary venous connection repair. J. Thorac. Cardiovasc. Surg. 2008, 136, 329–334. [Google Scholar] [CrossRef]
- Shahriari, A.; Rodefeld, M.D.; Turrentine, M.W.; Brown, J.W. Caval division technique for sinus venosus atrial septal defect with partial anomalous pulmonary venous connection. Ann. Thorac. Surg. 2006, 81, 224–229, discussion 229-30. [Google Scholar] [CrossRef]
- Chandra, D.; Gupta, A.; Nath, R.K.; Grover, V.; Gupta, V.K. Surgical management of anomalous pulmonary venous connection to the superior vena cava—Early results. Indian Heart J. 2013, 65, 561–565. [Google Scholar] [CrossRef][Green Version]
- Touray, M.; Bouchardy, J.; Ladouceur, M.; Schwerzmann, M.; Greutmann, M.; Tobler, D.; Harald, R.; Engel, R.; Pruvot, E.; Blanche, C.; et al. Long-term outcome of adult patients with partial anomalous pulmonary venous connection treated surgically and conservatively: Data from the SACHER registry and a French center. Eur. Heart J. 2020, 41, ehaa946.2222. [Google Scholar] [CrossRef]
- Rodríguez-Collado, J.; Attie, F.; Zabal, C.; Troyo, P.; Olvera, S.; Vázquez, J.; Gutiérrez, B.; Vargas-Barrón, J. Total anomalous pulmonary venous connection in adults. Long-term follow-up. J. Thorac. Cardiovasc. Surg. 1992, 103, 877–880. [Google Scholar] [CrossRef]
- Majdalany, D.S.; Phillips, S.D.; Dearani, J.A.; Connolly, H.M.; Warnes, C.A. Isolated partial anomalous pulmonary venous connections in adults: Twenty-year experience. Congenit. Heart Dis. 2010, 5, 537–545. [Google Scholar] [CrossRef]
- Griffeth, E.M.; Dearani, J.A.; Mathew, J.; Graham, G.C.; Connolly, H.M.; King, K.S.; Schaff, H.V.; Stephens, E.H. Early and Late Outcomes of the Warden and Modified Warden Procedure. Ann. Thorac. Surg. 2022, 114, 1723–1729. [Google Scholar] [CrossRef]

| Nr. | Male Sex | Age at Surgery (Years) | Pulmonary Veins | Left SVC | Acquired Disease | Surgical Procedure | Associated Procedure |
|---|---|---|---|---|---|---|---|
| 1 | Yes | 67.4 | PAPVC | Yes | MR + TR | Modified Warden | MVR + TVR |
| 2 | Yes | 71.3 | PAPVC | No | - | Transatrial | - |
| 3 | No | 67.7 | UTAPVC | Yes | MR + TR + CAD | TCR | MVR + TVR + CABG |
| 4 | Yes | 24.9 | UTAPVC | No | - | TCR | - |
| 5 | Yes | 68.6 | PAPVC | No | - | Modified Warden | LA closure |
| 6 | No | 55.2 | UTAPVC | No | - | TCR | - |
| 7 | Yes | 52.2 | UTAPVC | No | TR | TCR | TVR |
| 8 | Yes | 63.6 | PAPVC | No | CAD | TCR | CABG |
| 9 | No | 59 | PAPVC | No | - | TCR | - |
| 10 | Yes | 40 | PAPVC + DCRV | No | TR | TCR + PVR + ASD creation | TVR + LA closure |
| TCR 7 Patients | Modified Warden 2 Patients | Transatrial Repair 1 Patient | |
|---|---|---|---|
| SVC obstruction n (%) | 0 | 0 | 0 |
| Pulmonary vein obstruction n (%) | 0 | 0 | 0 |
| Sinus node dysfunction n (%) | 0 | 1 | 0 |
| MI n (%) | 0 | 0 | 0 |
| CPB time minutes (mean ± SD) | 87.25 ± 13.4 | 132.5 ± 35.2 | 185 |
| Aortic x-clamp time minutes mean | 54 | 85 | 161 |
| Infection n (%) | 1 (14.3%) | 0 | 0 |
| Type of infection | Urinary tract (E. coli) | 0 | 0 |
| NYHA class at discharge mean | II | II | III |
| Stroke/TIA n (%) | 0 | 0 | 0 |
| Cardiac deaths n (%) | 0 | 0 | 0 |
| Death from any other cause n (%) | 0 | 0 | 0 |
| Alive at discharge n (%) | 7 (100%) | 2 (100%) | 1 (100%) |
| Nr. | SVC Obstruction | Pulmonary Vein Obstruction | Sinus Node Dysfunction | MI | Infection | Type of Infection | Stroke or TIA | Cardiac Deaths | Death from Any Other Cause | Alive at Discharge |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | No | No | No | No | No | No | No | No | Yes |
| 2 | No | No | Yes | No | Yes | E. Coli | No | No | No | Yes |
| 3 | No | No | No | No | No | No | No | No | No | Yes |
| 4 | No | No | No | No | No | No | No | No | No | Yes |
| 5 | No | No | No | No | No | No | No | No | No | Yes |
| 6 | No | No | No | No | No | No | No | No | No | Yes |
| 7 | No | No | No | No | No | No | No | No | No | Yes |
| 8 | No | No | No | No | No | No | No | No | No | Yes |
| 9 | No | No | No | No | No | No | No | No | No | Yes |
| 10 | No | No | No | No | No | No | No | No | No | Yes |
| Transcaval Repair | Modified Warden | Transatrial Repair | |
|---|---|---|---|
| Number of Patients 7 | Number of Patients 2 | Number of Patients 1 | |
| SVC obstruction n (%) | 0 | 0 | 0 |
| Pulmonary vein obstruction n (%) | 0 | 0 | 0 |
| MI n (%) | 0 | 0 | 1 (100%) |
| PM implantation n (%) | 0 | 0 | 0 |
| Cardiac death n (%) | 0 | 0 | 1 (100%) |
| Death from any other cause n (%) | 0 | 0 | 0 |
| Alive at follow-up n (%) | 7 (100%) | 2 (100%) | 0 |
| Patients with EuroScore II ˃ 7 | Survival Time in Years |
|---|---|
| Patient #2 | 5.5 years |
| Study and Year | Country of Origin of the Study | Journal Published | Number of Patients | Age Years (SD) | Type of Anomaly | Qp:Qs | Pulmonary Stenosis N (%) | RVSP mmHg | Obstructed SVC n(%) | LVEF % | ASD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Touray et al. 2020 [27] | Switzerland | EHJ | 168 | 40 ± 17 | TAPVC + PAPVC | 1.2 ± 0.3 | 6 (5) | 16.8 ± 4.6 | 5 (4) | 62 ± 6 | 106 (82%) |
| Rodriguez-Collado 1997 [28] | Mexico | JCTVS | 19 | 26.2 ± 6.5 | TAPVC + PAPVC | NR | NR | 37.4 ± 14.4 | NR | 55 | NR |
| Majdalany et al. 2010 [29] | USA | Congenital Heart Dis. | 28 | 42 | PAPCV + TAPVC | 1.8 | 1 | 24–85 | NR | 55 | NR |
| Griffeth et al. 2022 [30] | USA | Annals | 75 | 39 (21–57) | PAPVC | NR | NR | 30 | NR | 63 | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dokollari, A.; Cameli, M.; Maccherini, M.; Kafazi, H.; Veshti, A.; Sicouri, S.; Bonacchi, M. Surgical Outcomes of Three Repair Techniques for Partial Anomalous Pulmonary Venous Connection in Adult Patients. Hearts 2022, 3, 137-146. https://doi.org/10.3390/hearts3040016
Dokollari A, Cameli M, Maccherini M, Kafazi H, Veshti A, Sicouri S, Bonacchi M. Surgical Outcomes of Three Repair Techniques for Partial Anomalous Pulmonary Venous Connection in Adult Patients. Hearts. 2022; 3(4):137-146. https://doi.org/10.3390/hearts3040016
Chicago/Turabian StyleDokollari, Aleksander, Matteo Cameli, Massimo Maccherini, Haxhire Kafazi, Altin Veshti, Serge Sicouri, and Massimo Bonacchi. 2022. "Surgical Outcomes of Three Repair Techniques for Partial Anomalous Pulmonary Venous Connection in Adult Patients" Hearts 3, no. 4: 137-146. https://doi.org/10.3390/hearts3040016
APA StyleDokollari, A., Cameli, M., Maccherini, M., Kafazi, H., Veshti, A., Sicouri, S., & Bonacchi, M. (2022). Surgical Outcomes of Three Repair Techniques for Partial Anomalous Pulmonary Venous Connection in Adult Patients. Hearts, 3(4), 137-146. https://doi.org/10.3390/hearts3040016








