Unexplained Syncope: The Importance of the Electrophysiology Study
Abstract
:1. Introduction
2. Significance
3. Epidemiology
4. Consequences
5. Etiology
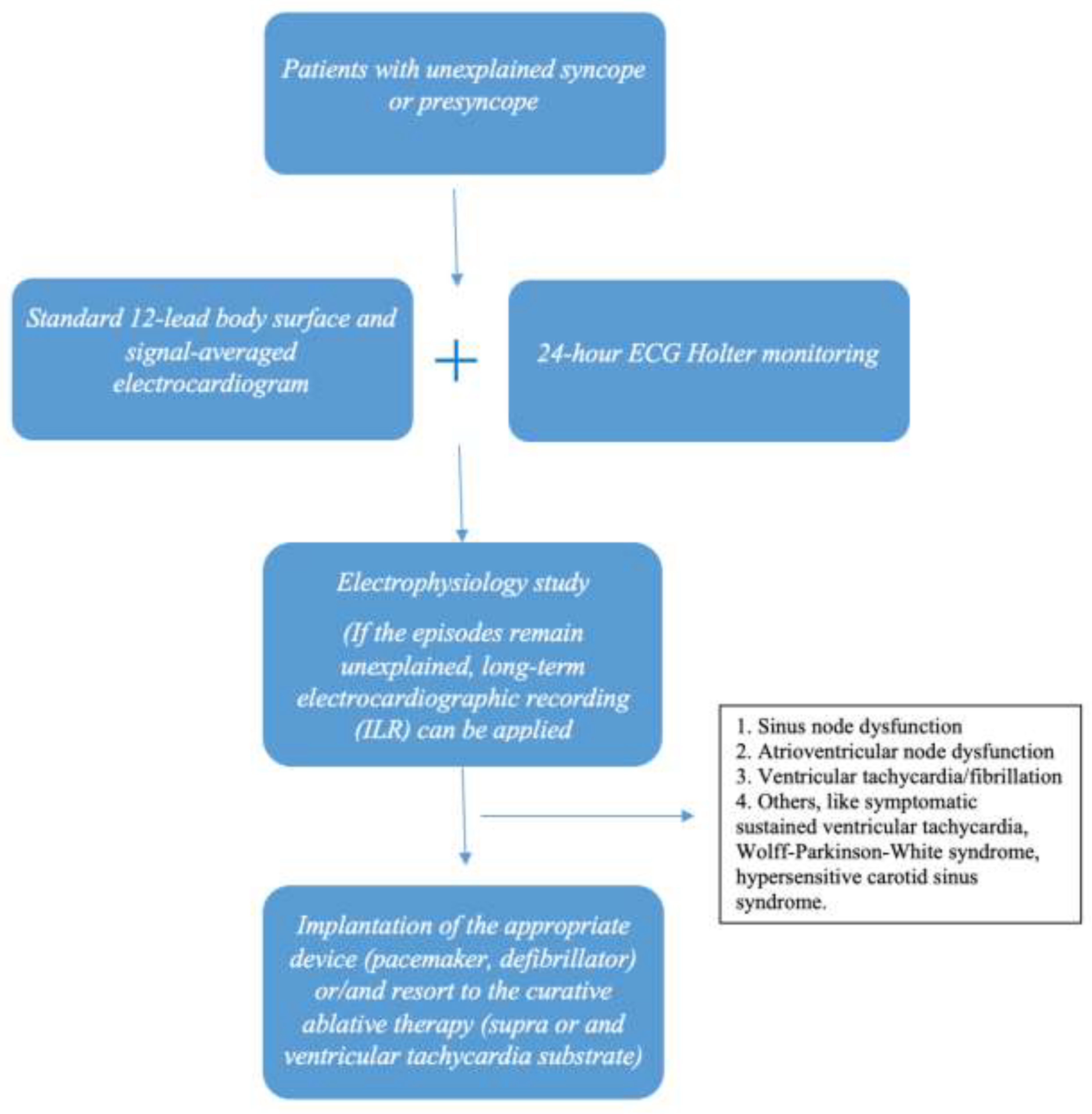
6. Diagnostic Approach
6.1. A. Standard 12-Lead Body Surface Electrocardiography (ECG)
- Sinus bradycardia with a heart rate <60 bpm.
- Presence of left anterior (LAFB) or left posterior fascicular block (LPFB) or complete right bundle brunch block (RBBB).
- Presence of left bundle branch block (LBBB).
- Presence of first degree atrioventricular block with PR interval >200 ms.
- Presence of bifascicular block (RBBB with either LAFB or LPFB, first degree AV block with either LAFB or LPFB).
- Presence of trifascicular block (RBBB and first degree atrioventricular block combined with either LAFB or LPFB).
- Presence of LBBB with first degree atrioventricular block.
- Presence of right ventricular repolarization abnormalities such as ST segment and T wave changes in right precordial leads V1-V2-V3, indicative of Brugada syndrome or arrhythmogenic right ventricular cardiomyopathy with epsilon wave (ARVC).
- Presence of delta waves.
- Presence of Q waves or poor R wave progression in the precordial leads, indicative of an old myocardial infarction.
- Presence of late potentials (LPs) in the signal-averaged electrocardiogram (SAECG).
- “Dagger-like” Q waves in inferior +/− lateral leads and deep inverted precordial T waves in apical hypertrophic cardiomyopathy.
6.2. Β. 24-h ECG Holter Monitoring
- Mean 24-h heart rate (day and night) <60 bpm, indicative of persistent sinus bradycardia pointing to sinus node disease as the arrhythmia cause of transient loss of consciousness [49].
- Presence of episodes of supraventricular tachycardia with a heart rate >140 bpm [41].
- Presence of sinus pauses of ≥2 and ≤2.5 s.
- Episodes of second degree atrioventricular block type I or type II, including 2:1 atrioventricular block [50].
- Presence of non-conducted premature atrial conductions.
6.3. C. Electrophysiology Study
- (a)
- Corrected sinus node recovery time (CSNRT) ≥525 ms.
- (b)
- Sinoatrial conduction time (SACT) ≥140 ms.
- (c)
- Chronotropic response to atropine or isoproterenol ≤90 bpm.
- (a)
- HV interval ≥ 60 ms.
- (b)
- Weckenbach phenomenon and 2:1 atrioventricular block ≥500 and 400 ms, respectively.
- (c)
- Atrioventricular node effective refractory period ≥450 ms.
- (d)
- Presence of split His potentials.
- (e)
- Induction of infranodal block in atrial pacing.
- (f)
- Induction of bifascicular or trifascicular block in atrial pacing.
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kapoor, W.N. Syncope. N. Engl. J. Med. 2000, 343, 1856–1862. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martin, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Kossyvakis, C.; Panagopoulou, V.; Tsiachris, D.; Doudoumis, K.; Mavri, M.; Vrachatis, D.; Letsas, K.; Efremidis, M.; Katsivas, A.; et al. Permanent pacemaker implantation in octogenarians with unexplained syncope and positive electrophysiologic testing. Heart Rhythm 2017, 14, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Bass, E.B.; Elson, J.J.; Fogoros, R.N.; Peterson, J.; Arena, V.C.; Kapoor, W.N. Long-term prognosis of patients undergoing electrophysiologic studies for syncope of unknown origin. Am. J. Cardiol. 1988, 62, 1186–1191. [Google Scholar] [CrossRef]
- Sutton, R.; Benditt, D.G. Epidemiology and economic impact of cardiac syncope in western countries. Future Cardiol. 2012, 8, 467–472. [Google Scholar] [CrossRef]
- Shiyovich, A.; Munchak, I.; Zelingher, J.; Grosbard, A.; Katz, A. Admission for syncope: Evaluation, cost and prognosis according to etiology. Isr. Med Assoc. J. IMAJ 2008, 10, 104–108. [Google Scholar]
- Malasana, G.; Brignole, M.; Daccarett, M.; Sherwood, R.; Hamdan, M.H. The prevalence and cost of the faint and fall problem in the state of Utah. Pacing Clin. Electrophysiol. PACE 2011, 34, 278–283. [Google Scholar] [CrossRef]
- Moya, A.; Sutton, R.; Ammirati, F.; Blanc, J.J.; Brignole, M.; Dahm, J.B.; Deharo, J.C.; Gajek, J.; Gjesdal, K.; Krahn, A.; et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur. Heart J. 2009, 30, 2631–2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Dwyer, C.; Hade, D.; Fan, C.W.; Cunningham, C.; Kenny, R.A. How well are European Society of Cardiology (ESC) guidelines adhered to in patients with syncope? Ir. Med J. 2010, 103, 11–14. [Google Scholar]
- Kapoor, W.N. Evaluation and outcome of patients with syncope. Medicine 1990, 69, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, H.E.; Linzer, M.; Gabelman, M.; Smoller, S.; Scheuer, J. Syncope in a general hospital patient population. Usefulness of the radionuclide brain scan, electroencephalogram, and 24-hour Holter monitor. N.Y. State J. Med. 1983, 83, 1161–1165. [Google Scholar]
- Day, S.C.; Cook, E.F.; Funkenstein, H.; Goldman, L. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am. J. Med. 1982, 73, 15–23. [Google Scholar] [CrossRef]
- Savage, D.D.; Corwin, L.; McGee, D.L.; Kannel, W.B.; Wolf, P.A. Epidemiologic features of isolated syncope: The Framingham Study. Stroke 1985, 16, 626–629. [Google Scholar] [CrossRef] [Green Version]
- Gatzoulis, K.; Sideris, S.; Theopistou, A.; Sotiropoulos, H.; Stefanadis, C.; Toutouzas, P. Long-term outcome of patients with recurrent syncope of unknown cause in the absence of organic heart disease and relation to results of baseline tilt table testing. Am. J. Cardiol. 2003, 92, 876–879. [Google Scholar] [CrossRef]
- Colman, N.; Nahm, K.; Ganzeboom, K.S.; Shen, W.K.; Reitsma, J.; Linzer, M.; Wieling, W.; Kaufmann, H. Epidemiology of reflex syncope. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2004, 14 (Suppl. 1), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, Z.; Voss, F.; Schoels, W. Results of invasive electrophysiologic evaluation in 268 patients with unexplained syncope. J. Huazhong Univ. Sci. Technol. 2003, 23, 278–279. [Google Scholar]
- Kapoor, W.N.; Karpf, M.; Wieand, S.; Peterson, J.R.; Levey, G.S. A prospective evaluation and follow-up of patients with syncope. N. Engl. J. Med. 1983, 309, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, W.N.; Peterson, J.; Wieand, H.S.; Karpf, M. Diagnostic and prognostic implications of recurrences in patients with syncope. Am. J. Med. 1987, 83, 700–708. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001, 345, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.K.; Decker, W.W.; Smars, P.A.; Goyal, D.G.; Walker, A.E.; Hodge, D.O.; Trusty, J.M.; Brekke, K.M.; Jahangir, A.; Brady, P.A.; et al. Syncope Evaluation in the Emergency Department Study (SEEDS): A multidisciplinary approach to syncope management. Circulation 2004, 110, 3636–3645. [Google Scholar] [CrossRef] [Green Version]
- Kenny, R.A.; Brignole, M.; Dan, G.A.; Deharo, J.C.; van Dijk, J.G.; Doherty, C.; Hamdan, M.; Moya, A.; Parry, S.W.; Sutton, R.; et al. Syncope Unit: Rationale and requirement--the European Heart Rhythm Association position statement endorsed by the Heart Rhythm Society. Ep Eur. 2015, 17, 1325–1340. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Mamarelis, I.I.; Theopistou, A.M.; Sideris, S.K.; Avgeropoulou, K.; Gialafos, J.H.; Toutouzas, P.K. Tilt-Table Testing in Syncopal Patients with Sick Sinus Syndrome: A Guide to Pathophysiology and Management? Ann. Noninvasive Electrocardiol. 1999, 4, 115–120. [Google Scholar] [CrossRef]
- McHenry, L.C., Jr.; Fazekas, J.F.; Sullivan, J.F. Cerebral hemodynamics of syncope. Am. J. Med Sci. 1961, 241, 173–178. [Google Scholar] [CrossRef]
- Gibson, G.E.; Pulsinelli, W.; Blass, J.P.; Duffy, T.E. Brain dysfunction in mild to moderate hypoxia. Am. J. Med. 1981, 70, 1247–1254. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Spanarkel, M.; Pitterman, A.; Toltzis, R.; Gratz, E.; Epstein, S.; Keiser, H.R. Circulatory control mechanisms in vasodepressor syncope. Am. Heart J. 1982, 104, 1071–1075. [Google Scholar] [CrossRef]
- Alboni, P.; Brignole, M.; Menozzi, C.; Raviele, A.; Del Rosso, A.; Dinelli, M.; Solano, A.; Bottoni, N. Diagnostic value of history in patients with syncope with or without heart disease. J. Am. Coll. Cardiol. 2001, 37, 1921–1928. [Google Scholar] [CrossRef] [Green Version]
- Gatzoulis, K.A.; Karystinos, G.; Gialernios, T.; Sotiropoulos, H.; Synetos, A.; Dilaveris, P.; Sideris, S.; Kalikazaros, I.; Olshansky, B.; Stefanadis, C.I. Correlation of noninvasive electrocardiography with invasive electrophysiology in syncope of unknown origin: Implications from a large syncope database. Ann. Noninvasive Electrocardiol. 2009, 14, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Krol, R.B.; Morady, F.; Flaker, G.C.; DiCarlo, L.A., Jr.; Baerman, J.M.; Hewett, J.; de Buitleir, M. Electrophysiologic testing in patients with unexplained syncope: Clinical and noninvasive predictors of outcome. J. Am. Coll. Cardiol. 1987, 10, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Gatzoulis, K.A.; Carlson, M.D.; Biblo, L.A.; Rizos, I.; Gialafos, J.; Toutouzas, P.; Waldo, A.L. Time domain analysis of the signal averaged electrocardiogram in patients with a conduction defect or a bundle branch block. Eur. Heart J. 1995, 16, 1912–1919. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Arsenos, P.; Trachanas, K.; Dilaveris, P.; Antoniou, C.; Tsiachris, D.; Sideris, S.; Kolettis, T.M.; Tousoulis, D. Signal-averaged electrocardiography: Past, present, and future. J. Arrhythmia 2018, 34, 222–229. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Karystinos, G.; Arsenos, P.; Gialernios, T.; Vouliotis, A.H.; Kritikos, A.; Papadopoulos, A.; Tousoulis, D. An electrophysiology guided approach is useful even among unexplained syncope patients without abnormal electrocardiographic findings or/and systolic ventricular dysfunction. In Proceedings of the (EHRA) EUROPACE–CARDIOSTIM, Milan, Italy, 21–24 June 2015. [Google Scholar]
- Abi-Samra, F.; Maloney, J.D.; Fouad-Tarazi, F.M.; Castle, L.W. The usefulness of head-up tilt testing and hemodynamic investigations in the workup of syncope of unknown origin. Pacing Clin. Electrophysiol. PACE 1988, 11, 1202–1214. [Google Scholar] [CrossRef]
- Fitzpatrick, A.; Sutton, R. Tilting towards a diagnosis in recurrent unexplained syncope. Lancet 1989, 1, 658–660. [Google Scholar] [CrossRef]
- Raviele, A.; Gasparini, G.; Di Pede, F.; Delise, P.; Bonso, A.; Piccolo, E. Usefulness of head-up tilt test in evaluating patients with syncope of unknown origin and negative electrophysiologic study. Am. J. Cardiol. 1990, 65, 1322–1327. [Google Scholar] [CrossRef]
- Grubb, B.P.; Temesy-Armos, P.; Hahn, H.; Elliott, L. Utility of upright tilt-table testing in the evaluation and management of syncope of unknown origin. Am. J. Med. 1991, 90, 6–10. [Google Scholar] [CrossRef]
- Grubb, B.P.; Wolfe, D.; Temesy-Armos, P.; Hahn, H.; Elliott, L. Reproducibility of head upright tilt table test results in patients with syncope. Pacing Clin. Electrophysiol. PACE 1992, 15, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Klein, G.J.; Yee, R.; Skanes, A.C. Randomized assessment of syncope trial: Conventional diagnostic testing versus a prolonged monitoring strategy. Circulation 2001, 104, 46–51. [Google Scholar] [CrossRef]
- Farwell, D.J.; Freemantle, N.; Sulke, N. The clinical impact of implantable loop recorders in patients with syncope. Eur. Heart J. 2006, 27, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulke, N.; Sugihara, C.; Hong, P.; Patel, N.; Freemantle, N. The benefit of a remotely monitored implantable loop recorder as a first line investigation in unexplained syncope: The EaSyAS II trial. 2016, 18, 912–918. Europace 2016, 18, 912–918. [Google Scholar] [CrossRef]
- Sideris, S.K.; Mousiama, T.A.; Stougiannos, P.N.; Skiadas, I.P.; Laiou, F.P.; Sotiropoulos, I.K.; Gatzoulis, K.A.; Stefanadis, C.; Kallikazaros, I.E. The role of the implantable loop recorder in the investigation of recurrent syncope. Hell. J. Cardiol. 2009, 50, 155–159. [Google Scholar]
- Soulaidopoulos, S.; Arsenos, P.; Doundoulakis, I.; Tsiachris, D.; Antoniou, C.K.; Dilaveris, P.; Fragakis, N.; Sotiriadou, M.; Sideris, S.; Kordalis, A.; et al. Syncope associated with supraventricular tachycardia: Diagnostic role of implantable loop recorders. Ann. Noninvasive Electrocardiol. 2021, e12850. [Google Scholar] [CrossRef]
- Kapoor, W.N.; Fortunato, M.; Hanusa, B.H.; Schulberg, H.C. Psychiatric illnesses in patients with syncope. Am. J. Med. 1995, 99, 505–512. [Google Scholar] [CrossRef]
- Katon, W. Panic disorder and somatization. Review of 55 cases. Am. J. Med. 1984, 77, 101–106. [Google Scholar] [CrossRef]
- Linzer, M.; Felder, A.; Hackel, A.; Perry, A.J.; Varia, I.; Lou Melville, M.; Ranga Krishnan, K. Psychiatric Syncope: A New Look at an Old Disease. Psychosomatics 1990, 31, 181–188. [Google Scholar] [CrossRef]
- Linzer, M.; Varia, I.; Pontinen, M.; Divine, G.W.; Grubb, B.P.; Estes, N.A.M. Medically unexplained syncope: Relationship to psychiatric illness. Am. J. Med. 1992, 92, S18–S25. [Google Scholar] [CrossRef]
- Gatzoulis, K.; Tsiachris, D.; Balta, G.; Antoniou, C.-K.; Arsenos, P.; Dilaveris, P.; Tousoulis, D. Cardiac rhythm management devices and ablation procedures in psychiatric patients: A case series and review of the literature. Heart Mind 2020, 4, 21–25. [Google Scholar] [CrossRef]
- Sowden, N.; Booth, C.; Kaye, G. Syncope, Epilepsy and Ictal Asystole: A Case Series and Narrative Review. Heart Lung Circ. 2021, 10.1016/j.hlc.2021.07.003. [Google Scholar] [CrossRef]
- Baranchuk, A.; Nault, M.A.; Morillo, C.A. The central nervous system and sudden cardiac death: What should we know? Cardiol. J. 2009, 16, 105–112. [Google Scholar] [PubMed]
- Doundoulakis, I.; Gatzoulis, K.A.; Arsenos, P.; Dilaveris, P.; Skiadas, I.; Tsiachris, D.; Antoniou, C.K.; Soulaidopoulos, S.; Karystinos, G.; Pylarinou, V.; et al. Permanent pacemaker implantation in unexplained syncope patients with borderline sinus bradycardia and electrophysiology study-proven sinus node disease. J. Arrhythmia 2021, 37, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Doundoulakis, I.; Gatzoulis, K.A.; Arsenos, P.; Dilaveris, P.; Tsiachris, D.; Antoniou, C.K.; Sideris, S.; Kordalis, A.; Soulaidopoulos, S.; Laina, A.; et al. Permanent pacemaker implantation in unexplained syncope patients with electrophysiology study-proven atrioventricular node disease. Hell. J. Cardiol. 2021. Ahead of Print. [Google Scholar]
- Gatzoulis, K.A.; Tsiachris, D.; Arsenos, P.; Archontakis, S.; Dilaveris, P.; Vouliotis, A.; Sideris, S.; Skiadas, I.; Kallikazaros, I.; Stefanadis, C. Prognostic value of programmed ventricular stimulation for sudden death in selected high risk patients with structural heart disease and preserved systolic function. Int. J. Cardiol. 2014, 176, 1449–1451. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Tsiachris, D.; Dilaveris, P.; Archontakis, S.; Arsenos, P.; Vouliotis, A.; Sideris, S.; Trantalis, G.; Kartsagoulis, E.; Kallikazaros, I.; et al. Implantable cardioverter defibrillator therapy activation for high risk patients with relatively well preserved left ventricular ejection fraction. Does it really work? Int. J. Cardiol. 2013, 167, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Teichman, S.L.; Felder, S.D.; Matos, J.A.; Kim, S.G.; Waspe, L.E.; Fisher, J.D. The value of electrophysiologic studies in syncope of undetermined origin: Report of 150 cases. Am. Heart J. 1985, 110, 469–479. [Google Scholar] [CrossRef]
- Hess, D.S.; Morady, F.; Scheinman, M.M. Electrophysiologic testing in the evaluation of patients with syncope of undetermined origin. Am. J. Cardiol. 1982, 50, 1309–1315. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Toutouzas, P.K. Neurocardiogenic syncope: Aetiology and management. Drugs 2001, 61, 1415–1423. [Google Scholar] [CrossRef]
- Scheinman, M.M.; Peters, R.W.; Suave, M.J.; Desai, J.; Abbott, J.A.; Cogan, J.; Wohl, B.; Williams, K. Value of the H-Q interval in patients with bundle branch block and the role of prophylactic permanent pacing. Am. J. Cardiol. 1982, 50, 1316–1322. [Google Scholar] [CrossRef]
- Morady, F.; Higgins, J.; Peters, R.W.; Schwartz, A.B.; Shen, E.N.; Bhandari, A.; Scheinman, M.M.; Sauvé, M.J. Electrophysiologic testing in bundle branch block and unexplained syncope. Am. J. Cardiol. 1984, 54, 587–591. [Google Scholar] [CrossRef]
- Moya, A.; García-Civera, R.; Croci, F.; Menozzi, C.; Brugada, J.; Ammirati, F.; Del Rosso, A.; Bellver-Navarro, A.; Garcia-Sacristán, J.; Bortnik, M.; et al. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur. Heart J. 2011, 32, 1535–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.K.; Sheldon, R.S.; Benditt, D.G.; Cohen, M.I.; Forman, D.E.; Goldberger, Z.D.; Grubb, B.P.; Hamdan, M.H.; Krahn, A.D.; Link, M.S.; et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017, 136, e25–e59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulamhusein, S.; Naccarelli, G.V.; Ko, P.T.; Prystowsky, E.N.; Zipes, D.P.; Barnett, H.J.; Heger, J.J.; Klein, G.J. Value and limitations of clinical electrophysiologic study in assessment of patients with unexplained syncope. Am. J. Med. 1982, 73, 700–705. [Google Scholar] [CrossRef]
| Reflex (Neurally Mediated) Syncope | Orthostatic | Cardiovascular |
|---|---|---|
| Vasovagal | Primary autonomic failure | Tachycardia |
|
|
|
| Situational | Secondary autonomic failure | Bradycardia |
|
|
|
| Carotid Sinus Syndrome (CSS) | Drug-induced | Structural cardiac |
|
| |
| Non-classical forms | Volume depletion | Cardiopulmonary and great vessels |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doundoulakis, I.; Soulaidopoulos, S.; Arsenos, P.; Dilaveris, P.; Tsiachris, D.; Antoniou, C.-K.; Sideris, S.; Kordalis, A.; Laina, A.; Kallinikidis, S.; et al. Unexplained Syncope: The Importance of the Electrophysiology Study. Hearts 2021, 2, 495-504. https://doi.org/10.3390/hearts2040038
Doundoulakis I, Soulaidopoulos S, Arsenos P, Dilaveris P, Tsiachris D, Antoniou C-K, Sideris S, Kordalis A, Laina A, Kallinikidis S, et al. Unexplained Syncope: The Importance of the Electrophysiology Study. Hearts. 2021; 2(4):495-504. https://doi.org/10.3390/hearts2040038
Chicago/Turabian StyleDoundoulakis, Ioannis, Stergios Soulaidopoulos, Petros Arsenos, Polychronis Dilaveris, Dimitris Tsiachris, Christos-Konstantinos Antoniou, Skevos Sideris, Athanasios Kordalis, Ageliki Laina, Sotirios Kallinikidis, and et al. 2021. "Unexplained Syncope: The Importance of the Electrophysiology Study" Hearts 2, no. 4: 495-504. https://doi.org/10.3390/hearts2040038
APA StyleDoundoulakis, I., Soulaidopoulos, S., Arsenos, P., Dilaveris, P., Tsiachris, D., Antoniou, C.-K., Sideris, S., Kordalis, A., Laina, A., Kallinikidis, S., Xydis, P., Archontakis, S., Tsioufis, K., & Gatzoulis, K. A. (2021). Unexplained Syncope: The Importance of the Electrophysiology Study. Hearts, 2(4), 495-504. https://doi.org/10.3390/hearts2040038









