1. Introduction
Physiologic monitoring, including electrocardiographic (ECG) monitoring, in the intensive care unit (ICU) remains unsatisfactory as evidenced by the well-known alarm fatigue problem. For example, in one study, a single ICU patient generated over 700 alarms per day [
1]. In the University of California San Francisco (UCSF) Alarm Study, an average of 187 audible alarms were generated per bed per day during a one-month assessment. Of note, 90% of the ECG arrhythmia alarms were determined to be false [
2,
3]. Alarm fatigue occurs when nurses and providers are desensitized by frequent alarms, most of which are false or clinically irrelevant (i.e., true, but with no action required). The multitude of alarm sounds become “background noise” that is assimilated into the normal ICU workflow. Over a decade ago, excessive alarm burden was exposed by the press as a significant patient safety concern with the highly publicized death of a patient who was being monitored at a prestigious medical center [
4]. Despite multiple heart rate alarms for bradycardia prior to the patient’s cardiac arrest, no one working on the unit that day recalled hearing the alarms. In the investigation that ensued, the Centers for Medicare and Medicaid Services reported: “Nurses not recalling hearing low heart rate alarms was indicative of alarm fatigue, which contributed to the patient’s death” [
4]. The most recent data from the past seven years, show that alarm fatigue was responsible for over 650 hospital deaths [
5,
6], a number believed to be a substantial underrepresentation due to non- or under-reporting. Over time, nurses cope with alarm fatigue by: (1) silencing alarms without assessing the patient; (2) lowering the alarm volume; (3) permanently disabling alarms; and/or (4) delay responding by assuming an alarm is false. These actions place patients at risk for adverse events, including death, as true alarms are missed.
A number of federal and national organizations have issued alerts about alarm fatigue. Since 2007, The Emergency Care Research Institute has placed alarm fatigue at or near the top of its list of top ten patient safety hazards [
7]. In 2014, The Joint Commission established National Patient Safety Goal 6, Reduce Harm Associated with Clinical Alarms [
5], which was updated in 2020 [
8]. The American Nurses Association and the American Association of Critical Care Nurses have issued practice alerts regarding alarm fatigue, emphasizing the need for evidence-based approaches to solve this complex problem [
9,
10]. While a strong desire to solve alarm fatigue exists from these mandates, over a decade has passed with no substantive progress towards a general solution.
Prior clinical studies designed to reduce false alarms have included: daily ECG skin electrode changes [
11,
12]; customizing alarm parameters and/or alarm settings [
11,
12,
13,
14,
15,
16,
17,
18]; disposable versus non-disposable ECG lead wires [
17,
19]; and educational initiatives [
12,
14,
17]. While these strategies have reduced the total number of alarms by 18% [
14,
19] to 90% [
12], these studies do not address the primary problem of false ECG arrhythmia alarms, namely deficient and outdated arrhythmia algorithms. The following review will discuss the research our group has completed to shed light on these deficiencies. In addition, we will briefly discuss an annotation effort our group has undertaken to improve arrhythmia algorithms for asystole, ventricular fibrillation, and ventricular tachycardia.
2. Overview of ECG False Arrhythmia Alarms
False ECG arrhythmia alarms are a major source of alarm fatigue. In the UCSF Alarm study, the frequency, types, and accuracy of ECG monitor alarms were examined in 461 ICU patients [
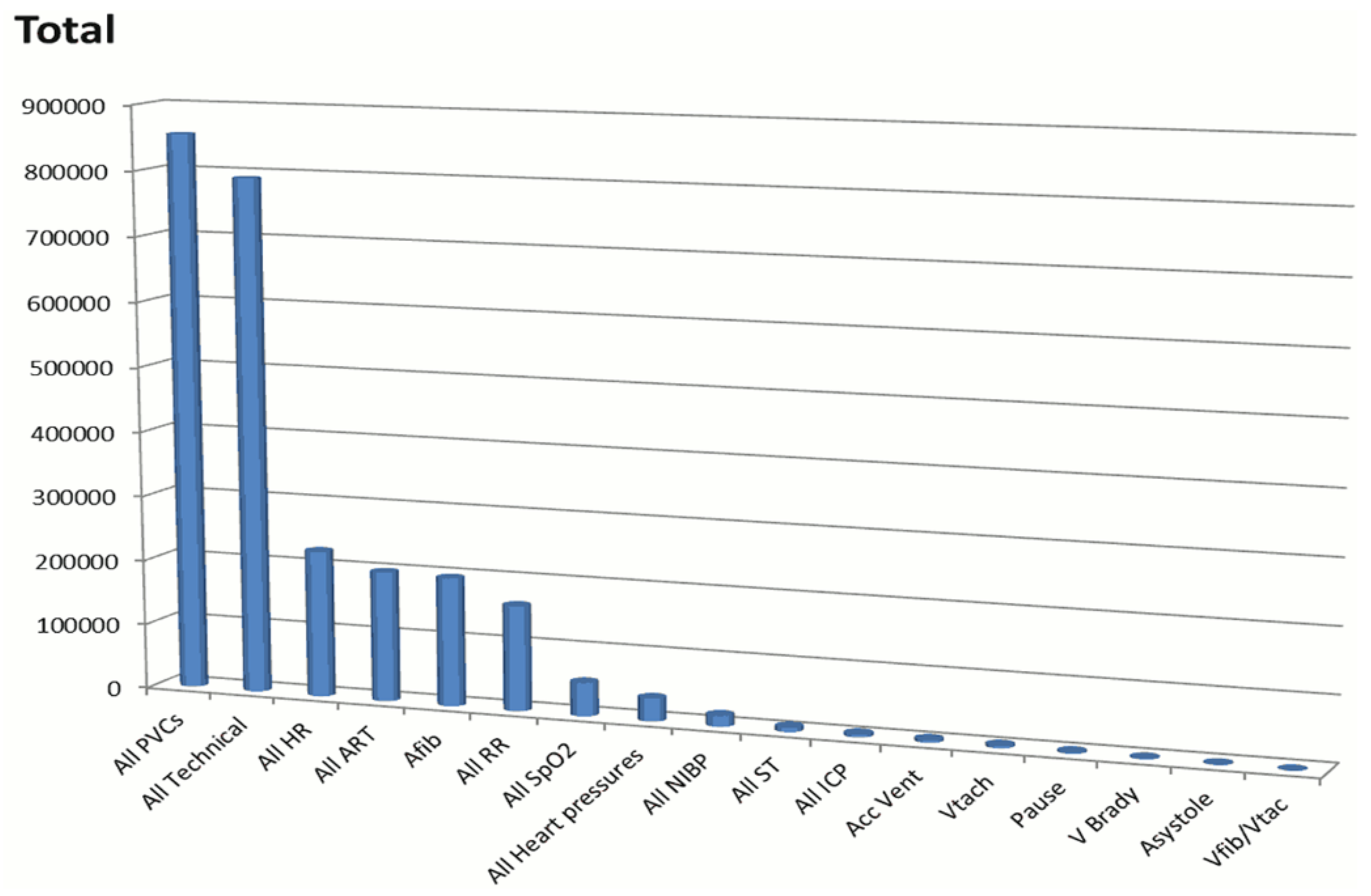
2]. A total of 2,558,760 unique alarms occurred in the 31-day study period: 1,154,201 (45%) arrhythmia; 612,927 (24%) parameter (i.e., too high, too low); and 791,632 (31%) technical (i.e., ECG leads off, artifact, or line/probe disconnect); these are shown in
Figure 1. There were 381,560 audible alarms for an audible alarm burden of 187/bed/day. Of 12,671 annotated ECG arrhythmia alarms, 90% were false positive. This study of consecutive ICU patients represents the largest study regarding alarm burden to date, and clearly illustrates the magnitude of alarm fatigue.
As noted in
Figure 1, the vast majority of the alarms were for premature ventricular complexes (PVC), followed by technical alarms (i.e., artifact or lead(s) off/fail). In addition to these observational data, the investigator of the UCSF Alarm study annotated six audible arrhythmia alarms as true or false using a standardized protocol [
2]. A total of 12,671 audible arrhythmia alarms were annotated. Inter-rater reliability of alarm annotations was tested by randomly selecting 300 alarms that were rated twice by pairs of the annotators. A Cohen’s Kappa was run to compare “Rater 1″ to “Rater 2”. There was 95% agreement as to whether the alarm was a true or false positive (Cohen’s Kappa score of 0.86).
Table 1 shows the accuracy of the arrhythmia alarms annotated.
Based on these findings, our group has published several studies examining patient, clinical, and ECG factors associated with false arrhythmia alarms. The following sections will describe these studies.
2.1. Patient and Clinical Factors Associated with False Arrhythmia Alarms
In a secondary data analysis of 461 patients enrolled in the UCSF Alarm study with 12,671 audible arrhythmia alarms, 250 (54%) of the patients had at least one of the six alarm types (
Table 1) [
3]. The number of false alarms per monitored hour for patients’ ranged from 0.0 to 7.7, and the duration of the false alarms per hour ranged from 0.0 to 158.8 s. Patient characteristics were compared in relation to: (1) the number and (2) the duration of false arrhythmia alarms per 24-h period, using nonparametric statistics to minimize the influence of outliers. Among the significant associations were the following: age > 60 years (
p = 0.013;
p = 0.034), confused mental status (
p = 0.001 for both comparisons), cardiovascular diagnoses (
p = 0.001 for both comparisons), electrocardiographic (ECG) features, including wide QRS complexes due to bundle branch block (BBB, both right or left) (
p = 0.003;
p = 0.004) or ventricular paced rhythm (
p = 0.002 for both comparisons), respiratory diagnoses (
p = 0.004 for both comparisons), and mechanical ventilation (
p = 0.001 for both comparisons). In a subsequent study, we found that patients with a left ventricular device also have high rates of false arrhythmia alarms, presumably due to the vibrations and artifact caused by this device [
20].
Signal quality as a source of false arrhythmia alarms was examined in the 12,671 audible arrhythmia alarms shown in
Table 1 [
2]. The following definitions were used: good; fair; or poor signal quality. Good signal quality was defined as a clearly visible P-QRS-T waveform across all available leads with little to no noise, baseline wander, or leads off. Fair signal quality was defined as moderate noise or baseline wander but having identifiable QRS complexes for basic rhythm/rate detection. Poor signal quality was defined as being unanalyzable due to excessive noise, baseline wander, or leads off. Of 12,671 audible arrhythmia alarms, ECG signal quality was rated as good in 73% of the false alarms and 93% of the true alarms, whereas poor signal quality was found in 18% of the false arrhythmia alarms and 6% of the true arrhythmia alarms. Hence, poor signal quality was not the source of the majority of the false arrhythmia alarms.
From our published studies we have found that the vast majority of false alarms occur in a small group of patients. For example, in the UCSF Alarm study, of 461 ICU patients, one patient generated nearly half of the alarms [
2], a problem described by others [
14,
17,
21,
22]. We found that many of these patients often have ECG features that contribute to false arrhythmia alarms, including: bundle branch block (BBB), ventricular paced rhythms, and low amplitude QRS complexes [
3,
23,
24]. The ECG features associated with false arrhythmia alarms will be described in more detail below.
2.2. Electrocardiographic Features Associated with False Arrhythmia Alarms
2.2.1. Right or Left BBB
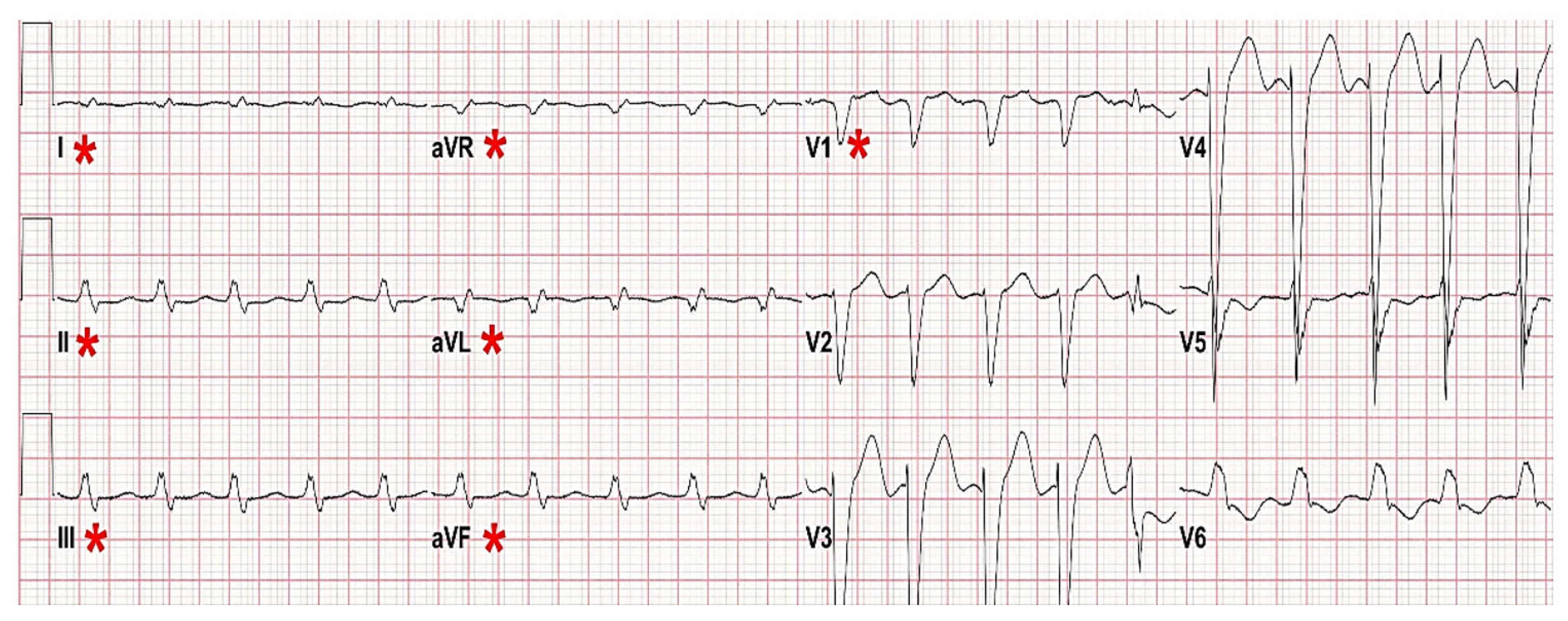
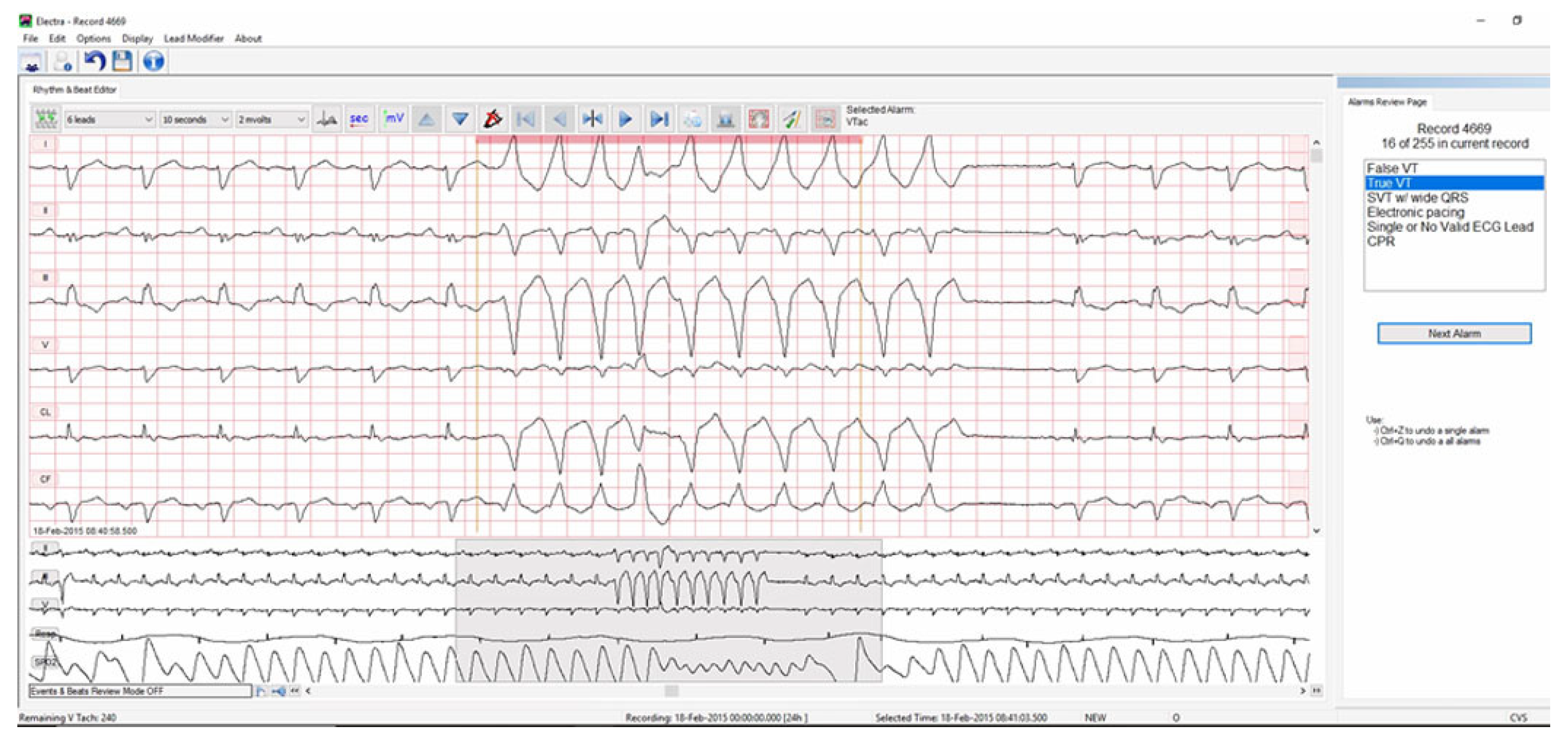
Patients with persistent or intermittent right or left BBB generate a high number of false alarms for ventricular tachycardia. This problem occurs as the wide QRSs associated with VT are mistaken as a ventricular rhythm. In a multivariate analysis, we found that patients with right or left BBB were 2.2 times more likely to generate false alarms when compared to patients without this ECG feature (
p = 0.020) [
3]. Current bedside monitor algorithms lack the ability to recognize right or left BBB. However, this ECG feature, which is present in <10% ICU patients [
4], causes a significant number of false alarms, as shown in
Figure 2.
2.2.2. Ventricular Paced Rhythms
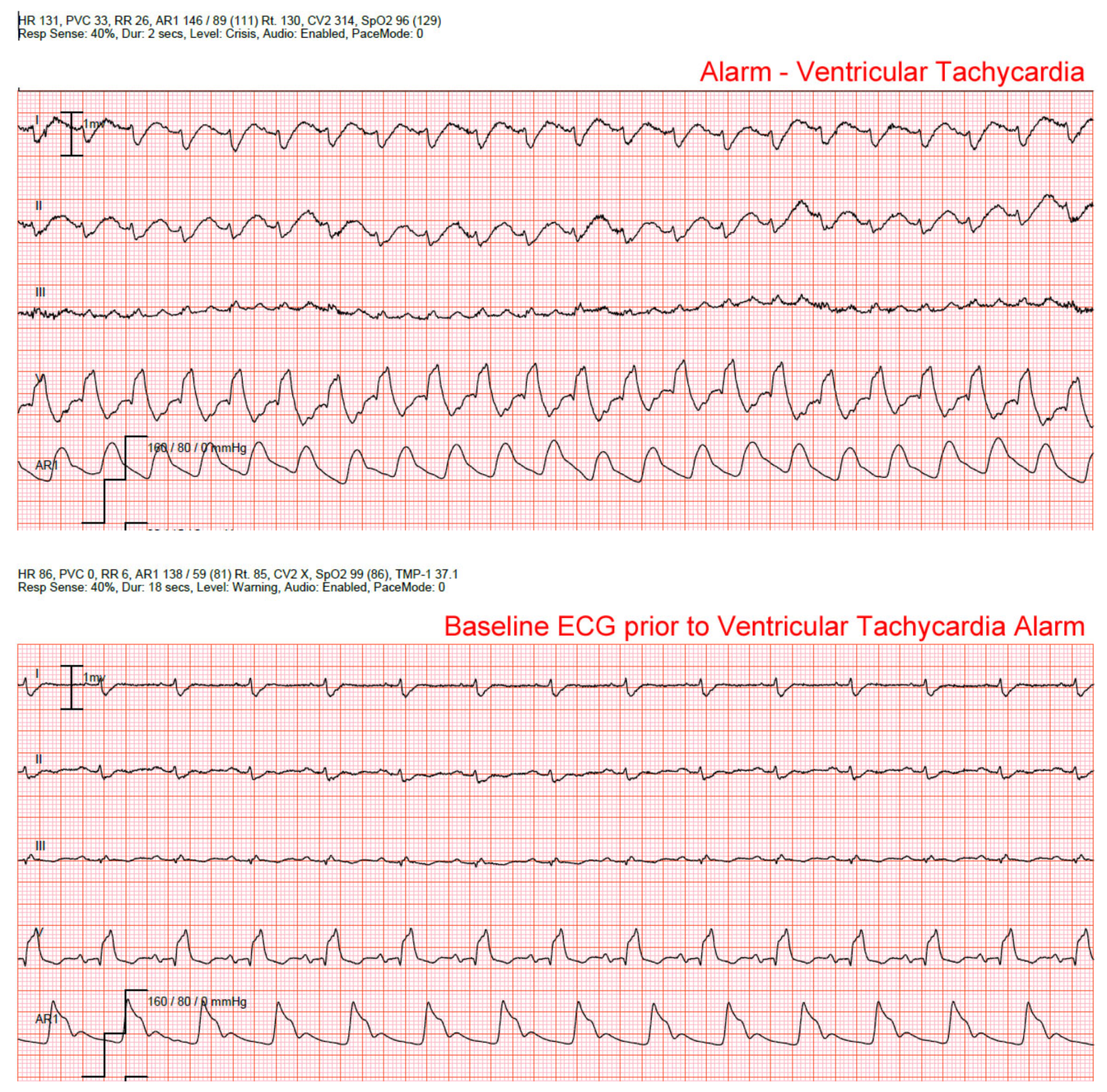
False alarms for ventricular arrhythmias (i.e., VT or accelerated ventricular rhythm) are common in patients with ventricular paced rhythms.
Figure 3 is a false alarm for accelerated ventricular rhythm (AVR; defines as a wide QRS < 100 beats/min) in a patient with a ventricular pacer. Note the pacer spikes before each QRS. The bedside monitor requires the nurse to activate the Pace Mode feature, which adjusts the filter settings in order to detect pacemaker stimuli (i.e., spikes). However, the Pacer Mode feature was not turned on (star) in this patient, which led to non-stop false AVR alarms. We found that the Pacer Mode had been activated in only 33% of the patients with a ventricular pacer [
2,
25].
2.2.3. Low Amplitude QRS Complexes
We found that low amplitude QRS complexes can cause false asystole alarms [
26]. This type of QRS feature can occur in morbidly obese patients, pericardial effusion, and/or BBB.
Figure 4 illustrates an outlier patient from the UCSF Alarm Study with low amplitude QRSs who generated 45% of the 12,671 annotated alarms. Note, low amplitude QRS complexes in the limb leads (*), but not in the precordial leads, due to left BBB [
2].
The American National Standard (ANS) for cardiac monitors, heart rate meters, and alarms states that ECG devices should not detect a QRS if the waveform is less than 0.15 mV (1.5 mm) in size [
27]. This standard was designed to prevent misdiagnosing P waves as QRSs during ventricular standstill. However, some manufacturers use higher QRS detection thresholds (e.g., 0.5 mV or 5 mm) and require that this higher threshold be present in more than one ECG lead. These stricter thresholds result in undercounting the heart rate and cause false asystole alarms [
24]. We found that when we examined all seven available ECG leads in patients with these types of alarms, a QRS was readily visible in one or more leads in 91% of false asystole alarms [
2].
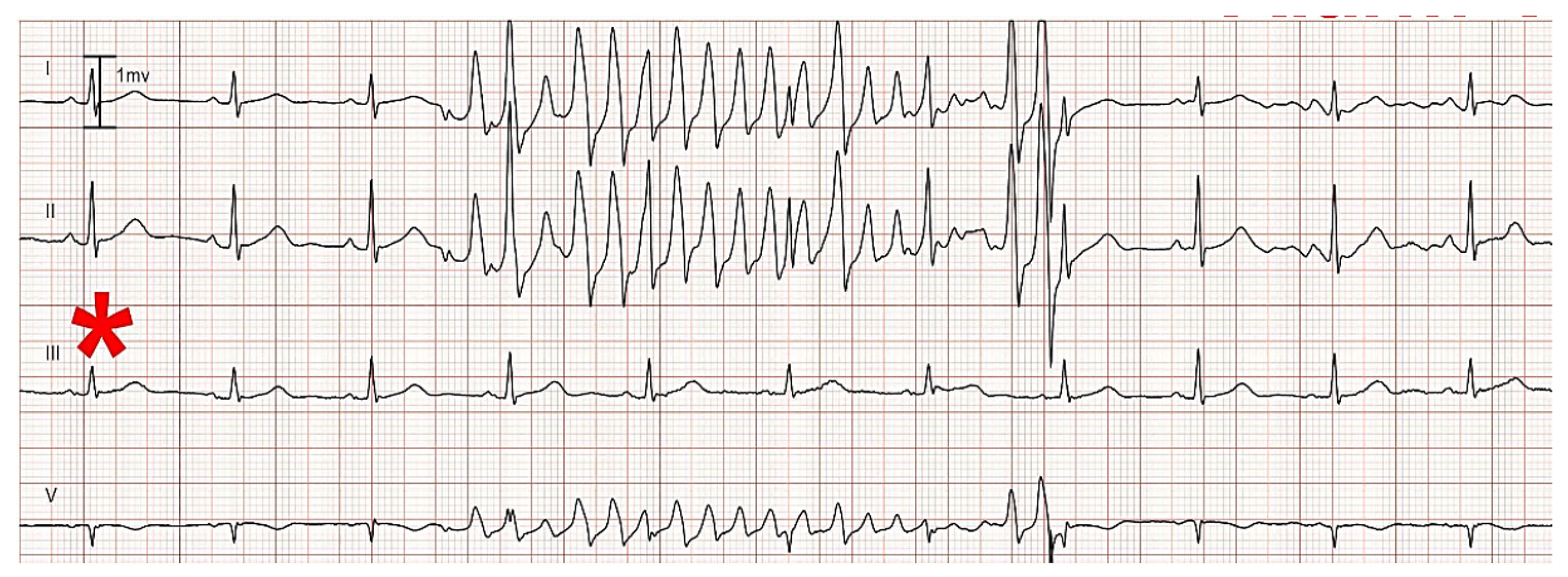
ECG Artifact: Motion artifact is a common cause of false alarms. Typically, the artifact is due to a specific skin electrode on the body surface. The artifact causes pseudo-ectopic beats to be detected, which in turn can trigger a false alarm.
Figure 5 illustrates a false V-fib alarm in a patient using their right hand to scratch their head; thus, causing artifact at the right arm (RA) electrode. Artifact in the RA appears in all of the ECG leads that use the RA in their definition. In this example, the leads affected are I, II, and V, the latter as the reference for V is the Wilson terminal [
28], which depends in part on the RA electrode. However, lead III (denoted by *) is not affected, as this lead uses the left arm and left leg electrodes, and not the RA.
Based on data from the above-mentioned studies, our group at the Center for Physiologic Research in the UCSF School of Nursing, are exploring new algorithms to improve arrhythmia detection of lethal arrhythmias for asystole, ventricular fibrillation (v-fib), and ventricular tachycardia (VT). In order to test the accuracy of these algorithms, our group has established an annotation protocol to examine true versus false alarms for lethal arrhythmias (i.e., asystole, V-fib, and VT) from a dataset of over 6100 ICU patients. Below, we will describe the dataset and the annotation protocol. The final annotation effort has not been published; hence, the focus of discussion in this chapter will be a description of the annotation protocol.
3. Data Capture System
Capturing bedside monitoring data is very challenging, and most frequently limited to a few parametric outputs, such as alarms and vital sign trends. The storage of the actual waveforms (raw data) only occurs under rare, typically research-based circumstances, and requires the implementation of costly and sophisticated data capture infrastructures. In addition, waveform storage typically requires the application of third-party software and hardware, making the workflow even more complex and cumbersome. Only recently have a handful of companies started to provide package solutions that allow seamless data flow from the patient bedside monitors to a hospital-based server and/or data storage center (on or off site).
The consequences of these complexities are that research is easily discouraged or, even worse, is based on bad data (i.e., with gaps or not linked to the correct patient). Indeed, based on the number of false alarms reported in the literature [
2,
3,
14,
15,
16,
17,
19,
21,
22,
29,
30,
31,
32,
33], it is hard to imagine clinically meaningful changes coming out of unsupervised physiologic and alarm data generated by current bedside monitor systems. This is a particularly important point due to the use of artificial intelligence methods and models that are currently very popular approaches used for predicting untoward patient events (i.e., code blue, sepsis). However, these approaches require high quality input data in order to train and test the underlying model being used. Unfortunately, these approaches are being used widely and are being introduced into clinical care.
In the above-described observational study from 2013 [
2], all of the available physiologic data were collected using a sophisticated closed network system that connected data from all 77 ICU monitors and the central monitoring station via a gateway system. The waveform data were ultimately sent to a secure server in our research lab for off-line analysis. The following data were collected from each ICU monitor: (1) all available waveforms (e.g., ECG, arterial BP, central venous pressure, intracranial pressure, and SpO2); (2) vital signs (e.g., heart rate, non-invasive BP, and respiratory rate); (3) alarm settings (i.e., crisis, warning or advisory, and message/technical); as well as (4) audible and inaudible alarms. Our hospital’s Institutional Review Board approved the study waiving patient consent as bedside monitoring is a standard of care, and we examined the data retrospectively; hence, we collected data from consecutive ICU patients.
While we captured an extremely robust dataset, several challenges had to be overcome in order to use the data in a meaningful way. For example, due to the need for third party hardware to store the data, the data were often not well organized, making it difficult to synchronize, not only the physiologic data, but the electronic health record data with individual patients. In addition, as ICU patients are often in the unit for multiple days and even weeks, the size of the files was very large. The above issues were overcome by our group using several approaches. Our Center has developed an automated procedure that re-organizes all the waveform and alarm data into 24-h time blocks. This standardization makes it much easier to analyze both individual and cohorts of patients based upon the research question. The waveforms are also converted and assembled into a binary public format that is suitable for processing by any algorithm; this fosters data sharing, whenever applicable. In addition, we performed an audit of physiologic alarms captured in an individual patient to that of the electronic health record to verify that our data matched with the patient’s electronic health record. As of today, our center has collected and stored bedside monitoring data from all of the adult UCSF ICUs since the pioneering research that started in March 2013; thus, making this the largest database of continuous physiologic data in consecutive ICU patients.
4. Tackling Alarm Fatigue: The Establishment of an Annotated Database Using Bedside Monitoring Data from Consecutive Intensive Care Unit Patients
Alarm fatigue is a substantial problem which is difficult to deal with. Part of the reason is certainly the poor motivation from the industry to invest in and improve existing algorithms. However, even more importantly, the most critical aspect is the lack of a robust database for use as a benchmark for testing of existing and newly designed algorithms. The latter problem has actually raised the attention of regulators, in particular the Food and Drug Administration (FDA), who are seeking an adequate (large, digitized, multi-signal) database to develop, test, and qualify new algorithms. As of today, the benchmark dataset used for medical device testing consists of small number of 30-min 2-channel ECG recordings. In addition, these data are more than 30 years old and from analog Holter recorders with only a few critical arrhythmia events. Therefore, any meaningful answers to alarm fatigue from currently available databases will be limited. Thus, there is a need for an adequate, ideally robust, benchmark dataset from bedside monitors that are currently used in the hospital setting in order to solve this critical problem. Such a dataset should include all of the waveforms typically acquired (i.e., not limited to only ECG) so as to enhance algorithms using multiple signals. To meet this important and yet unmet need, and building on the well-established UCSF Alarm Study that was started in March 2013, our Center is taking on this ambitious challenge.
Using a subset of the existing database, we have assembled 20 months of data (100 patient years) that has been dedicated towards this effort. For this subset of data, we acquired all seven ECG leads (i.e., I, II, III, aVR, AVL, aVF, and a V lead (V1), the latter being the hospital default) and the following waveform data: plethysmograph; transthoracic impedance; and arterial blood pressure. The final sample includes 6143 ICU patients with one or more lethal arrhythmia types (i.e., asystole, V-fib, and VT). The sample was comprised of 46% women and 31% ethnic minorities. Three ICU types were included: Cardiac (16 beds), Medical/Surgical (32 beds), and Neurologic (29 beds). As mentioned previously, the UCSF Institutional Review Board approved the study with waiver of signed patient consent as physiologic monitoring is standard care in the ICU and our data were analyzed off-line and retrospectively; hence, the data are in consecutively enrolled ICU patients.
The seven ECG channels, as well as the other waveform data, were processed by the Center for Physiologic Research lethal arrhythmia algorithms. The physiologic data were converted into a suitable public domain format employing a lossless compression architecture capable of achieving a compression rate of 4:1. For example, the size of a 24 h record inclusive of both ECG and non-ECG waveforms, after compression, is about 100 MB. To make the files more manageable for annotating, multi-day ECG recordings will be separated into 24-h blocks, using midnight to 23:59 PM as the timeframe reference. A HIPAA compliant patient de-identification procedure was applied to the files for annotation, and each record was assigned a unique study ID number. In addition to demographic anonymization, a random date shift was applied to the start date, but not to the start time, for each recording.
The de-identified data were loaded into the Continuous ECG Recording Suite (CER-S, AMPS-LLC) platform for review. A customized version of CER-S has been specifically designed for annotating the database by five PhD prepared ECG expert nurse scientists (protocol described below). Annotators were able to connect remotely to the UCSF network via a dedicated and secure server. Each of the five annotators was assigned arrhythmia alarms to annotate as shown in
Figure 6.
5. Annotation Protocol
The annotation protocol was designed as a multi-tiered, multi-expert, ground truth, manual annotation protocol used for triple ascertainment of lethal arrhythmia events with disagreements resolved by a fourth expert. The annotation team (A-Team) was made up of five nurse scientists with decades of experience as bedside clinical nurses and interpreting hospital based-ECG bedside monitoring data. Each of the lethal arrhythmia alarms was randomly assigned to three of the five annotators. Alarms with consensus (all three annotators agree), were finalized. Alarms for which only two annotators agreed were re-assigned to a fourth annotator. If the disagreement was resolved (i.e., fourth annotators concurred with the two initial annotators) the annotation was finalized. If the disagreement was not resolved, the alarm was reviewed by two independent reviewers for a final decision.
Assessment of False Negatives
In addition to the annotation effort described above using the Center’s lethal arrhythmia algorithm, the incidence of false-negative alarms (i.e., undetected alarms) will be assessed in a subsequent study. For each 24-h data block, a 15-min data segment will be randomly selected (i.e., regardless of whether it contained any true alarm already identified). The same five annotators reviewed each of the 15-min segments, marking the presence of any lethal cardiac arrhythmia.
The annotation effort using the 20-month database (both true versus false and false negatives) is still underway, and the final data will be published at a later date. The goal of this effort is to improve current algorithms used in bedside physiologic monitors by reducing false arrhythmia alarms that are associated with alarm fatigue in clinicians and the associated patient hazards related to missed true events. Furthermore, both monitoring manufacturers and regulatory agencies will benefit from this work, as they work towards solutions to this complex problem.
6. Discussion
In this review we discuss the issue of false arrhythmia alarms that contribute to alarm fatigue in clinicians working in hospitals. While nurses/physicians experience alarm fatigue from repeated exposure to alarms, patients are subjected to stress, both psychological (i.e., fear, anxiety) [
34,
35] and physiological (i.e., increased heart rate and blood pressure, sleep deprivation, and delirium) [
36,
37] from alarm noise. Patients report being frightened by frequent alarms that often go unanswered [
34]. Sentinel events have also been reported due to missed true events that are buried among high numbers of false alarms, with resultant mortality and morbidity among hospitalized patients. As such, a number of federal and professional organizations have issued alerts about alarm fatigue, yet very few interventions have had a substantial and sustained impact on this problem [
7,
8,
9,
10,
13].
A widely held belief is that poor skin electrode contact causes false arrhythmia alarms. However, we found that only 9% of false arrhythmia alarms were due to poor signal quality (i.e., unanalyzable due to excessive noise, baseline wander, or leads off). Rather, our group has shown that false arrhythmia alarms are more common in patients with ECG features such as bundle branch block, a ventricular pacemaker, and/or low amplitude QRS complexes [
3,
24,
25,
38]. Of note, these features, present in only a few patients, were shown to be responsible for 60% of false alarms. Our published research suggests that clinical interventions (i.e., skin electrode changes, alarm adjustments, and education) will not address the vast majority of false arrhythmia alarms as the central problem are deficiencies in currently used arrhythmia algorithms. Importantly, algorithms used in current monitors were developed using three existing databases created in the mid 1970s (i.e., AHA-ECRI, the CUBD, and the MIT/BIH Databases). None of these databases include digitally acquired ECGs, or any other signals currently acquired in modern patient monitors (Sp02 or arterial blood pressure). Additionally, only a small number of patients and arrhythmias were captured during only hours of ECG monitoring. Therefore, new algorithm development will remain stagnant until more robust data sets are available to test and improve arrhythmia algorithms.
Alternative Approaches and Future Directions
In this chapter, we describe an effort to improve the detection of lethal arrhythmias (i.e., asystole, V-fib, and VT) during in-hospital ECG monitoring by annotating a large database (
n > 6100 ICU patients) using currently available bedside monitors (i.e., digitized multi-channel ECG and all available physiologic signals). The ultimate goal of this effort is to create a database that can be used to develop and test new algorithms that incorporate not only ECG, but existing physiologic signals (i.e., SpO2 or arterial blood pressure). It should be noted that a number of studies have been published using varied algorithm-based approaches, mostly machine learning, to address false lethal arrhythmia alarms [
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49]. While several of these studies have shown improved detection of VT, all of these studies have used existing databases, which have limitations as stated above (i.e., decades old, non-digitized, two-channel ECG and one or two physiologic signals, small sample of patients and arrhythmias, sampling bias, and recordings of short duration). Therefore, future studies are needed to examine these novel approaches using contemporary data.
In addition to algorithm-based solutions, there is a need to develop and test new technologies (wearables and sensors) as well as “smart alarms” that integrate multiple physiologic parameters [
50]. Clinicians would also benefit from the integration of multiple data elements (i.e., ECG, physiologic data, electronic health record, laboratory and pharmacologic data) displayed in such a manner that the interpretation of complex multi-layered data elements can be completed promptly. Lastly, there is a need to pair algorithm-based solutions with patient outcomes, which could be used to guide default settings in bedside monitors to differentiate actionable from non-actionable arrhythmias.
7. Conclusions
In this chapter a number of research studies that have examined false arrhythmia alarms during in-hospital ECG monitoring in the intensive care unit were reviewed. The central problem that is created from false arrhythmia alarms is alarm fatigue (i.e., desensitization), which directly impacts nurses and providers who are exposed to high numbers of false alarms but also patient and their families who hear the alarms and wonder if something is wrong. Discussed were a number of patient, clinical and ECG waveform factors associated with false arrhythmia alarms. Our research group at the UCSF Center for Physiologic Research is using a very large database of over 6100 ICU patients with multi-lead and multi-parameter data to develop and test new algorithms to identify lethal arrhythmias, namely asystole, V-fib, and VT. We described the protocol that will be used to annotate these data which we anticipate will serve as a benchmark database that will move the science forward towards meaningful change.
Author Contributions
Conceptualization, M.M.P., D.M. and F.B.; writing—original draft preparation, M.M.P.; and writing—review and editing, D.M. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The studies reviewed in this chapter were conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of The University of California, San Francisco California (IRB #12-09723 original approval 08/29/2012 with ongoing approval).
Informed Consent Statement
For all of the published studies presented in this review, patient consent was waived because physiologic monitoring is used as a standard of care in all of the intensive care units and the data were analyzed off-line and retrospectively and therefore, were not used for clinical decision making.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cvach, M. Monitor alarm fatigue: An integrative review. Biomed. Instrum. Technol. 2012, 46, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.J.; Harris, P.; Zegre-Hemsey, J.K.; Mammone, T.; Schindler, D.; Salas-Boni, R.; Bai, Y.; Tinoco, A.; Ding, Q.; Hu, X. Insights into the problem of alarm fatigue with physiologic monitor devices: A comprehensive observational study of consecutive intensive care unit patients. PLoS ONE 2014, 9, e110274. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.R.; Zegre-Hemsey, J.K.; Schindler, D.; Bai, Y.; Pelter, M.M.; Hu, X. Patient characteristics associated with false arrhythmia alarms in intensive care. Ther. Clin. Risk Manag. 2017, 13, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, L. MGH Death Spurs Review of Patient Monitors. Available online: http://www.boston.com/news/health/articles/2010/02/21/mgh_death_spurs_review_of_patient_monitors/ (accessed on 6 January 2016).
- The Joint Commission. National Patient Safety Goal. Available online: http://www.jointcommission.org/assets/1/18/JCP0713_Announce_New_NSPG.pdf (accessed on 15 July 2021).
- Ruskin, K.J.; Hueske-Kraus, D. Alarm fatigue: Impacts on patient safety. Curr. Opin. Anaesthesiol. 2015, 28, 685–690. [Google Scholar] [CrossRef]
- Keller, J.P., Jr. Clinical alarm hazards: A “top ten” health technology safety concern. J. Electrocardiol. 2012, 45, 588–591. [Google Scholar] [CrossRef] [PubMed]
- The Joint Commission. National Patient Safety Goals. Available online: https://www.jointcommission.org/-/media/tjc/documents/standards/national-patient-safety-goals/2020/npsg_chapter_hap_jul2020.pdf (accessed on 9 June 2021).
- American Nurses Association. Medical Alarm Safety in Hospitals. Available online: http://www.nursingworld.org/MainMenuCategories/ThePracticeofProfessionalNursing/Improving-Your-Practice/One-Strong-Voice-Clinically-Speaking/Medical-Alarm-Safety-in-Hospitals.html (accessed on 15 July 2021).
- American Association of Critical Care Nurses. Alarm Fatigue. Available online: https://www.aacn.org (accessed on 15 July 2021).
- Phillips, J.; Barnsteiner, J.H. Clinical alarms: Improving efficiency and effectiveness. Crit. Care Nurs. Q. 2005, 28, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Whalen, D.A.; Covelle, P.M.; Piepenbrink, J.C.; Villanova, K.L.; Cuneo, C.L.; Awtry, E.H. Novel approach to cardiac alarm management on telemetry units. J. Cardiovasc. Nurs. 2014, 29, E13–E22. [Google Scholar] [CrossRef]
- A Siren Call to Action: Priority Issues from the Medical Device Alarms Summit. Available online: https://www.aami.org/anthology-alarm-management-solutions/alarm-anthology-siren-call (accessed on 15 March 2021).
- Graham, K.C.; Cvach, M. Monitor alarm fatigue: Standardizing use of physiological monitors and decreasing nuisance alarms. Am. J. Crit. Care 2010, 19, 28–34, quiz 35. [Google Scholar] [CrossRef]
- Gross, B.; Dahl, D.; Nielsen, L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed. Instrum. Technol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, H.; Funk, M.; Whittemore, R. Measurement of Physiological Monitor Alarm Accuracy and Clinical Relevance in Intensive Care Units. Am. J. Crit. Care 2018, 27, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sendelbach, S.; Wahl, S.; Anthony, A.; Shotts, P. Stop the Noise: A Quality Improvement Project to Decrease Electrocardiographic Nuisance Alarms. Crit. Care Nurse 2015, 35, 15–22, quiz 11p following 22. [Google Scholar] [CrossRef]
- Sowan, A.K.; Vera, A.G.; Fonseca, E.I.; Reed, C.C.; Tarriela, A.F.; Berndt, A.E. Nurse Competence on Physiologic Monitors Use: Toward Eliminating Alarm Fatigue in Intensive Care Units. Open Med. Inform. J. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Cvach, M.M.; Biggs, M.; Rothwell, K.J.; Charles-Hudson, C. Daily electrode change and effect on cardiac monitor alarms: An evidence-based practice approach. J. Nurs. Care Qual. 2013, 28, 265–271. [Google Scholar] [CrossRef]
- Watanakeeree, K.; Suba, S.; Mackin, L.; Badinili, F.; Pelter, M.M. ECG Arrhythmia and Tehcnical Alarms during Left Ventricular Assist Device (LVAD) Therapy in the ICU. Heart Lung 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Cvach, M.; Kitchens, M.; Smith, K.; Harris, P.; Flack, M.N. Customizing Alarm Limits Based on Specific Needs of Patients. Biomed. Instrum. Technol. 2017, 51, 227–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cvach, M.; Rothwell, K.J.; Cullen, A.M.; Nayden, M.G.; Cvach, N.; Pham, J.C. Effect of altering alarm settings: A randomized controlled study. Biomed. Instrum. Technol. 2015, 49, 214–222. [Google Scholar] [CrossRef][Green Version]
- Nguyen, S.C.; Suba, S.; Hu, X.; Pelter, M.M. Double Trouble: Patients With Both True and False Arrhythmia Alarms. Crit. Care Nurse 2020, 40, 14–23. [Google Scholar] [CrossRef]
- Pelter, M.M.; Fidler, R.; Hu, X. Research: Association of Low-Amplitude QRSs with False-Positive Asystole Alarms. Biomed. Instrum. Technol. 2016, 50, 329–335. [Google Scholar] [CrossRef]
- Suba, S.; Sandoval, C.P.; Zegre-Hemsey, J.K.; Hu, X.; Pelter, M.M. Contribution of Electrocardiographic Accelerated Ventricular Rhythm Alarms to Alarm Fatigue. Am. J. Crit. Care 2019, 28, 222–229. [Google Scholar] [CrossRef]
- Pelter, M.M.; Adams, M.G.; Drew, B.J. Computer versus manual measurement of ST-segment deviation. J. Electrocardiol. 1996. [Google Scholar] [CrossRef]
- Association for the Advancement of Medical Instrumentation Center for Radiological Devices & Health U S A Food & Drug Administration. American National Standard: Cardiac Monitors, Heart Rate Meters, and Alarms; Association for the Advancement of Medical Instrumentation Arlington: Virginia, VA, USA, 2002; corrected 2008; Volume EC13:2002/(R)2007.
- Gargiulo, G.D. True unipolar ECG machine for Wilson Central Terminal measurements. Biomed. Res. Int. 2015, 2015, 586397. [Google Scholar] [CrossRef] [PubMed]
- Bonafide, C.P.; Localio, A.R.; Holmes, J.H.; Nadkarni, V.M.; Stemler, S.; MacMurchy, M.; Zander, M.; Roberts, K.E.; Lin, R.; Keren, R. Video Analysis of Factors Associated With Response Time to Physiologic Monitor Alarms in a Children’s Hospital. JAMA Pediatr. 2017, 171, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Dandoy, C.E.; Davies, S.M.; Flesch, L.; Hayward, M.; Koons, C.; Coleman, K.; Jacobs, J.; McKenna, L.A.; Olomajeye, A.; Olson, C.; et al. A team-based approach to reducing cardiac monitor alarms. Pediatrics 2014, 134, e1686–e1694. [Google Scholar] [CrossRef] [PubMed]
- Feder, S.; Funk, M. Over-monitoring and alarm fatigue: For whom do the bells toll? Heart Lung 2013, 42, 395–396. [Google Scholar] [CrossRef]
- Khademi, G.; Roudi, M.; Shah Farhat, A.; Shahabian, M. Noise pollution in intensive care units and emergency wards. Iran. J. Otorhinolaryngol. 2011, 23, 141–148. [Google Scholar]
- Siebig, S.; Kuhls, S.; Imhoff, M.; Gather, U.; Scholmerich, J.; Wrede, C.E. Intensive care unit alarms—How many do we need? Crit. Care Med. 2010, 38, 451–456. [Google Scholar] [CrossRef]
- Johansson, L.; Bergbom, I.; Lindahl, B. Meanings of being critically ill in a sound-intensive ICU patient room—A phenomenological hermeneutical study. Open Nurs. J. 2012, 6, 108–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johansson, L.; Bergbom, I.; Waye, K.P.; Ryherd, E.; Lindahl, B. The sound environment in an ICU patient room—A content analysis of sound levels and patient experiences. Intensive Crit. Care Nurs. 2012, 28, 269–279. [Google Scholar] [CrossRef]
- Berglund, B.; Lindvall, T. Guidlines for Community Noise. Available online: http://www.wholint/docstore/peh/noise/Noiseold.html (accessed on 20 May 2021).
- Figueroa-Ramos, M.I.; Arroyo-Novoa, C.M.; Lee, K.A.; Padilla, G.; Puntillo, K.A. Sleep and delirium in ICU patients: A review of mechanisms and manifestations. Intensive Care Med. 2009, 35, 781–795. [Google Scholar] [CrossRef]
- Nguyen, S.C.; Suba, S.; Hu, X.; Pelter, M.M. Double trouble: Patients with both true and false ECG arrhythmia alarms and the impact on alarm fatigue. Crit. Care Nurse 2019, in press. [Google Scholar]
- Aboukhalil, A.; Nielsen, L.; Saeed, M.; Mark, R.G.; Clifford, G.D. Reducing false alarm rates for critical arrhythmias using the arterial blood pressure waveform. J. Biomed. Inform. 2008, 41, 442–451. [Google Scholar] [CrossRef]
- Baumgartner, B.; Rodel, K.; Knoll, A. A data mining approach to reduce the false alarm rate of patient monitors. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 5935–5938. [Google Scholar] [CrossRef]
- Desai, K.; Lexa, M.; Matthews, B.; Genc, S. Hemodynamic-impact-based prioritization of ventricular tachycardia alarms. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 2014, 3456–3459. [Google Scholar] [CrossRef]
- Lehman, E.P.; Krishnan, R.G.; Zhao, X.; Mark, R.G.; Lehman, L.H. Representation Learning Approaches to Detect False Arrhythmia Alarms from ECG Dynamics. Proc. Mach. Learn. Res. 2018, 85, 571–586. [Google Scholar]
- Li, Q.; Clifford, G.D. Signal quality and data fusion for false alarm reduction in the intensive care unit. J. Electrocardiol. 2012, 45, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Salas-Boni, R.; Bai, Y.; Harris, P.R.; Drew, B.J.; Hu, X. False ventricular tachycardia alarm suppression in the ICU based on the discrete wavelet transform in the ECG signal. J. Electrocardiol. 2014, 47, 775–780. [Google Scholar] [CrossRef]
- Clifford, G.D.; Behar, J.; Li, Q.; Rezek, I. Signal quality indices and data fusion for determining clinical acceptability of electrocardiograms. Physiol. Meas. 2012, 33, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Fotoohinasab, A.; Afghah, F. Single-modal and multi-modal false arrhythmia alarm reduction using attention-based convolutional and recurrent neural networks. PLoS ONE 2020, 15, e0226990. [Google Scholar] [CrossRef]
- Rodrigues, R.; Couto, P. Detection of false arrhythmia alarms with emphasis on ventricular tachycardia. Physiol. Meas. 2016, 37, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, W.M.; Sahani, A.K.; Isselbacher, E.M.; Armoundas, A.A. Reduction of false alarms in the intensive care unit using an optimized machine learning based approach. NPJ Digit. Med. 2019, 2, 86. [Google Scholar] [CrossRef]
- Sadr, N.; Huvanandana, J.; Nguyen, D.T.; Kalra, C.; McEwan, A.; de Chazal, P. Reducing false arrhythmia alarms in the ICU using multimodal signals and robust QRS detection. Physiol. Meas. 2016, 37, 1340–1354. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.D.; Cvach, M.M.; Bonafide, C.P.; Hu, X.; Konkani, A.; O’Connor, M.F.; Rothschild, J.M.; Selby, N.M.; Pelter, M.M.; McLean, B.; et al. Technologic Distractions (Part 2): A Summary of Approaches to Manage Clinical Alarms With Intent to Reduce Alarm Fatigue. Crit. Care Med. 2017. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).