Cardiac Stimulation in the Third Millennium: Where Do We Head from Here?
Abstract
1. Introduction
- Atrial-based pacing and maintenance of atrioventricular (AV) coupling;
- The detrimental effect of right ventricular (RV) stimulation;
- The role of AV timing and of RV pacing minimization;
- His bundle and conduction system pacing to preserve physiologic AV timing and ventricular activation;
- Minimization of device related complications by leadless cardiac stimulation.
2. Indications for Cardiac Pacing
2.1. Atrial Pacing: Interatrial and Atrioventricular Coupling
2.2. AV Conduction and Ventricular Pacing
2.2.1. The Role of Ventricular Activation
2.2.2. Minimization of Ventricular Pacing
2.2.3. The Tradeoff between RV Pacing Minimization and Suboptimal AV Coupling
3. Conduction System Pacing
3.1. What Do We Already Know? Several Studies Have Shown the Feasibility, Safety, and Positive Clinical Outcomes of His Bundle Pacing (HBP) Compared with Customary RV Apical Pacing
3.2. Which Possible Indications for CSP?
- Brady-arrhythmia therapy, including SND with 1st, 2nd and 3rd-degree AVB;
- “Ablate and pace” strategy for AF;
- Cardiac resynchronization therapy in patients with HF and systolic dysfunction, by restoring AV synchrony in those with AVB first and/or BBB, either right or left.
4. Leadless Cardiac Stimulation: Another Step into the Future?
5. Conclusions
- Avoid unnecessary atrial stimulation to prevent interatrial dyssynchrony and excessive prolongation of the AV interval;
- Avoid unnecessary ventricular stimulation until mild prolongation of the PR interval;
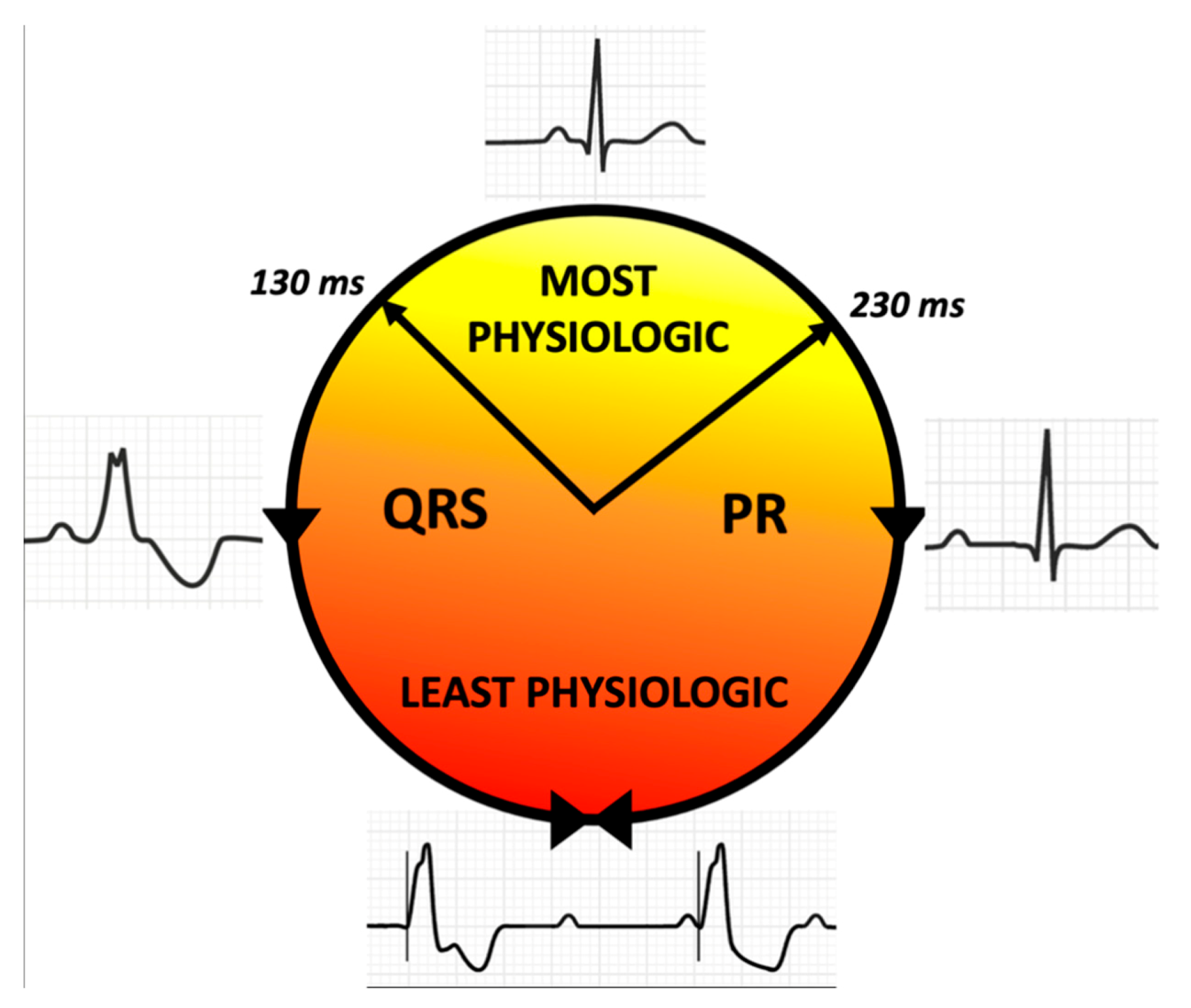
- Treat advanced AV block by His bundle pacing in patients with <130 ms QRS duration;
- Correct BBB by CSP at any level or CRT in broad QRS complex patients;
- Apply ventricular stimulation to correct intraventricular conduction delay residual to CSP, or CRT when CSP is not effective/not feasible.
Funding
Conflicts of Interest
References
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varma, N.; Ricci, R.P. Telemedicine and cardiac implants: What is the benefit? Eur. Heart J. 2013, 34, 1885–1895. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, H.; Siu, C.W.; Yiu, K.H.; Chan, W.S.; Lee, K.L.; Chan, H.W.; Lee, S.W.; Fu, G.S.; Lau, C.P.; et al. New-onset heart failure after permanent right ventricular apical pacing in patients with acquired high-grade atrioventricular block and normal left ventricular function. J. Cardiovasc. Electrophysiol. 2008, 19, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Proclemer, A.; Zecchin, M.; D’Onofrio, A.; Boriani, G.; Ricci, R.P.; Rebellato, L.; Ghidina, M.; Bianco, G.; Bernardelli, E.; Miconi, A.; et al. The Pacemaker and Implantable Cardioverter-Defibrillator Registry of the Italian Association of Arrhythmology and Cardiac Pacing—Annual report 2018. G. Ital. Cardiol. 2020, 21, 157–169. [Google Scholar] [CrossRef]
- Stabile, G.; Senatore, G.; Simone, A.; Turco, P.; Coltorti, F.; Nocerino, P.; Vitale, D.F.; Chiariello, M. Determinants of Efficacy of Atrial Pacing in Preventing Atrial Fibrillation Recurrences. J. Cardiovasc. Electrophysiol. 1999, 10, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Ammann, P.; Sticherling, C.; Burger, P.; Schaer, B.; Rocca, H.P.B.-L.; Eckstein, J.; Kiencke, S.; Kaiser, C.; Linka, A.; et al. Right Atrial Pacing Impairs Cardiac Function During Resynchronization Therapy. J. Am. Coll. Cardiol. 2005, 45, 1482–1487. [Google Scholar] [CrossRef]
- Healey, J.S.; Martin, J.L.; Duncan, A.; Connolly, S.J.; Ha, A.H.; Morillo, C.A.; Nair, G.M.; Eikelboom, J.; Divakaramenon, S.; Dokainish, H. Pacemaker-Detected Atrial Fibrillation in Patients with Pacemakers: Prevalence, Predictors, and Current Use of Oral Anticoagulation. Can. J. Cardiol. 2013, 29, 224–228. [Google Scholar] [CrossRef]
- Bukari, A.; Wali, E.; Deshmukh, A.; Aziz, Z.; Broman, M.; Beaser, A.; Upadhyay, G.; Nayak, H.; Tung, R.; Ozcan, C. Prevalence and predictors of atrial arrhythmias in patients with sinus node dysfunction and atrial pacing. J. Interv. Card. Electrophysiol. 2018, 53, 365–371. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef]
- Sławuta, A.; Kliś, M.; Skoczyński, P.; Bańkowski, T.; Moszczyńska-Stulin, J.; Gajek, J. Bachmann’s Bundle Pacing not Only Improves Interatrial Conduction but Also Reduces the Need for Ventricular Pacing. Adv. Clin. Exp. Med. 2016, 25, 845–850. [Google Scholar] [CrossRef]
- Verlato, R.; Botto, G.L.; Massa, R.; Amellone, C.; Perucca, A.; Bongiorni, M.G.; Bertaglia, E.; Ziacchi, V.; Piacenti, M.; Del Rosso, A.; et al. Efficacy of Low Interatrial Septum and Right Atrial Appendage Pacing for Prevention of Permanent Atrial Fibrillation in Patients with Sinus Node Disease: Results From the Electrophysiology-Guided Pacing Site Selection (EPASS) Study. Circ. Arrhythmia Electrophysiol. 2011, 4, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Bailin, S.J.; Adler, S.; Giudici, M. Prevention of chronic atrial fibrillation by pacing in the region of Bachmann’s bundle: Results of a multicenter randomized trial. J. Cardiovasc. Electrophysiol. 2001, 12, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.G.; Katsivas, A.G.; Vassilopoulos, C.; Koutsogeorgis, D.; Louvros, N.E. Prevention of atrial fibrillation by inter-atrial septum pacing guided by electrophysiological testing, in patients with delayed interatrial conduction. Europace 2002, 4, 165–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johner, N.; Namdar, M.; Shah, D.C. Intra- and interatrial conduction abnormalities: Hemodynamic and arrhythmic significance. J. Interv. Card. Electrophysiol. 2018, 52, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Fontenla, A.; Salguero, R.; Martinez-Ferrer, J.B.; Rodriguez, A.; Alzueta, J.; Garcia, E.; Basterra, N.; Romero, R.; De La Concha, J.F.; Viñolas, X.; et al. Atrial Rate-Responsive Pacing and Incidence of Sustained Atrial Arrhythmias in Patients with Implantable Cardioverter Defibrillators. Pacing Clin. Electrophysiol. 2016, 39, 548–556. [Google Scholar] [CrossRef]
- Biffi, M.; D’Onofrio, A.; Pignalberi, C.; Pisanò, E.C.; Iacopino, S.; Curnis, A.; Senatore, G.; Capucci, A.; Della Bella, P.; Calvi, V.; et al. Rate-responsive pacing and atrial high rate episodes in cardiac resynchronization therapy patients: Is low heart rate the key? Clin. Cardiol. 2019, 42, 820–828. [Google Scholar] [CrossRef]
- Liao, J.-N.; Chao, T.-F.; Tuan, T.-C.; Kong, C.-W.; Chen, S.-A. Long-term outcome in patients receiving permanent pacemaker implantation for atrioventricular block. Medicine 2016, 95, e4668. [Google Scholar] [CrossRef]
- Biffi, M.; Massaro, G.; Candelora, A.; Angeletti, A.; Valzania, C.; Martignani, C.; Grassini, D.; Diemberger, I.; Ziacchi, M. Less is more: Can we achieve cardiac resynchronization with 2 leads only? Int. J. Cardiol. 2017, 249, 184–190. [Google Scholar] [CrossRef]
- Ziacchi, M.; Palmisano, P.; Biffi, M.; Ricci, R.P.; Landolina, M.; Zoni-Berisso, M.; Occhetta, E.; Maglia, G.; Botto, G.; Padeletti, L.; et al. Clinically oriented device programming in bradycardia patients: Part 1 (sinus node disease). Proposals from AIAC (Italian association of arrhythmology and cardiac pacing). J. Cardiovasc. Med. 2018, 19, 161–169. [Google Scholar] [CrossRef]
- Barold, S.S.; Ilercil, A.; Herweg, B. Echocardiographic optimization of the atrioventricular and interventricular intervals during cardiac resynchronization. EP Eur. 2008, 10 (Suppl. 3), iii88–iii95. [Google Scholar] [CrossRef]
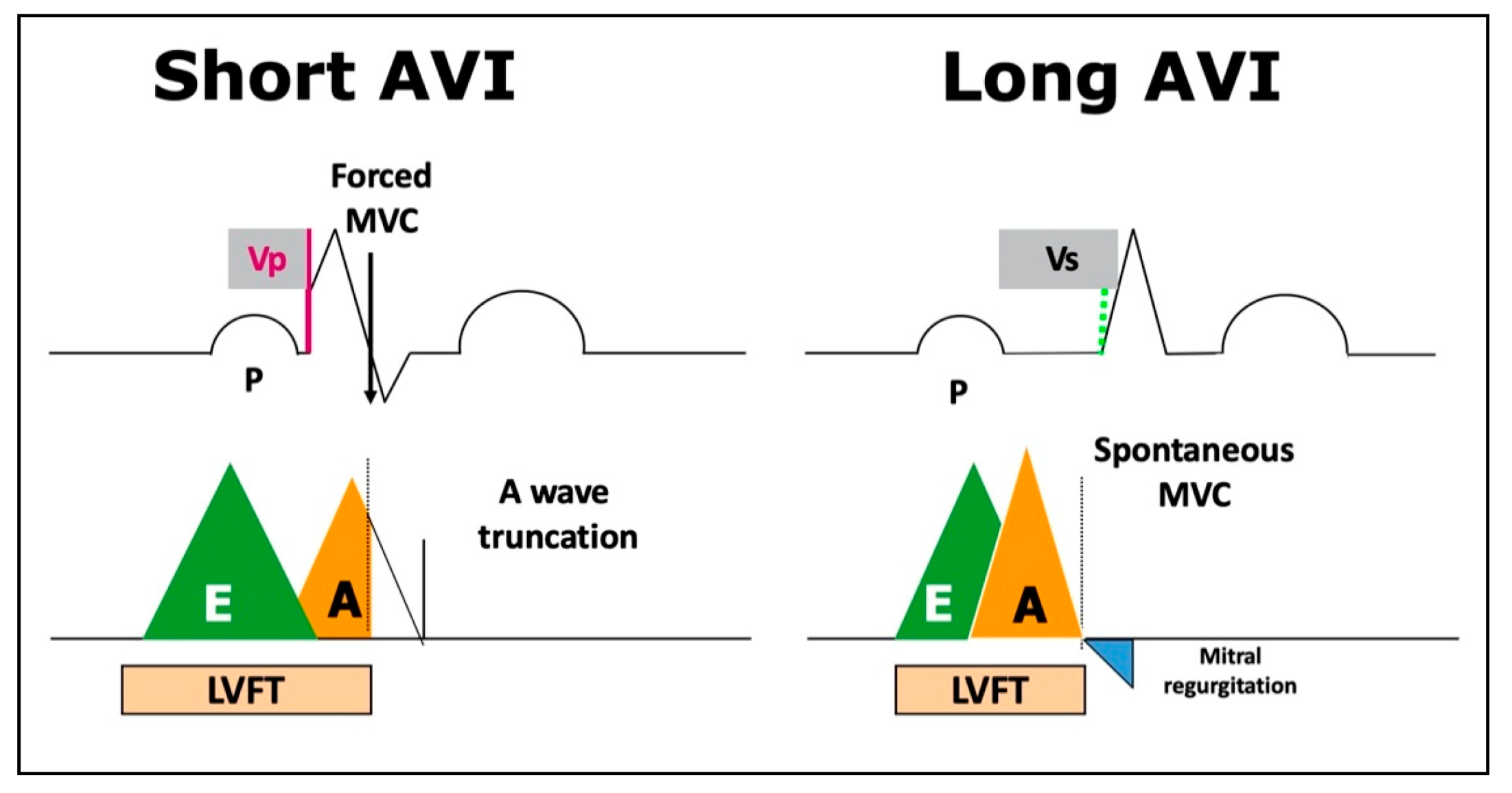
- Ishikawa, T.; Kimura, K.; Miyazaki, N.; Tochikubo, O.; Usui, T.; Kashiwagi, M.; Ishii, M. Diastolic mitral regurgitation in patients with first-degree atrioventricular block. Pacing Clin. Electrophysiol. 1992, 15, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Sumita, S.; Kimura, K.; Kuji, N.; Nakayama, R.; Nagura, T.; Miyazaki, N.; Tochikubo, O.; Usui, T.; Kashiwagi, M.; et al. Critical PQ Interval for the Appearance of Diastolic Mitral Regurgitation and Optimal PQ Interval in Patients Implanted with DDD Pacemakers. Pacing Clin. Electrophysiol. 1994, 17, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Kerr, C.R.; Gent, M.; Roberts, R.S.; Yusuf, S.; Gillis, A.M.; Sami, M.H.; Talajic, M.; Tang, A.S.L.; Klein, G.J.; et al. Effects of Physiologic Pacing versus Ventricular Pacing on the Risk of Stroke and Death Due to Cardiovascular Causes. New Engl. J. Med. 2000, 342, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.R.; Connolly, S.J.; Abdollah, H.; Roberts, R.S.; Gent, M.; Yusuf, S.; Gillis, A.M.; Tang, A.S.L.; Talajic, M.; Klein, G.J.; et al. Canadian Trial of Physiological Pacing. Circulation 2004, 109, 357–362. [Google Scholar] [CrossRef]
- Lamas, G.A.; Lee, K.L.; Sweeney, M.O.; Silverman, R.; Leon, A.; Yee, R.; Marinchak, R.A.; Flaker, G.; Schron, E.; Orav, E.J.; et al. Ventricular Pacing or Dual-Chamber Pacing for Sinus-Node Dysfunction. New Engl. J. Med. 2002, 346, 1854–1862. [Google Scholar] [CrossRef]
- Toff, W.D.; Camm, A.J.; Skehan, J.D. Single-Chamber versus Dual-Chamber Pacing for High-Grade Atrioventricular Block. New Engl. J. Med. 2005, 353, 145–155. [Google Scholar] [CrossRef]
- Healey, J.S.; Toff, W.D.; Lamas, G.A.; Andersen, H.R.; Thorpe, K.E.; Ellenbogen, K.A.; Lee, K.L.; Skene, A.M.; Schron, E.B.; Skehan, J.D.; et al. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: Meta-analysis of randomized trials, using individual patient data. Circulation 2006, 114, 11–17. [Google Scholar] [CrossRef]
- The DAVID Trial Investigators. Dual-Chamber Pacing or Ventricular Backup Pacing in Patients with an Implantable Defibrillator. JAMA 2002, 288, 3115–3123. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Fischer, A.; Wang, P.; Schuger, C.; Daubert, J.; Mcnitt, S.; Andrews, M.; Brown, M.; Hall, W.J.; Zareba, W.; et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J. Cardiovasc. Electrophysiol. 2005, 16, 359–365. [Google Scholar] [CrossRef]
- Stierle, U.; Krüger, D.; Mitusch, R.; Potratz, J.; Taubert, G.; Sheikhzadeh, A. Adverse Pacemaker Hemodynamics Evaluated by Pulmonary Venous Flow Monitoring. Pacing Clin. Electrophysiol. 1995, 18, 2028–2034. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Hellkamp, A.S.; Ellenbogen, K.A.; Greenspon, A.J.; Freedman, R.A.; Lee, K.L.; Lamas, G.A. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003, 107, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.D.; Rizo-Patron, C.; Hallstrom, A.P.; O’Neill, G.P.; Rothbart, S.; Martins, J.B.; Roelke, M.; Steinberg, J.S.; Greene, H.L. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005, 2, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Barsheshet, A.; Moss, A.J.; McNitt, S.; Jons, C.; Glikson, M.; Klein, H.U.; Huang, D.T.; Steinberg, J.S.; Brown, M.W.; Zareba, W.; et al. Long-term implications of cumulative right ventricular pacing among patients with an implantable cardioverter-defibrillator. Heart Rhythm 2011, 8, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.O.; Prinzen, F.W. Ventricular pump function and pacing: Physiological and clinical integration. Circ. Arrhythmia Electrophysiol. 2008, 1, 127–139. [Google Scholar] [CrossRef]
- Tang, A.S.L.; Roberts, R.S.; Kerr, C.; Gillis, A.M.; Green, M.S.; Talajic, M.; Yusuf, S.; Abdollah, H.; Gent, M.; Connolly, S.J. Relationship between pacemaker dependency and the effect of pacing mode on cardiovascular outcomes. Circulation 2001, 103, 3081–3085. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Bank, A.J.; Nsah, E.; Koullick, M.; Zeng, Q.C.; Hettrick, D.; Sheldon, T.; Lamas, G.A. Minimizing Ventricular Pacing to Reduce Atrial Fibrillation in Sinus-Node Disease. N. Engl. J. Med. 2007, 357, 1000–1008. [Google Scholar] [CrossRef]
- Boriani, G.; Tukkie, R.; Manolis, A.S.; Mont, L.; Pürerfellner, H.; Santini, M.; Inama, G.; Serra, P.; De Sousa, J.; Botto, G.L.; et al. Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: The MINERVA randomized multicentre international trial. Eur. Heart J. 2014, 35, 2352–2362. [Google Scholar] [CrossRef]
- Stockburger, M.; Boveda, S.; Moreno, J.; Da Costa, A.; Hatala, R.; Brachmann, J.; Butter, C.; Seara, J.G.; Rolando, M.; Defaye, P. Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur. Heart J. 2015, 36, 151–157. [Google Scholar] [CrossRef]
- Thibault, B.; Ducharme, A.; Baranchuk, A.; Dubuc, M.; Dyrda, K.; Guerra, P.G.; Macle, L.; Mondésert, B.; Rivard, L.; Roy, D.; et al. Very Low Ventricular Pacing Rates Can Be Achieved Safely in a Heterogeneous Pacemaker Population and Provide Clinical Benefits: The CANadian Multi-Centre Randomised Study-Spontaneous AtrioVEntricular Conduction pReservation (CAN-SAVE R) Trial. J. Am. Heart Assoc. 2015, 4, e001983. [Google Scholar] [CrossRef]
- Shurrab, M.; Healey, J.S.; Haj-Yahia, S.; Kaoutskaia, A.; Boriani, G.; Carrizo, A.; Botto, G.; Newman, D.; Padeletti, L.; Connolly, S.J.; et al. Reduction in unnecessary ventricular pacing fails to affect hard clinical outcomes in patients with preserved left ventricular function: A meta-analysis. Europace 2017, 19, 282–288. [Google Scholar] [CrossRef]
- Nikolaidou, T.; Ghosh, J.M.; Clark, A.L. Outcomes Related to First-Degree Atrioventricular Block and Therapeutic Implications in Patients with Heart Failure. JACC Clin. Electrophysiol. 2016, 2, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Ellenbogen, K.A. Reducing Ventricular Pacing Frequency in Patients with Atrioventricular Block. Circ. Arrhythmia Electrophysiol. 2016, 9, e004404. [Google Scholar] [CrossRef]
- Cheng, S.; Keyes, M.J.; Larson, M.G.; McCabe, E.L.; Newton-Cheh, C.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Wang, T.J. Long-term Outcomes in Individuals with Prolonged PR Interval or First-Degree Atrioventricular Block. JAMA 2009, 301, 2571–2577. [Google Scholar] [CrossRef]
- Uhm, J.-S.; Shim, J.; Wi, J.; Mun, H.-S.; Park, J.; Park, S.-H.; Joung, B.; Pak, H.-N.; Lee, M.-H. First-degree atrioventricular block is associated with advanced atrioventricular block, atrial fibrillation and left ventricular dysfunction in patients with hypertension. J. Hypertens. 2014, 32, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Crisel, R.K.; Farzaneh-Far, R.; Na, B.; Whooley, M.A. First-degree atrioventricular block is associated with heart failure and death in persons with stable coronary artery disease: Data from the Heart and Soul Study. Eur. Heart J. 2011, 32, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, M.; Birnie, D.; Lemke, B.; Aonuma, K.; Lee, K.L.-F.; Gorcsan, J.; Landolina, M.; Klepfer, R.; Meloni, S.; Cicconelli, M.; et al. Adaptive Cardiac Resynchronization Therapy Reduces Atrial Fibrillation Incidence in Heart Failure Patients with Prolonged AV Conduction. Circ. Arrhythmia Electrophysiol. 2019, 12, e007260. [Google Scholar] [CrossRef]
- Eicher, J.C.; Laurent, G.; Mathé, A.; Barthez, O.; Bertaux, G.; Philip, J.L.; Dorian, P.; Wolf, J.E. Atrial dyssynchrony syndrome: An overlooked phenomenon and a potential cause of “diastolic” heart failure. Eur. J. Heart Fail. 2012, 14, 248–258. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Ellenbogen, K.A.; Tang, A.S.L.; Whellan, D.; Mortensen, P.T.; Giraldi, F.; Sandler, D.A.; Sherfesee, L.; Sheldon, T. Atrial pacing or ventricular backup–only pacing in implantable cardioverter-defibrillator patients. Heart Rhythm 2010, 7, 1552–1560. [Google Scholar] [CrossRef]
- Boriani, G.; Pieragnoli, P.; Botto, G.L.; Puererfellner, H.; Mont, L.; Ziacchi, M.; Manolis, A.S.; Gulizia, M.; Tukkie, R.; Landolina, M.; et al. Effect of PR interval and pacing mode on persistent atrial fibrillation incidence in dual chamber pacemaker patients: A sub-study of the international randomized MINERVA trial. EP Eur. 2019, 21, 636–644. [Google Scholar] [CrossRef]
- Stockburger, M.; Moss, A.J.; Klein, H.U.; Zareba, W.; Goldenberg, I.; Biton, Y.; McNitt, S.; Kutyifa, V. Sustained clinical benefit of cardiac resynchronization therapy in non-LBBB patients with prolonged PR-interval: MADIT-CRT long-term follow-up. Clin. Res. Cardiol. 2016, 105, 944–952. [Google Scholar] [CrossRef]
- Lin, J.; Buhr, K.A.; Kipp, R. Effect of PR Interval on Outcomes Following Cardiac Resynchronization Therapy: A Secondary Analysis of the COMPANION Trial. J. Cardiovasc. Electrophysiol. 2017, 28, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gervais, R.; Leclercq, C.; Shankar, A.; Jacobs, S.; Eiskjær, H.; Johannessen, A.; Freemantle, N.; Cleland, J.G.F.; Tavazzi, L.; Daubert, C.; et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: A sub-analysis of the CARE-HF trial. Eur. J. Heart Fail. 2009, 11, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.P.; Stopper, M.M.; Li, J.; Beshai, J.F.; Pavri, B.B. Impact of baseline PR interval on cardiac resynchronization therapy outcomes in patients with narrow QRS complexes: An analysis of the ReThinQ Trial. J. Interv. Card. Electrophysiol. 2015, 43, 145–149. [Google Scholar] [CrossRef]
- Botto, G.L.; Iuliano, A.; Occhetta, E.; Belotti, G.; Russo, G.; Campari, M.; Valsecchi, S.; Stabile, G. A randomized controlled trial of cardiac resynchronization therapy in patients with prolonged atrioventricular interval: The REAL-CRT pilot study. Europace 2020, 22, 299–305. [Google Scholar] [CrossRef]
- Zanon, F.; Ellenbogen, K.A.; Dandamudi, G.; Sharma, P.S.; Huang, W.; Lustgarten, D.L.; Tung, R.; Tada, H.; Koneru, J.N.; Bergemann, T.; et al. Permanent His-bundle pacing: A systematic literature review and meta-analysis. Europace 2018, 20, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Shimony, A.; Eisenberg, M.J.; Filion, K.B.; Amit, G. Beneficial effects of right ventricular non-apical vs. apical pacing: A systematic review and meta-analysis of randomized-controlled trials. Europace 2012, 14, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Sadoul, N.; Mont, L.; Defaye, P.; Osca, J.; Mouton, E.; Isnard, R.; Habib, G.; Zamorano, J.; Derumeaux, G.; et al. Comparison of right ventricular septal pacing and right ventricular apical pacing in patients receiving cardiac resynchronization therapy defibrillators: The SEPTAL CRT Study. Eur. Heart J. 2016, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Ploux, S.; Eschalier, R.; Whinnett, Z.I.; Lumens, J.; Derval, N.; Sacher, F.; Hocini, M.; Jaïs, P.; Dubois, R.; Ritter, P.; et al. Electrical dyssynchrony induced by biventricular pacing: Implications for patient selection and therapy improvement. Heart Rhythm 2015, 12, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Cazeau, S.; Le Breton, H.; Ritter, P.; Mabo, P.; Gras, D.; Pavin, D.; Lazarus, A.; Daubert, J.C. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J. Am. Coll. Cardiol. 1998, 32, 1825–1831. [Google Scholar] [CrossRef]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J.; Gras, D.; et al. Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. New Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef]
- Dandamudi, G.; Vijayaraman, P. The Complexity of the His Bundle: Understanding Its Anatomy and Physiology through the Lens of the Past and the Present. Pacing Clin. Electrophysiol. 2016, 39, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Beer, D.; Sharma, P.S.; Subzposh, F.A.; Naperkowski, A.; Pietrasik, G.M.; Durr, B.; Qureshi, M.; Panikkath, R.; Abdelrahman, M.; Williams, B.A.; et al. Clinical Outcomes of Selective Versus Nonselective His Bundle Pacing. JACC Clin. Electrophysiol. 2019, 5, 766–774. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Herweg, B.; Dandamudi, G.; Mittal, S.; Bhatt, A.G.; Marcantoni, L.; Naperkowski, A.; Sharma, P.S.; Zanon, F. Outcomes of His-bundle pacing upgrade after long-term right ventricular pacing and/or pacing-induced cardiomyopathy: Insights into disease progression. Heart Rhythm 2019, 16, 1554–1561. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Subzposh, F.A.; Beer, D.; Durr, B.; Naperkowski, A.; Sun, H.; Oren, J.W.; Dandamudi, G.; Vijayaraman, P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J. Am. Coll. Cardiol. 2018, 71, 2319–2330. [Google Scholar] [CrossRef]
- Lustgarten, D.L.; Calame, S.; Crespo, E.M.; Calame, J.; Lobel, R.; Spector, P.S. Electrical resynchronization induced by direct His-bundle pacing. Heart Rhythm 2010, 7, 15–21. [Google Scholar] [CrossRef]
- Teng, A.E.; Lustgarten, D.L.; Vijayaraman, P.; Tung, R.; Shivkumar, K.; Wagner, G.S.; Ajijola, O.A. Usefulness of His Bundle Pacing to Achieve Electrical Resynchronization in Patients wWith Complete Left Bundle Branch Block and the Relation Between Native QRS Axis, Duration, and Normalization. Am. J. Cardiol. 2016, 118, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ajijola, O.A.; Upadhyay, G.A.; Macias, C.; Shivkumar, K.; Tung, R. Permanent His-bundle pacing for cardiac resynchronization therapy: Initial feasibility study in lieu of left ventricular lead. Heart Rhythm 2017, 14, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Barba-Pichardo, R.; Sánchez, A.M.; Fernández-Gómez, J.M.; Moriña-Vázquez, P.; Venegas-Gamero, J.; Herrera-Carranza, M. Ventricular resynchronization therapy by direct His-bundle pacing using an internal cardioverter defibrillator. Europace 2013, 15, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Dandamudi, G.; Herweg, B.; Wilson, D.; Singh, R.; Naperkowski, A.; Koneru, J.N.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm 2018, 15, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, D.L.; Crespo, E.M.; Arkhipova-Jenkins, I.; Lobel, R.; Winget, J.; Koehler, J.; Liberman, E.; Sheldon, T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm 2015, 12, 1548–1557. [Google Scholar] [CrossRef]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Naperkowski, A.; Bauch, T.D.; Chan, J.Y.S.; Arnold, A.D.; Whinnett, Z.I.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His Bundle Pacing for Cardiac Resynchronization Therapy in Patients with Heart Failure and Right Bundle Branch Block. Circ. Arrhythmia Electrophysiol. 2018, 11, e006613. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.D.; Shun-Shin, M.J.; Keene, D.; Howard, J.P.; Sohaib, S.M.A.; Wright, I.J.; Cole, G.D.; Qureshi, N.A.; Lefroy, D.C.; Koa-Wing, M.; et al. His Resynchronization Versus Biventricular Pacing in Patients with Heart Failure and Left Bundle Branch Block. J. Am. Coll. Cardiol. 2018, 72, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Jastrzebski, M.; Vijayaraman, P. Electrocardiographic Analysis for His Bundle Pacing at Implantation and Follow-Up. JACC Clin. Electrophysiol. 2020, 6, 883–900. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Vijayaraman, P.; Nayak, H.M.; Verma, N.; Dandamudi, G.; Sharma, P.S.; Saleem, M.; Mandrola, J.; Genovese, D.; Tung, R. His Corrective Pacing or Biventricular Pacing for Cardiac Resynchronization in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 157–159. [Google Scholar] [CrossRef]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A Novel Pacing Strategy with Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can. J. Cardiol. 2017, 33, 1736.e1–1736.e3. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Subzposh, F.A.; Naperkowski, A.; Panikkath, R.; John, K.; Mascarenhas, V.; Bauch, T.D.; Huang, W. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm 2019, 16, 1774–1782. [Google Scholar] [CrossRef]
- Huang, W.; Wu, S.; Vijayaraman, P.; Su, L.; Chen, X.; Cai, B.; Zou, J.; Lan, R.; Fu, G.; Mao, G.; et al. Cardiac Resynchronization Therapy in Patients with Nonischemic Cardiomyopathy Using Left Bundle Branch Pacing. JACC Clin. Electrophysiol. 2020, 6, 849–858. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Chung, M.K.; Dandamudi, G.; Upadhyay, G.A.; Krishnan, K.; Crossley, G.; Campbell, K.B.; Lee, B.K.; Refaat, M.M.; Saksena, S.; et al. His Bundle Pacing. J. Am. Coll. Cardiol. 2018, 72, 927–947. [Google Scholar] [CrossRef]
- Udo, E.O.; Zuithoff, N.P.A.; Van Hemel, N.M.; De Cock, C.C.; Hendriks, T.; Doevendans, P.A.; Moons, K.G.M. Incidence and predictors of short- and long-term complications in pacemaker therapy: The FOLLOWPACE study. Heart Rhythm 2012, 9, 728–735. [Google Scholar] [CrossRef]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jørgensen, O.D.; Nielsen, J.C. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur. Heart J. 2014, 35, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.P.; Cronin, E.M.; Wazni, O.; Baranowski, B.; Saliba, W.I.; Sabik, J.F.; Lindsay, B.D.; Wilkoff, B.L.; Tarakji, K.G. Outcomes of patients requiring emergent surgical or endovascular intervention for catastrophic complications during transvenous lead extraction. Heart Rhythm 2014, 11, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, K.G.; Wazni, O.M.; Harb, S.; Hsu, A.; Saliba, W.; Wilkoff, B.L. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: The impact of the infection type and the presence of vegetation on survival. Europace 2014, 16, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Knops, R.E.; Sperzel, J.; Miller, M.A.; Petru, J.; Simon, J.; Sediva, L.; De Groot, J.R.; Tjong, F.V.Y.; Jacobson, P.; et al. Permanent leadless cardiac pacing: Results of the LEADLESS trial. Circulation 2014, 129, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Exner, D.V.; Cantillon, D.J.; Doshi, R.; Bunch, T.J.; Tomassoni, G.F.; Friedman, P.A.; Estes, N.A.M.; Ip, J.; Niazi, I.; et al. Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker. N. Engl. J. Med. 2015, 373, 1125–1135. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Knops, R.; Atwater, B.; Neužil, P.; Ip, J.; Gonzalez, E.; Friedman, P.; Defaye, P.; Exner, D.; Aonuma, K.; et al. A worldwide experience of the management of battery failures and chronic device retrieval of the Nanostim leadless pacemaker. Heart Rhythm 2017, 14, 1756–1763. [Google Scholar] [CrossRef]
- Richter, S.; Döring, M.; Ebert, M.; Bode, K.; Müssigbrodt, A.; Sommer, P.; Husser, D.; Hindricks, G. Battery Malfunction of a Leadless Cardiac Pacemaker: Worrisome Single-Center Experience. Circulation 2018, 137, 2408–2410. [Google Scholar] [CrossRef]
- Jung, W.; Sadeghzadeh, G.; Jäckle, S.; Roggenbuck-Schwilk, B.; Zvereva, V.; Kohler, J. Successful implant of a leadless pacemaker with tine-based fixation next to an abandoned battery-depleted screw-in helix fixation leadless device. EP Eur. 2018, 20, 500. [Google Scholar] [CrossRef]
- Reynolds, D.; Duray, G.Z.; Omar, R.; Soejima, K.; Neuzil, P.; Zhang, S.; Narasimhan, C.; Steinwender, C.; Brugada, J.; Lloyd, M.; et al. A Leadless Intracardiac Transcatheter Pacing System. N. Engl. J. Med. 2016, 374, 533–541. [Google Scholar] [CrossRef]
- Roberts, P.R.; Clementy, N.; Al Samadi, F.; Garweg, C.; Martinez-Sande, J.L.; Iacopino, S.; Johansen, J.B.; Prat, X.V.; Kowal, R.C.; Klug, D.; et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm 2017, 14, 1375–1379. [Google Scholar] [CrossRef]
- Vaidya, V.R.; Dai, M.; Asirvatham, S.J.; Rea, R.F.; Thome, T.M.; Srivathsan, K.; Mulpuru, S.K.; Kusumoto, F.; Venkatachalam, K.L.; Ryan, J.D.; et al. Real-world experience with leadless cardiac pacing. Pacing Clin. Electrophysiol. 2019, 42, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Biffi, M.; Melissano, D.; Rossi, P.; Kaliska, G.; Havlíĉek, A.; Pelargonio, G.; Romero, R.; Guastaferro, C.; Menichelli, M.; Vireca, E.; et al. The OPTI-MIND study: A prospective, observational study of pacemaker patients according to pacing modality and primary indications. Europace 2014, 16, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Biffi, M.; Bertini, M.; Saporito, D.; Belotti, G.; Quartieri, F.; Piancastelli, M.; Pucci, A.; Boggian, G.; Mazzocca, G.F.; Giorgi, D.; et al. Automatic management of atrial and ventricular stimulation in a contemporary unselected population of pacemaker recipients: The ESSENTIAL Registry. Europace 2016, 18, 1551–1560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boveda, S.; Lenarczyk, R.; Haugaa, K.H.; Iliodromitis, K.; Finlay, M.; Lane, D.; Prinzen, F.W.; Dagres, N. Use of leadless pacemakers in Europe: Results of the European Heart Rhythm Association survey. Europace 2018, 20, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Leadless Cardiac Pacemakers Back to the Future. J. Am. Coll. Cardiol. 2015, 66, 1179–1189. [Google Scholar] [CrossRef]
- Chinitz, L.; Ritter, P.; Khelae, S.K.; Iacopino, S.; Garweg, C.; Grazia-Bongiorni, M.; Neuzil, P.; Johansen, J.B.; Mont, L.; Gonzalez, E.; et al. Accelerometer-based atrioventricular synchronous pacing with a ventricular leadless pacemaker: Results from the Micra atrioventricular feasibility studies. Heart Rhythm 2018, 15, 1363–1371. [Google Scholar] [CrossRef]
- Steinwender, C.; Khelae, S.K.; Garweg, C.; Chan, J.Y.S.; Ritter, P.; Johansen, J.B.; Sagi, V.; Epstein, L.M.; Piccini, J.P.; Pascual, M.; et al. Atrioventricular Synchronous Pacing Using a Leadless Ventricular Pacemaker: Results from the MARVEL 2 Study. JACC Clin. Electrophysiol. 2020, 6, 94–106. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, C.; Cao, M.; Li, H. Atrioventricular block can be used as a risk predictor of clinical atrial fibrillation. Clin. Cardiol. 2019, 42, 452–458. [Google Scholar] [CrossRef]
- Lamas, G.A.; Ellenbogen, K.A.; Hennekens, C.H.; Montanez, A. Evidence Base for Peacemaker Mode Selection from Physiology to Randomized Trials. Circulation 2004, 109, 443–451. [Google Scholar] [CrossRef]





| Leadless | Leadless II | Micra Transcatheter Pacing Trial (IDE) | Micra Post Approval Registry (PAR-Ongoing) | |
|---|---|---|---|---|
| Enrollment period (from–to) | Dec 2012–April 2013 | Feb 2014–June 2015 | Dec 2013–May 2015 | July 2015–ongoing |
| System | Nanostim | Nanostim | Micra | Micra |
| Population (n) | 33 | 526 | 725 | 795 (intended 1830) |
| Follow-up (days) | 90 | 180 | 180 | 30 (ongoing) |
| Successful implant rate (%) | 97% | 95.8% | 99.2% | 99.6% |
| Acute major complication rate (%) | 6% | 6.7% | 3,4% | 1.5% |
| Leadless Pacing Systems | Transvenous Pacing Systems | |
|---|---|---|
| Total short terms complications | 3.4–4.8% | 6.4–12% |
| Pericardial effusion | 0.6–1.5% | 0.3–1.2% |
| Cardiac perforation | 1.3–1.5% | 0.1–0.8% |
| Access site | 0.7–1.2% | 1.2–2.2% |
| High pacing threshold | 0.3–1.3% | 0.8–1.6% |
| Conventional TV Pacemaker | Leadless Pacemaker | Conduction System Pacing | CRT-P | |
|---|---|---|---|---|
| Occluded superior access to the heart | − | +++ | − | − |
| Superior access to be preserved | − | ++ | − | − |
| Indwelling TV catheters | − | ++ | − | − |
| Frail patients prone to bleeding | + | ++ | + | − |
| Previous infection & CIED extraction | − | ++ | − | − |
| <20% Vp expected | + | + | + | − |
| Baseline EF <50% | − | − | + | + |
| Narrow QRS at baseline | − | − | ++ | + |
| BBB at baseline | + | + | ++ | ++ |
| History of heart failure | − | − | ++ | ++ |
| Paced QRS duration > 160 ms | − | − | ++ | ++ |
| Cost | +++ | − | ++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biffi, M.; Spadotto, A.; Piemontese, G.P.; Toniolo, S.; Bartoli, L.; Sorrentino, S.; Minguzzi, A.; Massaro, G.; Capobianco, C.; Statuto, G. Cardiac Stimulation in the Third Millennium: Where Do We Head from Here? Hearts 2021, 2, 15-35. https://doi.org/10.3390/hearts2010003
Biffi M, Spadotto A, Piemontese GP, Toniolo S, Bartoli L, Sorrentino S, Minguzzi A, Massaro G, Capobianco C, Statuto G. Cardiac Stimulation in the Third Millennium: Where Do We Head from Here? Hearts. 2021; 2(1):15-35. https://doi.org/10.3390/hearts2010003
Chicago/Turabian StyleBiffi, Mauro, Alberto Spadotto, Giuseppe Pio Piemontese, Sebastiano Toniolo, Lorenzo Bartoli, Sergio Sorrentino, Alessandro Minguzzi, Giulia Massaro, Claudio Capobianco, and Giovanni Statuto. 2021. "Cardiac Stimulation in the Third Millennium: Where Do We Head from Here?" Hearts 2, no. 1: 15-35. https://doi.org/10.3390/hearts2010003
APA StyleBiffi, M., Spadotto, A., Piemontese, G. P., Toniolo, S., Bartoli, L., Sorrentino, S., Minguzzi, A., Massaro, G., Capobianco, C., & Statuto, G. (2021). Cardiac Stimulation in the Third Millennium: Where Do We Head from Here? Hearts, 2(1), 15-35. https://doi.org/10.3390/hearts2010003






