Non-Invasive Assessment of Pulmonary Vasculopathy
Abstract
:1. Introduction
2. Methods
2.1. Study Endpoints
- A reduction of pulmonary peak and mean systolic velocity, peak flow, stroke volume, and wall shear stress, with an increase in pulmonary artery pulse-wave velocity.
- An increased stiffness of the pulmonary arteries, with a decreased pulsatility.
- A lower DLCO.
2.2. Design
2.3. Patient Selection and Follow-Up
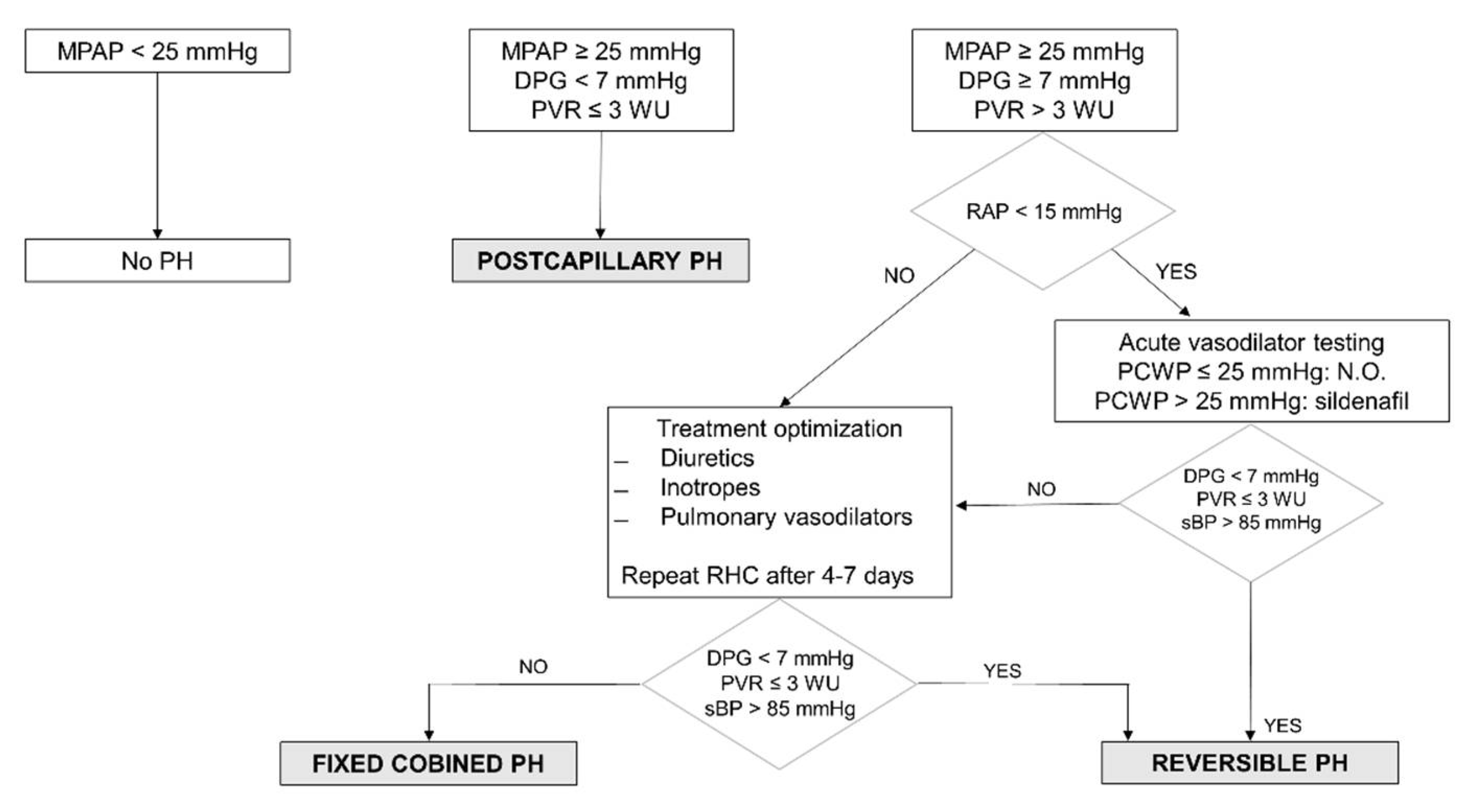
2.4. Pulmonary Hypertension Definition and Hemodynamic Evaluation
2.5. Magnetic Resonance Imaging
- Standard segmented cine steady-state free-precession sequence (repetition time/echo time/flip angle (TR/TE/α) = 2.7 ms/1.35 ms/40°) to provide high-quality anatomical references to evaluate ventricular mass, volume, thickness, and ejection fraction. Field of view (FOV) of 320 × 320 mm, slice thickness of 8 mm with no gaps, and in plane resolution of 1.8 × 1.8 mm2 and 30 acquired cardiac phases.
- T1 (modified look-locker inversion recovery (MOLLI)) pre- and post-contrast (15 min after gadolinium injection) sequences based on a 5(3)3 scheme using a single shot steady-state free precession readout sequence (TR/TE/Flip angle = 2.1 ms/1.05 ms/35°) with an in-plane acquisition resolution of 1.5 × 1.8 mm2 and an 8 mm slice thickness. These sequences will allow a quantitative evaluation of myocardial fibrosis and extracellular volume.
- Lung perfusion will be acquired using dynamic multi-slice acquisition using saturation recovery spoiled turbo field echo sequence during the first pass of contrast injection. Image resolution of the sequence will be 4 × 4 in plane resolution and 25 slices with a slice thickness of 10 mm and no gap between slices covering both lungs. Image volume will be acquired in coronal orientation to allow a higher parallel acceleration factor in the left–right direction (SENSE factor of 2.2), allowing to acquire a new imaging volume every 2.2 s. Saturation delay time was adjusted to improve the signal intensity to contrast concentration (100 ms). The injection rate will be 3 mL/s to avoid strong T2* effects during contrast administration.
- Late gadolinium enhancement sequence: performed 10 to 15 min after intravenous administration of 0.20 mmol of gadopentetate dimeglumine contrast agent per kg of body weight (30) using a 2D inversion-recovery spoiled turbo field echo (IR-T1TFE) sequence with the following parameters: FOV of 320 × 320 mm, with in plane resolution of 1.6 × 1.6 mm2, end-diastolic acquisition, thickness of 8 mm with no gap, TR 5.6 ms, TE 2.8 ms, inversion delay time will be optimized to null normal myocardium, and 2 number of excitations. The same sequence will be acquired in a short axis with as many slices as required to cover the entire cardiac muscle without a gap between slices and one slice in 2, 3, and 4 chambers views.
- Four-dimensional flow will be acquired using a 3D spoiled turbo field echo sequence (TR/TE/α = 3.6 ms/2.2 ms/7°) with isotropic resolution of 2.5 × 2.5 × 2.5 mm3 and 20 acquired cardiac phases covering an imaging volume of 320 × 300 × 300 mm3 (cranial–caudal, left–right (LR), and anterior–posterior (AP) direction, respectively). Images were acquired in three velocity encoding directions and the maximum velocity was adjusted according to the maximum velocity. A parallel acceleration factor of 5.7 (1.9 in AP and 3 in LR direction, respectively) will be applied to reduce the total acquisition time.
2.6. Study Organization
2.7. Sample Size and Statistical Analysis
2.8. Funding
3. Discussion
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vachiery, J.-L.; Adir, Y.; Barberà, J.A.; Champion, H.C.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, J.S.R.; et al. Pulmonary Hypertension Due to Left Heart Diseases. J. Am. Coll. Cardiol. 2013, 62, D100–D108. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.; Recusani, F.; Tavazzi, L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Guazzi, M.; Naeije, R. Pulmonary Hypertension in Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1718–1734. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.D.; Conde, E.; Sánchez, V.; López-Ríos, F.; Gómez-Sánchez, M.A.; Escribano, P.; Sotelo, T.; De La Cámara, A.G.; Cortina, J.; De La Calzada, C.S. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur. J. Heart Fail. 2005, 7, 1011–1016. [Google Scholar]
- Freed, B.H.; Collins, J.D.; François, C.J.; Barker, A.J.; Cuttica, M.J.; Chesler, N.C.; Markl, M.; Shah, S.J. MR and CT Imaging for the Evaluation of Pulmonary Hypertension. JACC Cardiovasc. Imaging 2016, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Reiter, U.; Reiter, G.; Kovacs, G.; Adelsmayr, G.; Greiser, A.; Olschewski, H.; Fuchsjäger, M. Native myocardial T1 mapping in pulmonary hypertension: Correlations with cardiac function and hemodynamics. Eur. Radiol. 2017, 27, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Y.; Yun, H.; Jin, H.; Kong, D.H.; Long, Y.L.; Fu, C.X.; Yang, S.; Zeng, M. Association of native T1 times with biventricular function and hemodynamics in precapillary pulmonary hypertension. Int. J. Cardiovasc. Imaging 2017, 33, 1179–1189. [Google Scholar] [CrossRef]
- Nitsche, C.; Kammerlander, A.A.; Binder, C.; Duca, F.; Aschauer, S.; Koschutnik, M.; Snidat, A.; Beitzke, D.; Loewe, C.; Bonderman, D.; et al. Native T1 time of right ventricular insertion points by cardiac magnetic resonance: Relation with invasive haemodynamics and outcome in heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2019, 21, 683–691. [Google Scholar] [CrossRef]
- Swift, A.J.; Telfer, A.; Rajaram, S.; Condliffe, R.; Marshall, H.; Capener, D.; Hurdman, J.; Elliot, C.; Kiely, D.G.; Wild, J.M. Dynamic contrast-enhanced magnetic resonance imaging in patients with pulmonary arterial hypertension. Pulm. Circ. 2014, 4, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Barker, A.J.; Roldan-Alzate, A.; Entezari, P.; Shah, S.J.; Chesler, N.C.; Wieben, O.; Markl, M.; Francois, C.J. 4D Flow Assessment of Pulmonary Artery Flow and Wall Shear Stress in Adult Pulmonary Arterial Hypertension: Results from Two Institutions. Magn. Reson. Med. 2015, 73, 1904–1913. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Shah, S.J.; Thenappan, T.; Archer, S.L.; Rich, S.; Gomberg-Maitland, M. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J. Heart Lung Transplant. 2010, 29, 181–187. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Meyer, K.; Rademacher, J.; Fuge, J.; Welte, T.; Olsson, K.M. Diffusion Capacity and Mortality in Patients with Pulmonary Hypertension Due to Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.; et al. 2016 ESC GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF ACUTE AND CHRONIC HEART FAILURE. Russ. J. Cardiol. 2016, 37, 2129–2200. [Google Scholar]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Respir. J. 2015, 46, 1855–1856. [Google Scholar] [CrossRef]
- Houston, B.A.; Tedford, R.J. What We Talk About When We Talk About the Wedge Pressure. Circ. Heart Fail. 2017, 10, e004450. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Lestache, E.B.; Gómez-Sánchez, M.A.; de la Cruz, J.; Gonzalez–Trevilla, A.A.; Martín, M.T.V.; Cano, M.J.R.; Jiménez, J.F.D.; Subias, P.E.; López-Guarch, C.J.; Peiretti, M.A.C.; et al. Pulmonary Hypertension “Out of Proportion” in Patients who are Candidates for Heart Transplant: Does Acute Vasodilator Response to Sildenafil Predict Survival after Transplant? J. Pulmon. Resp. Med. 2013, S4, 003. [Google Scholar]
- Jeong, H.J.; Vakil, P.; Sheehan, J.J.; Shah, S.J.; Cuttica, M.; Carr, J.C.; Carroll, T.J.; Davarpanah, A. Time Resolved MRA: Evaluation of Intrapulmonary Circulation Parameters in Pulmonary Arterial Hypertension. J. Magn. Reson. Imaging 2011, 33, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Chäfer, M.; Kheyfets, V.O.; Schroeder, J.D.; Dunning, J.; Shandas, R.; Buckner, J.K.; Browning, J.; Hertzberg, J.; Hunter, K.S.; Fenster, B.E. Main Pulmonary Arterial Wall Shear Stress Correlates with Invasive Hemodynamics and Stiffness in Pulmonary Hypertension. Pulm. Circ. 2016, 6, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, J.F.; Jiménez, J.D. La circulación pulmonar en la insuficiencia cardiaca. Rev. Española Cardiol. 2010, 63, 334–345. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S. The 2016 International Society for Heart LungTransplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, E.; D’Elia, E.; La Rovere, M.T.; Gronda, E. Remote heart function monitoring: Role of the CardioMEMS HF System. J. Cardiovasc. Med. 2016, 17, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Zajac, J.; Eriksson, J.; Dyverfeldt, P.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Turbulent kinetic energy in normal and myopathic left ventricles. J. Magn. Reson. Imaging 2015, 41, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, K.; Nagai, S.; Tanaka, S.; Handa, T.; Shigematsu, M.; Nagao, T.; Mishima, M.; Kitaichi, M.; Izumi, T. Significance of Pulmonary Arterial Pressure and Diffusion Capacity of the Lung as Prognosticator in Patients with Idiopathic Pulmonary Fibrosis. Chest 2007, 131, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kawut, S.M.; Taichman, D.B.; Archer-Chicko, C.L.; Palevsky, H.I.; Kimmel, S.E. Hemodynamics and Survival in Patients with Pulmonary Arterial Hypertension Related to Systemic Sclerosis*. Chest 2003, 123, 344–350. [Google Scholar] [CrossRef] [Green Version]

| Inclusion Criteria |
|
|
|
|
| Exclusion Criteria |
|
|
|
|
|
|
|
| Pulmonary Hypertension | Definition |
|---|---|
| PH | MPAP ≥ 25 mmHg |
PH due to left heart disease
| MPAP ≥ 25 mmHg and PWCP > 15 mmHg
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponz, I.; Nuche, J.; Sanchez Sanchez, V.; Sanchez-Gonzalez, J.; Blazquez-Bermejo, Z.; Caravaca Perez, P.; Garcia-Cosio Carmena, M.D.; de Juan Baguda, J.S.; Rodríguez Chaverri, A.; Sarnago Cebada, F.; et al. Non-Invasive Assessment of Pulmonary Vasculopathy. Hearts 2021, 2, 5-14. https://doi.org/10.3390/hearts2010002
Ponz I, Nuche J, Sanchez Sanchez V, Sanchez-Gonzalez J, Blazquez-Bermejo Z, Caravaca Perez P, Garcia-Cosio Carmena MD, de Juan Baguda JS, Rodríguez Chaverri A, Sarnago Cebada F, et al. Non-Invasive Assessment of Pulmonary Vasculopathy. Hearts. 2021; 2(1):5-14. https://doi.org/10.3390/hearts2010002
Chicago/Turabian StylePonz, Ines, Jorge Nuche, Violeta Sanchez Sanchez, Javier Sanchez-Gonzalez, Zorba Blazquez-Bermejo, Pedro Caravaca Perez, Maria Dolores Garcia-Cosio Carmena, Javier S. de Juan Baguda, Adriana Rodríguez Chaverri, Fernando Sarnago Cebada, and et al. 2021. "Non-Invasive Assessment of Pulmonary Vasculopathy" Hearts 2, no. 1: 5-14. https://doi.org/10.3390/hearts2010002
APA StylePonz, I., Nuche, J., Sanchez Sanchez, V., Sanchez-Gonzalez, J., Blazquez-Bermejo, Z., Caravaca Perez, P., Garcia-Cosio Carmena, M. D., de Juan Baguda, J. S., Rodríguez Chaverri, A., Sarnago Cebada, F., Arribas Ynsaurriaga, F., Ibañez, B., & Delgado Jiménez, J. F. (2021). Non-Invasive Assessment of Pulmonary Vasculopathy. Hearts, 2(1), 5-14. https://doi.org/10.3390/hearts2010002






