Clinical Significance of Endotypes of Asian Chronic Rhinosinusitis: A Review and Expert Commentary
Abstract
1. Introduction
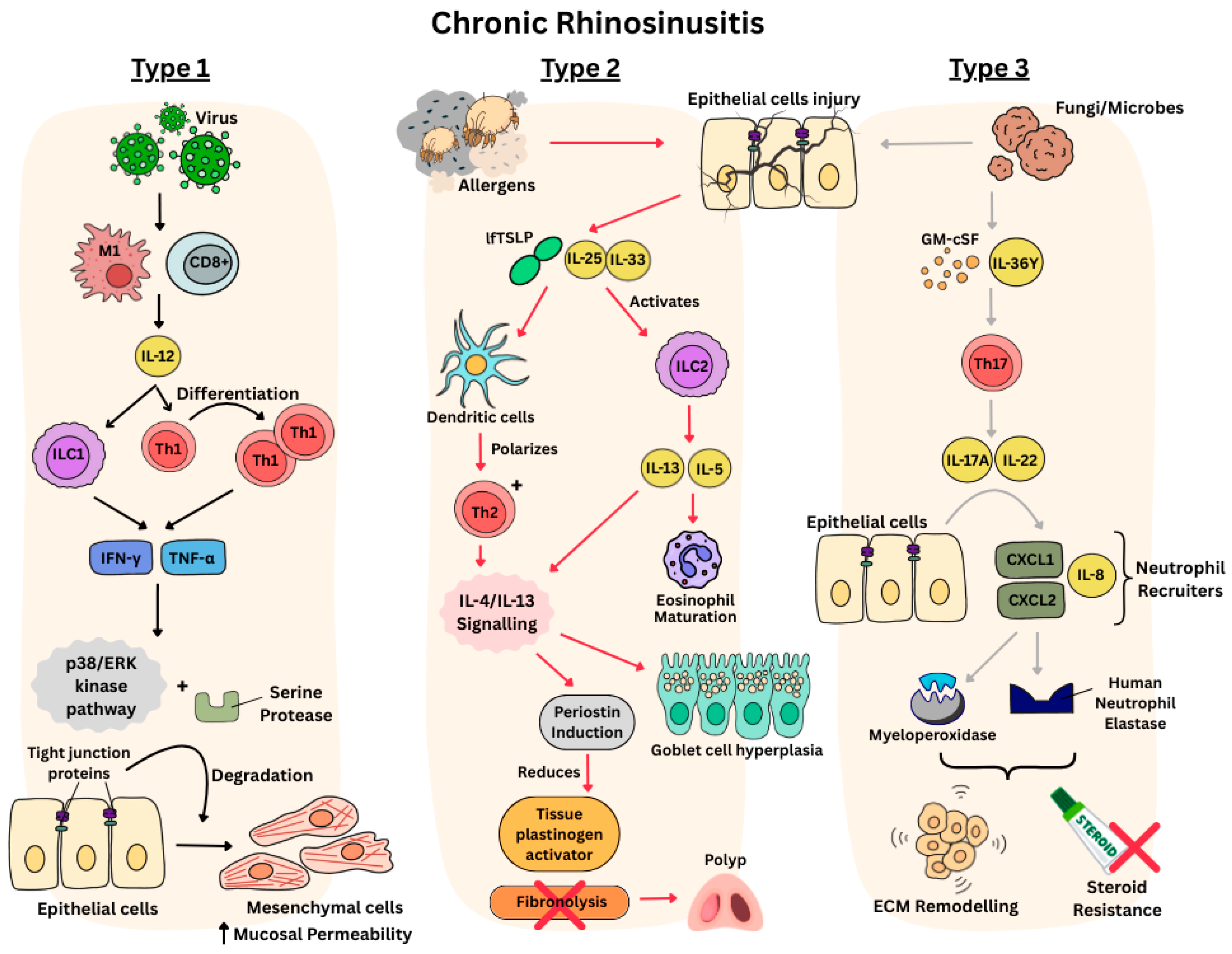
Physiology of Endotypes in CRS
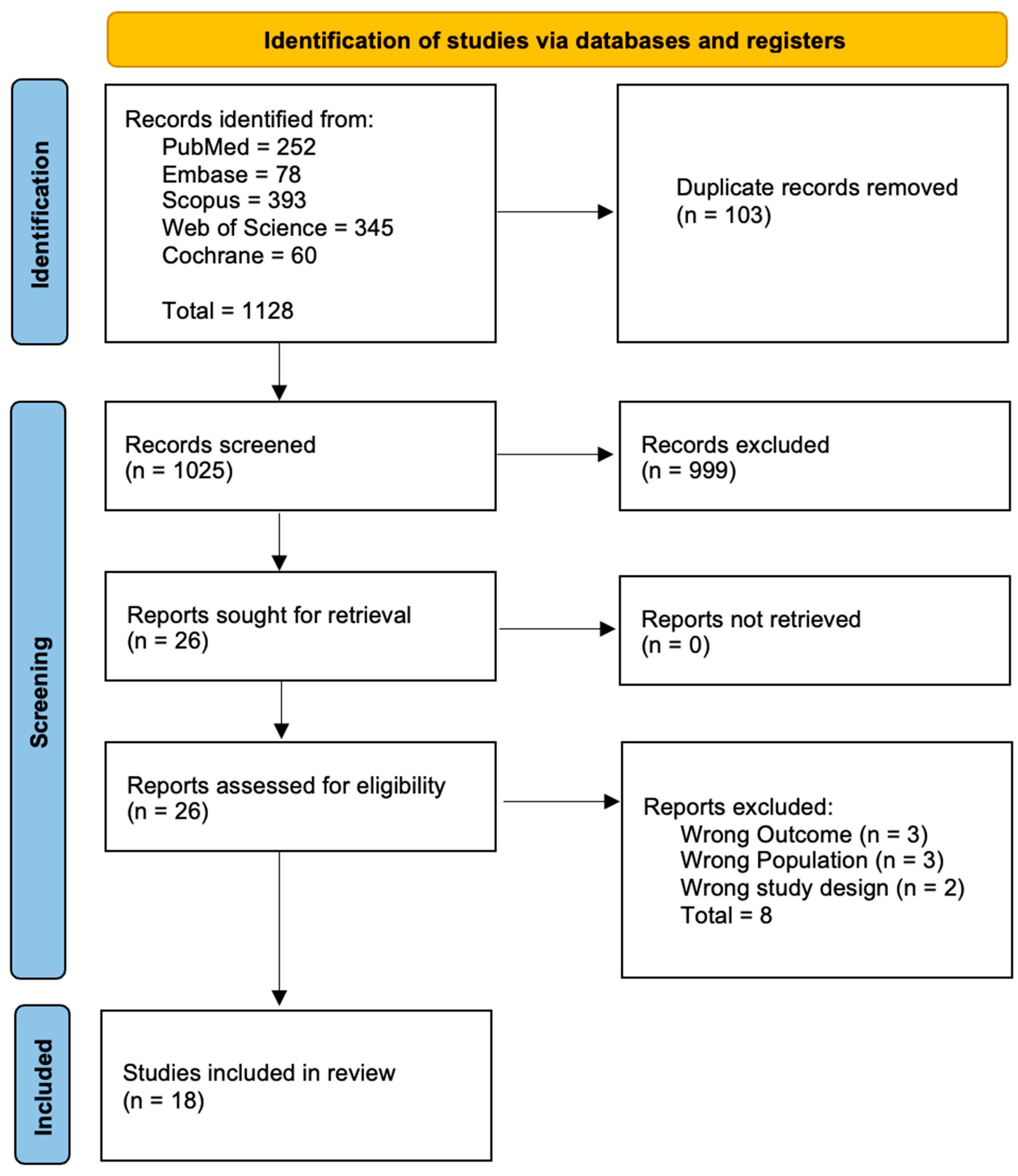
2. Methods
3. Results
3.1. Clinical Implications
3.2. The Case for Endotype-Driven Care
3.3. Endotype-Driven Care in an Asian Population
3.4. Current Evidence Base and Limitations
3.4.1. Lack of Research on Treatment for Non-T2 CRS
3.4.2. Diagnostic Gaps
4. Future Research and Direction
4.1. Establish Asian-Centric Endotype Criteria
4.2. Develop Accessible Diagnostics
4.3. Accelerate Targeted Therapies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 50, 1–464. [Google Scholar] [CrossRef]
- Min, H.K.; Lee, S.; Kim, S.; Son, Y.; Park, J.; Kim, H.J.; Lee, J.; Lee, H.; Smith, L.; Rahmati, M.; et al. Global Incidence and Prevalence of Chronic Rhinosinusitis: A Systematic Review. Clin. Exp. Allergy 2025, 55, 52–66. [Google Scholar] [CrossRef]
- Smith, K.A.; Orlandi, R.R.; Rudmik, L. Cost of Adult Chronic Rhinosinusitis: A Systematic Review. Laryngoscope 2015, 125, 1547–1556. [Google Scholar] [CrossRef]
- Suzuki, M.; Cooksley, C.; Suzuki, T.; Ramezanpour, M.; Nakazono, A.; Nakamaru, Y.; Homma, A.; Vreugde, S. TLR Signals in Epithelial Cells in the Nasal Cavity and Paranasal Sinuses. Front. Allergy 2021, 2, 780425. [Google Scholar] [CrossRef]
- Abidin, M.R.; Alpan, O.; Plassmeyer, M.; Kozhaya, L.; Loizou, D.; Dogan, M.; Upchurch, Z.; Manes, N.P.; Nita-Lazar, A.; Unutmaz, D.; et al. STAT4 Phosphorylation of T-Helper Cells Predicts Surgical Outcomes in Refractory Chronic Rhinosinusitis. medRxiv 2023. [Google Scholar] [CrossRef]
- Soklic, T.K.; Rijavec, M.; Silar, M.; Koren, A.; Kern, I.; Hocevar-Boltezar, I.; Korosec, P. Transcription Factors Gene Expression in Chronic Rhinosinusitis with And Without Nasal Polyps. Radiol. Oncol. 2019, 53, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Majak, J. The Role of Innate Lymphoid Cells in Chronic Rhinosinusitis Severity in Children: A Cross-Sectional Study. Alergol. Pol. Pol. J. Allergol. 2023, 10, 100–106. [Google Scholar] [CrossRef]
- Drake, L.Y.; Iijima, K.; Kita, H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy 2014, 69, 1300–1307. [Google Scholar] [CrossRef]
- Choi, T.; Ryu, S.; Bae, J.-S.; Yoo, S.H.; Mo, J.-H. Epithelial-Mesenchymal Transition in Chronic Rhinosinusitis. J. Rhinol. 2024, 31, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Mo, J.-H.; Shin, H.-W. Epithelial-to-Mesenchymal Transition in Neutrophilic Chronic Rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 30–37. [Google Scholar] [CrossRef]
- Youakim, A.; Ahdieh, M. Interferon-Gamma Decreases Barrier Function in T84 Cells by Reducing ZO-1 Levels and Disrupting Apical Actin. Am. J. Physiol. 1999, 276, G1279–G1288. [Google Scholar] [CrossRef] [PubMed]
- Klingler, A.I.; Stevens, W.W.; Tan, B.K.; Peters, A.T.; Poposki, J.A.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; Kern, R.C.; et al. Mechanisms and Biomarkers of Inflammatory Endotypes in Chronic Rhinosinusitis without Nasal Polyps. J. Allergy Clin. Immunol. 2021, 147, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Wu, Y.; Li, P.; Li, C.; Jiang, T.; Zhang, Q.; Liu, S.; Shi, L. An Integrated Analysis of Inflammatory Endotypes and Clinical Characteristics in Chronic Rhinosinusitis with Nasal Polyps. J. Inflamm. Res. 2022, 15, 5557–5565. [Google Scholar] [CrossRef]
- Van Bruaene, N.; Pérez-Novo, C.A.; Basinski, T.M.; Van Zele, T.; Holtappels, G.; De Ruyck, N.; Schmidt-Weber, C.; Akdis, C.; Van Cauwenberge, P.; Bachert, C.; et al. T-Cell Regulation in Chronic Paranasal Sinus Disease. J. Allergy Clin. Immunol. 2008, 121, 1435–1441.e3. [Google Scholar] [CrossRef]
- Nagarkar, D.R.; Poposki, J.A.; Tan, B.K.; Comeau, M.R.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Norton, J.; Harris, K.E.; Grammer, L.C.; et al. Thymic Stromal Lymphopoietin Activity Is Increased in Nasal Polyps of Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 2013, 132, 593–600.e12. [Google Scholar] [CrossRef]
- Lam, M.; Hull, L.; Imrie, A.; Snidvongs, K.; Chin, D.; Pratt, E.; Kalish, L.; Sacks, R.; Earls, P.; Sewell, W.; et al. Interleukin-25 and Interleukin-33 as Mediators of Eosinophilic Inflammation in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2015, 29, 175–181. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Menzies-Gow, A.; Caveney, S.; Han, J.K.; Martin, N.; Israel, E.; Lee, J.K.; Llanos, J.-P.; Martin, N.; Megally, A.; et al. Tezepelumab Efficacy in Patients with Severe, Uncontrolled Asthma with Comorbid Nasal Polyps in NAVIGATOR. J. Asthma Allergy 2023, 16, 915. [Google Scholar] [CrossRef]
- Walford, H.H.; Lund, S.J.; Baum, R.E.; White, A.A.; Bergeron, C.M.; Husseman, J.; Bethel, K.J.; Scott, D.R.; Khorram, N.; Miller, M.; et al. Increased ILC2s in the Eosinophilic Nasal Polyp Endotype Are Associated with Corticosteroid Responsiveness. Clin. Immunol. 2014, 155, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Bliźniewska, H.; Paprocka-Zjawiona, M.; Merecz-Sadowska, A.; Zajdel, R.; Bliźniewska-Kowalska, K.; Malinowska, K. Serum IL-5, POSTN and IL-33 Levels in Chronic Rhinosinusitis with Nasal Polyposis Correlate with Clinical Severity. BMC Immunol. 2022, 23, 33. [Google Scholar] [CrossRef]
- Danielides, G.; Lygeros, S.; Kanakis, M.; Naxakis, S. Periostin as a Biomarker in Chronic Rhinosinusitis: A Contemporary Systematic Review. Int. Forum Allergy Rhinol. 2022, 12, 1535–1550. [Google Scholar] [CrossRef]
- Asano, T.; Kanemitsu, Y.; Takemura, M.; Yokota, M.; Fukumitsu, K.; Takeda, N.; Ichikawa, H.; Uemura, T.; Takakuwa, O.; Ohkubo, H.; et al. Serum Periostin as a Biomarker for Comorbid Chronic Rhinosinusitis in Patients with Asthma. Ann. Am. Thorac. Soc. 2017, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-C.; Lee, T.-J.; Huang, C.-C.; Chang, P.-H.; Chen, Y.-W.; Fu, C.-H. Serum Eosinophil Cationic Protein: A Prognostic Factor for Early Postoperative Recurrence of Nasal Polyps. Int. Forum Allergy Rhinol. 2021, 11, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Bae, J.-S.; Kim, J.H.; Kim, E.H.; Lyu, L.; Chung, Y.-J.; Mo, J.-H. Role of IL-17A in Chronic Rhinosinusitis with Nasal Polyp. Allergy Asthma Immunol. Res. 2020, 12, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, M.; Xu, Y.; Guo, H.; Zhao, C. Expression of Interleukin-22 and Its Significance in the Pathogenesis of Chronic Rhinosinusitis. Int. J. Clin. Exp. Pathol. 2014, 7, 5709–5716. [Google Scholar]
- Sun, Z.; Yang, P. Role of Imbalance between Neutrophil Elastase and Alpha 1-Antitrypsin in Cancer Development and Progression. Lancet Oncol. 2004, 5, 182–190. [Google Scholar] [CrossRef]
- Stevens, W.W.; Peters, A.T.; Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Hulse, K.E.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; et al. Associations between Inflammatory Endotypes and Clinical Presentations in Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2019, 7, 2812–2820.e3. [Google Scholar] [CrossRef]
- Min, J.-Y.; Kim, J.Y.; Sung, C.M.; Kim, S.T.; Cho, H.-J.; Mun, S.J.; Cho, S.-W.; Hong, S.D.; Ryu, G.; Cho, K.R.; et al. Inflammatory Endotypes of Chronic Rhinosinusitis in the Korean Population: Distinct Expression of Type 3 Inflammation. Allergy Asthma Immunol. Res. 2023, 15, 437–450. [Google Scholar] [CrossRef]
- Yu, J.; Xian, M.; Piao, Y.; Zhang, L.; Wang, C. Changes in Clinical and Histological Characteristics of Nasal Polyps in Northern China over the Past 2-3 Decades. Int. Arch. Allergy Immunol. 2021, 182, 615–624. [Google Scholar] [CrossRef]
- Pan, L.; Liao, B.; Guo, C.-L.; Liu, J.-X.; Wang, H.; Long, X.-B.; Liu, Z. Inflammatory Features and Predictors for Postsurgical Outcomes in Patients with Nasal Polyps Stratified by Local and Systemic Eosinophilia. Int. Forum Allergy Rhinol. 2021, 11, 846–856. [Google Scholar] [CrossRef]
- Liao, B.; Liu, J.-X.; Li, Z.-Y.; Zhen, Z.; Cao, P.-P.; Yao, Y.; Long, X.-B.; Wang, H.; Wang, Y.; Schleimer, R.; et al. Multidimensional Endotypes of Chronic Rhinosinusitis and Their Association with Treatment Outcomes. Allergy 2018, 73, 1459–1469. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of TH Cytokine Profiles in Patients with Chronic Rhinosinusitis: A Multicenter Study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef]
- Lou, H.; Meng, Y.; Piao, Y.; Zhang, N.; Bachert, C.; Wang, C.; Zhang, L. Cellular Phenotyping of Chronic Rhinosinusitis with Nasal Polyps. Rhinology 2016, 54, 150–159. [Google Scholar] [CrossRef]
- Ikeda, K.; Shiozawa, A.; Ono, N.; Kusunoki, T.; Hirotsu, M.; Homma, H.; Saitoh, T.; Murata, J. Subclassification of Chronic Rhinosinusitis with Nasal Polyp Based on Eosinophil and Neutrophil. Laryngoscope 2013, 123, E1–E9. [Google Scholar] [CrossRef]
- Nakayama, T.; Yoshikawa, M.; Asaka, D.; Okushi, T.; Matsuwaki, Y.; Otori, N.; Hama, T.; Moriyama, H. Mucosal Eosinophilia and Recurrence of Nasal Polyps—New Classification of Chronic Rhinosinusitis. Rhinology 2011, 49, 392–396. [Google Scholar] [CrossRef]
- Yoshimura, K.; Kawata, R.; Haruna, S.; Moriyama, H.; Hirakawa, K.; Fujieda, S.; Masuyama, K.; Takenaka, H. Clinical Epidemiological Study of 553 Patients with Chronic Rhinosinusitis in Japan. Allergol. Int. 2011, 60, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.-P.; Li, H.-B.; Wang, B.-F.; Wang, S.-B.; You, X.-J.; Cui, Y.-H.; Wang, D.-Y.; Desrosiers, M.; Liu, Z. Distinct Immunopathologic Characteristics of Various Types of Chronic Rhinosinusitis in Adult Chinese. J. Allergy Clin. Immunol. 2009, 124, 478–484.e1–2. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, Y.; Wang, Z.; Jiang, Y.; Yuan, F.; Zhang, T. Single Cell Transcriptomic Analysis Reveals Transcriptome Differences of Different Cells between Eosinophilic Chronic Rhinosinusitis with Nasal Polyps and Non-Eosinophilic Chronic Rhinosinusitis with Nasal Polyps. PLoS ONE 2025, 20, e0328241. [Google Scholar] [CrossRef]
- Jin, J.; Guo, B.; Zhang, W.; Chen, J.-J.; Deng, Y.-Q.; Xiang, R.; Tan, L.; Qin, D.-X.; Zheng, L.; Chen, Z.; et al. Diagnostic Value of Myeloperoxidase and Eosinophil Cationic Protein in Nasal Secretions for Endotypes of Chronic Rhinosinusitis. Eur. Arch. Otorhinolaryngol. 2023, 280, 3707–3720. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, J.; Ren, Y.; Qiu, H.; Yuan, L.; Deng, H.; Zhang, Y.; Zheng, R.; Hong, H.; Sun, Y.; et al. Artificial Intelligence for Cellular Phenotyping Diagnosis of Nasal Polyps by Whole-Slide Imaging. EBioMedicine 2021, 66, 103336. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.Y.; Han, Y.E.; Kim, J.K.; Lim, H.S.; Eun, K.M.; Yang, S.K.; Kim, D.W. Elastase-Positive Neutrophils Are Associated with Refractoriness of Chronic Rhinosinusitis with Nasal Polyps in an Asian Population. Allergy Asthma Immunol. Res. 2020, 12, 42–55. [Google Scholar] [CrossRef]
- Kim, D.W.; Eun, K.M.; Roh, E.Y.; Shin, S.; Kim, D.-K. Chronic Rhinosinusitis without Nasal Polyps in Asian Patients Shows Mixed Inflammatory Patterns and Neutrophil-Related Disease Severity. Mediat. Inflamm. 2019, 2019, 7138643. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.-Y.; Jiang, W.-X.; Liao, B.; Zhai, G.-T.; Wang, N.; Zhen, Z.; Ruan, J.-W.; Long, X.-B.; Wang, H.; et al. The Activation and Function of IL-36γ in Neutrophilic Inflammation in Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 2018, 141, 1646–1658. [Google Scholar] [CrossRef]
- Xia, W.; Bai, J.; Wu, X.; Wei, Y.; Feng, S.; Li, L.; Zhang, J.; Xiong, G.; Fan, Y.; Shi, J.; et al. Interleukin-17A Promotes MUC5AC Expression and Goblet Cell Hyperplasia in Nasal Polyps via the Act1-Mediated Pathway. PLoS ONE 2014, 9, e98915. [Google Scholar] [CrossRef]
- Bachert, C.; Zhang, N.; Cavaliere, C.; Weiping, W.; Gevaert, E.; Krysko, O. Biologics for Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 2020, 145, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Alaraifi, A.K.; Alanizy, B.; Alsalamah, S.; Alraddadi, J.; Alhedaithy, R. Predictors and Time Interval of Chronic Rhinosinusitis Recurrence After Endoscopic Sinus Surgery. Turk. Arch. Otorhinolaryngol. 2023, 61, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Chapurin, N.; Schlosser, R.J.; Gutierrez, J.; Mace, J.C.; Smith, T.L.; Bodner, T.E.; Khan, S.; Mulligan, J.K.; Mattos, J.L.; Alt, J.A.; et al. All Chronic Rhinosinusitis Endotype Clusters Demonstrate Improvement in Patient-Reported and Clinical Outcome Measures after Endoscopic Sinus Surgery. Int. Forum Allergy Rhinol. 2024, 14, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Delemarre, T.; Bachert, C. Neutrophilic Inflammation in Chronic Rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 14–21. [Google Scholar] [CrossRef]
- Kato, A.; Peters, A.T.; Stevens, W.W.; Schleimer, R.P.; Tan, B.K.; Kern, R.C. Endotypes of Chronic Rhinosinusitis: Relationships to Disease Phenotypes, Pathogenesis, Clinical Findings, and Treatment Approaches. Allergy 2022, 77, 812–826. [Google Scholar] [CrossRef]
- Smith, K.A.; Orlandi, R.R.; Oakley, G.; Meeks, H.; Curtin, K.; Alt, J.A. Long-Term Revision Rates for Endoscopic Sinus Surgery. Int. Forum Allergy Rhinol. 2019, 9, 402–408. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and Safety of Dupilumab in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from Two Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Phase 3 Trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Russo, P.; Bassano, E.; Menichetti, M.; Lucidi, D.; Minniti, R.M.; Cigarini, E.; Menabue, S.; Marchioni, D.; Perano, D.; Ghidini, A. Long-Term Effectiveness of Dupilumab in Severe Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: A Multicenter Retrospective Study. Am. J. Rhinol. Allergy 2025, 39, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.; Mannent, L.P.; Amin, N.; Canonica, G.W.; Hellings, P.W.; Gevaert, P.; Mullol, J.; Lee, S.E.; Fujieda, S.; Han, J.K.; et al. Dupilumab Reduces Systemic Corticosteroid Use and Sinonasal Surgery Rate in CRSwNP. Rhinology 2021, 59, 301–311. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Mullol, J.; Kennedy, D.; Philpott, C.; Seccia, V.; Kern, R.C.; Coste, A.; Sousa, A.R.; Howarth, P.H.; Benson, V.S.; et al. Mepolizumab for Chronic Rhinosinusitis with Nasal Polyps (SYNAPSE): In-Depth Sinus Surgery Analysis. Allergy 2023, 78, 812–821. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B.; Shen, S.; Song, X.; Jiang, Y.; Shi, L.; Zhao, C.; Yang, Y.; Jiang, L.; Li, J.; et al. Efficacy and Safety of CM310 in Severe Eosinophilic Chronic Rhinosinusitis with Nasal Polyps (CROWNS-1): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. eClinicalMedicine 2023, 61, 102076. [Google Scholar] [CrossRef] [PubMed]
- Wautlet, A.; Bachert, C.; Desrosiers, M.; Hellings, P.W.; Peters, A.T. The Management of Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) With Biologics. J. Allergy Clin. Immunol. Pract. 2023, 11, 2642–2651. [Google Scholar] [CrossRef]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.-O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G.; et al. Inflammatory Endotypes of Chronic Rhinosinusitis Based on Cluster Analysis of Biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef]
- Gevaert, P.; Calus, L.; Van Zele, T.; Blomme, K.; De Ruyck, N.; Bauters, W.; Hellings, P.; Brusselle, G.; De Bacquer, D.; van Cauwenberge, P.; et al. Omalizumab Is Effective in Allergic and Nonallergic Patients with Nasal Polyps and Asthma. J. Allergy Clin. Immunol. 2013, 131, 110–116.e1. [Google Scholar] [CrossRef]
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J.; et al. Reduced Need for Surgery in Severe Nasal Polyposis with Mepolizumab: Randomized Trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031.e14. [Google Scholar] [CrossRef]
- Grimm, D.; Hwang, P.H.; Lin, Y.-T. The Link between Allergic Rhinitis and Chronic Rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2023, 31, 3–10. [Google Scholar] [CrossRef]
- Bachert, C.; Mannent, L.; Naclerio, R.M.; Mullol, J.; Ferguson, B.J.; Gevaert, P.; Hellings, P.; Jiao, L.; Wang, L.; Evans, R.R.; et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients with Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Stevens, W.W.; Peters, A.T.; Suh, L.A.; Norton, J.; Carter, R.G.; Hulse, K.E.; Harris, K.E.; et al. Heterogeneous Inflammatory Patterns in Chronic Rhinosinusitis without Nasal Polyps in Chicago, Illinois. J. Allergy Clin. Immunol. 2017, 139, 699–703.e7. [Google Scholar] [CrossRef]
- Zhang, N.; Van Zele, T.; Perez-Novo, C.; Van Bruaene, N.; Holtappels, G.; DeRuyck, N.; Van Cauwenberge, P.; Bachert, C. Different Types of T-Effector Cells Orchestrate Mucosal Inflammation in Chronic Sinus Disease. J. Allergy Clin. Immunol. 2008, 122, 961–968. [Google Scholar] [CrossRef]
- Yao, Y.; Zeng, M.; Liu, Z. Revisiting Asian Chronic Rhinosinusitis in the Era of Type 2 Biologics. Clin. Exp. Allergy 2022, 52, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gevaert, E.; Lou, H.; Wang, X.; Zhang, L.; Bachert, C.; Zhang, N. Chronic Rhinosinusitis in Asia. J. Allergy Clin. Immunol. 2017, 140, 1230–1239. [Google Scholar] [CrossRef]
- Chen, C.-L.; Yao, Y.; Pan, L.; Hu, S.-T.; Ma, J.; Wang, Z.-C.; Kern, R.C.; Schleimer, R.P.; Liu, Z. Common Fibrin Deposition and Tissue Plasminogen Activator Downregulation in Nasal Polyps with Distinct Inflammatory Endotypes. J. Allergy Clin. Immunol. 2020, 146, 677–681. [Google Scholar] [CrossRef]
- Staudacher, A.G.; Peters, A.T.; Kato, A.; Stevens, W.W. Use of Endotypes, Phenotypes, and Inflammatory Markers to Guide Treatment Decisions in Chronic Rhinosinusitis. Ann. Allergy Asthma Immunol. 2020, 124, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Ng, C.L.; Li, C.; Li, Y.Y.; Duan, C.; Shen, L.; Jiao, Y.F.; Liu, M.; Wang, D.Y. Smoking Is an Independent Association of Squamous Metaplasia in Chinese Nasal Polyps. Int. Forum Allergy Rhinol. 2016, 6, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, C.W.; Jin, P.; Ng, C.L.; Lin, Z.B.; Li, Y.Y.; Li, T.Y.; Petersson, B.F.; Shi, L.; Wang, D.Y. Histopathological Features of Sinonasal Inverted Papillomas in Chinese Patients. Laryngoscope 2016, 126, E141. [Google Scholar] [CrossRef]
- Bossé, Y.; Bacot, F.; Montpetit, A.; Rung, J.; Qu, H.-Q.; Engert, J.C.; Polychronakos, C.; Hudson, T.J.; Froguel, P.; Sladek, R.; et al. Identification of Susceptibility Genes for Complex Diseases Using Pooling-Based Genome-Wide Association Scans. Hum. Genet. 2009, 125, 305–318. [Google Scholar] [CrossRef]
- Zhang, Y.; Endam, L.M.; Filali-Mouhim, A.; Zhao, L.; Desrosiers, M.; Han, D.; Zhang, L. Polymorphisms in RYBP and AOAH Genes Are Associated with Chronic Rhinosinusitis in a Chinese Population: A Replication Study. PLoS ONE 2012, 7, e39247. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Z.; Fu, Y.; Zhang, N.; Liu, W.; Shi, L.; Lu, T.; Li, Z.; Tu, Z.; Li, J.; et al. Association of Long-Term Air Pollution and Allergen Exposure with Endotype Shift in Chronic Rhinosinusitis with Nasal Polyps. Ann. Allergy Asthma Immunol. 2025, 135, 171–179.e2. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.M.; Soler, Z.M.; Rathi, V.K.; Schlosser, R.J. Spending, Utilization, and Coverage for Chronic Rhinosinusitis with Nasal Polyposis Therapies among Medicare Advantage Beneficiaries. Int. Forum Allergy Rhinol. 2024, 14, 1643–1646. [Google Scholar] [CrossRef]
- World Bank Open Data. Available online: https://data.worldbank.org (accessed on 13 September 2025).
- Wen, W.; Liu, W.; Zhang, L.; Bai, J.; Fan, Y.; Xia, W.; Luo, Q.; Zheng, J.; Wang, H.; Li, Z.; et al. Increased Neutrophilia in Nasal Polyps Reduces the Response to Oral Corticosteroid Therapy. J. Allergy Clin. Immunol. 2012, 129, 1522–1528.e5. [Google Scholar] [CrossRef]
- Videler, W.J.; Badia, L.; Harvey, R.J.; Gane, S.; Georgalas, C.; van der Meulen, F.W.; Menger, D.J.; Lehtonen, M.T.; Toppila-Salmi, S.K.; Vento, S.I.; et al. Lack of Efficacy of Long-Term, Low-Dose Azithromycin in Chronic Rhinosinusitis: A Randomized Controlled Trial. Allergy 2011, 66, 1457–1468. [Google Scholar] [CrossRef]
- Haxel, B.R.; Clemens, M.; Karaiskaki, N.; Dippold, U.; Kettern, L.; Mann, W.J. Controlled Trial for Long-Term Low-Dose Erythromycin after Sinus Surgery for Chronic Rhinosinusitis. Laryngoscope 2015, 125, 1048–1055. [Google Scholar] [CrossRef]
- Wallwork, B.; Coman, W.; Mackay-Sim, A.; Greiff, L.; Cervin, A. A Double-Blind, Randomized, Placebo-Controlled Trial of Macrolide in the Treatment of Chronic Rhinosinusitis. Laryngoscope 2006, 116, 189–193. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, J.; Yan, B.; Wang, C.; Zhang, L.; Lan, F. New Insights into the Endotypes of Chronic Rhinosinusitis in the Biologic Era. J. Allergy Clin. Immunol. 2025, 156, 51–60. [Google Scholar] [CrossRef]
- Toro, M.D.C.; Antonio, M.A.; Alves Dos Reis, M.G.; de Assumpcao, M.S.; Sakano, E. Achieving the Best Method to Classify Eosinophilic Chronic Rhinosinusitis: A Systematic Review. Rhinology 2021, 59, 330–339. [Google Scholar] [CrossRef]
- Soler, Z.M.; Sauer, D.; Mace, J.; Smith, T.L. Impact of Mucosal Eosinophilia and Nasal Polyposis on Quality-of-Life Outcomes after Sinus Surgery. Otolaryngol. –Head Neck Surg. 2010, 142, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Karpischenko, S.; Jung, Y.G.; Kim, D.-W.; Spriggs, K.; Tsang, R.K.-Y.; Yeh, T.-H. Management of Chronic Rhinosinusitis with Nasal Polyps in the Asia-Pacific Region and Russia: Recommendations from an Expert Working Group. Asia Pac. Allergy 2024, 14, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zhang, N.; Bachert, C.; Zhang, L. Highlights of Eosinophilic Chronic Rhinosinusitis with Nasal Polyps in Definition, Prognosis, and Advancement. Int. Forum Allergy Rhinol. 2018, 8, 1218–1225. [Google Scholar] [CrossRef]
- Yoo, S.H.; Mo, J.-H. The Different Features of Inflammatory Endotypes in Korean Patients with Chronic Rhinosinusitis. Allergy Asthma Immunol. Res. 2023, 15, 413–415. [Google Scholar] [CrossRef]
- Mahdavinia, M.; Suh, L.A.; Carter, R.G.; Stevens, W.W.; Norton, J.E.; Kato, A.; Tan, B.K.; Kern, R.C.; Conley, D.B.; Chandra, R.; et al. Increased Non-Eosinophilic Nasal Polyps in Chronic Rhinosinusitis in U.S. Second-Generation Asians Suggests Genetic Regulation of Eosinophilia. J. Allergy Clin. Immunol. 2015, 135, 576–579. [Google Scholar] [CrossRef]
- Ryu, G.; Kim, H.Y.; Jung, Y.G.; Hong, S.D. Endotypes of Asian Chronic Rhinosinusitis with Nasal Polyps: A Narrative Review. Precis. Future Med. 2022, 6, 170–176. [Google Scholar] [CrossRef]
- Xu, S.; Vallei, M.; Hwang Siok Gek, J.; Tze Choong, C.; Wei Yang Teo, N. Endotyping of Nasal Polyps in a Multiracial Asian Population. Rhinol. Online 2022, 5, 142–148. [Google Scholar] [CrossRef]
- Bystrom, J.; Patel, S.Y.; Amin, K.; Bishop-Bailey, D. Dissecting the Role of Eosinophil Cationic Protein in Upper Airway Disease. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Linder, A.; Venge, P.; Deuschl, H. Eosinophil Cationic Protein and Myeloperoxidase in Nasal Secretion as Markers of Inflammation in Allergic Rhinitis. Allergy 1987, 42, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Liu, F.; Zhang, J.; Liu, Y.; Du, J.; Liu, S.; Zhang, N.; Bachert, C.; Meng, J. Multivariate Analysis of Inflammatory Endotypes in Recurrent Nasal Polyposis in a Chinese Population. Rhinology 2018, 56, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Deng, Y.-Q.; Li, H.-D.; Jiao, W.-E.; Chen, J.; Chen, J.-J.; Tao, Z.-Z. Diagnostic Value of a Novel Eosinophil Cationic Protein-Myeloperoxidase Test Paper Before and After Treatment for Allergic Rhinitis. J. Asthma Allergy 2022, 15, 1005–1019. [Google Scholar] [CrossRef]
- Xi, Y.; Deng, Y.-Q.; Li, H.-D.; Jiao, W.-E.; Chen, J.; Chen, J.-J.; Tao, Z.-Z. Evaluation of the Correlation Between Nasal Secretion ECP-MPO Test Papers and Immune Markers in Subcutaneous Immunotherapy of Dust Mites. J. Asthma Allergy 2024, 17, 847–862. [Google Scholar] [CrossRef]
- Ding, J.; Yue, C.; Wang, C.; Liu, W.; Zhang, L.; Chen, B.; Shen, S.; Piao, Y.; Zhang, L. Machine Learning Method for the Cellular Phenotyping of Nasal Polyps from Multicentre Tissue Scans. Expert Rev. Clin. Immunol. 2023, 19, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Plaas, M.; Brazovskaja, A.; Kisand, K.; Tserel, L.; Peterson, P. Secukinumab (Anti-IL-17A Therapeutic Antibody) Improves Clinical Outcome for a Mixed Endotype CRS. Clin. Case Rep. 2024, 12, e9692. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Burgel, P.-R.; Daley, C.L.; De Soyza, A.; Haworth, C.S.; Mauger, D.; Loebinger, M.R.; McShane, P.J.; Ringshausen, F.C.; Blasi, F.; et al. Phase 3 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N. Engl. J. Med. 2025, 392, 1569–1581. [Google Scholar] [CrossRef]
- Okano, M.; Yamada, M.; Oka, A. Personalized Medicine in Chronic Rhinosinusitis: Treatable Traits Using Biologics for Unmet Needs. Allergy Asthma Immunol. Res. 2025, 17, 8–21. [Google Scholar] [CrossRef] [PubMed]
| Search Parameters | Specifications |
|---|---|
| Date of search | 15 July 2025 |
| Databases searched | PubMed, Cochrane, Web of Science, Embase, Scopus |
| Search terms utilized | “Chronic Rhinosinusitis”, “Endotypes”, “CRS endotyping”, “Asian populations”, “Precision Medicine”, “personalized treatment”, “Biomarkers”, “Biologics” and “Research gaps” |
| Timeframe | January 2000 to July 2025 |
| Inclusion criteria | Clinical and experimental studies investigating CRS endotypes, studies exploring current barriers to implementation, studies exploring inflammatory patterns or biomarkers related to CRS in Asian populations |
| Excusion criteria | Non-Asian studies, case reports, conference abstracts |
| Author, Year | Title of Study | Study Design | Sample Size (n) | Key Findings | Study Strengths | Study Limitations | Study Quality (NOS) |
|---|---|---|---|---|---|---|---|
| Min et al., 2023 [27] | Inflammatory endotypes of chronic rhinosinusitis in the Korean population: Distinct expression of type 3 inflammation | Prospective cohort study | 244 CRS patients |
|
|
| High (8/9) |
| Jiang W et al., 2021 [28] | Changes in clinical and histological characteristics of nasal polyps in Northern China | Retrospective cohort study | 300 Chinese CRS patients |
|
|
| Medium (6/9) |
| Pan L et al., 2021 [29] | Inflammatory features and predictors for postsurgical outcomes in patients with nasal polyps stratified by local and systemic eosinophilia | Prospective cohort study | 535 Asian CRS patients |
|
|
| High (8/9) |
| Liao B et al., 2018 [30] | Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes | Prospective cohort study | 246 Chinese CRS patients |
|
|
| High (8/9) |
| Wang XD et al., 2016 [31] | Diversity of TH cytokine profiles in patients with chronic rhinosinusitis | Cross-sectional study | 435 Chinese CRS patients; 138 Western controls |
|
|
| High (7/9) |
| Lou H et al., 2016 [32] | Cellular phenotyping of chronic rhinosinusitis with nasal polyps | Retrospective cohort study | 366 Chinese CRS patients |
|
|
| Medium (6/9) |
| Ikeda et al., 2013 [33] | Subclassification of CRSwNP based on eosinophil and neutrophil infiltration | Cross-sectional Study | 130 Japanese CRS patients |
|
|
| Medium (5/9) |
| Nakayama T et al., 2011 [34] | Mucosal eosinophilia and recurrence of nasal polyps—new classification of CRS | Prospective cohort study | 175 Japanese CRS patients |
|
|
| High (7/9) |
| Yoshimura K et al., 2011 [35] | Clinical epidemiological study of 553 patients with chronic rhinosinusitis in Japan | Cross-sectional study | 553 Japanese CRS patients |
|
|
| Medium (6/9) |
| Cao PP et al., 2009 [36] | Distinct immunopathologic characteristics of chronic rhinosinusitis in adult Chinese | Cross-sectional study | 245 Chinee CRS patients, 50 Chinese controls |
|
|
| Medium (6/9) |
| Author, Year | Title of Study | Methodology | Key Findings | Study Strengths | Study Limitations |
|---|---|---|---|---|---|
| Jin et al., 2025 [37] | Single cell transcriptomic analysis reveals transcriptome differences of different cells between eosinophilic chronic rhinosinusitis with nasal polyps and non-eosinophilic chronic rhinosinusitis with nasal polyps |
|
|
|
|
| Jin et al., 2023 [38] | Diagnostic Value of Myeloperoxidase and Eosinophil Cationic Protein in Nasal Secretions for Endotypes of Chronic Rhinosinusitis |
|
|
|
|
| Wu et al., 2021 [39] | Artificial Intelligence for Cellular Phenotyping Diagnosis of Nasal Polyps by Whole-Slide Imaging |
|
|
|
|
| Kim et al., 2020 [40] | Elastase-Positive Neutrophils Are Associated With Refractoriness of Chronic Rhinosinusitis With Nasal Polyps in an Asian Population |
|
|
|
|
| Ryu et al., 2020 [23] | Role of IL-17A in Chronic Rhinosinusitis With Nasal Polyp |
|
|
|
|
| Kim et al., 2019 [41] | Chronic Rhinosinusitis without Nasal Polyps in Asian Patients Shows Mixed Inflammatory Patterns and Neutrophil-Related Disease Severity |
|
|
|
|
| Wang et al., 2018 [42] | The activation and function of IL-36γ in neutrophilic inflammation in chronic rhinosinusitis |
|
|
|
|
| Xia et al., 2014 [43] | Interleukin-17A promotes MUC5AC expression and goblet cell hyperplasia in nasal polyps via the Act1-mediated pathway |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loh, S.B.J.; Chua, N.Y.M.; Ang, L.F.; Di Pierro, F.; Ng, C.L. Clinical Significance of Endotypes of Asian Chronic Rhinosinusitis: A Review and Expert Commentary. Sinusitis 2025, 9, 19. https://doi.org/10.3390/sinusitis9020019
Loh SBJ, Chua NYM, Ang LF, Di Pierro F, Ng CL. Clinical Significance of Endotypes of Asian Chronic Rhinosinusitis: A Review and Expert Commentary. Sinusitis. 2025; 9(2):19. https://doi.org/10.3390/sinusitis9020019
Chicago/Turabian StyleLoh, Sean Bo Jie, Nevin Yi Meng Chua, Lee Fang Ang, Francesco Di Pierro, and Chew Lip Ng. 2025. "Clinical Significance of Endotypes of Asian Chronic Rhinosinusitis: A Review and Expert Commentary" Sinusitis 9, no. 2: 19. https://doi.org/10.3390/sinusitis9020019
APA StyleLoh, S. B. J., Chua, N. Y. M., Ang, L. F., Di Pierro, F., & Ng, C. L. (2025). Clinical Significance of Endotypes of Asian Chronic Rhinosinusitis: A Review and Expert Commentary. Sinusitis, 9(2), 19. https://doi.org/10.3390/sinusitis9020019






