Apical Periodontitis and Maxillary Sinus Alterations: Results of an Exploratory Cross-Sectional Tomographic In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
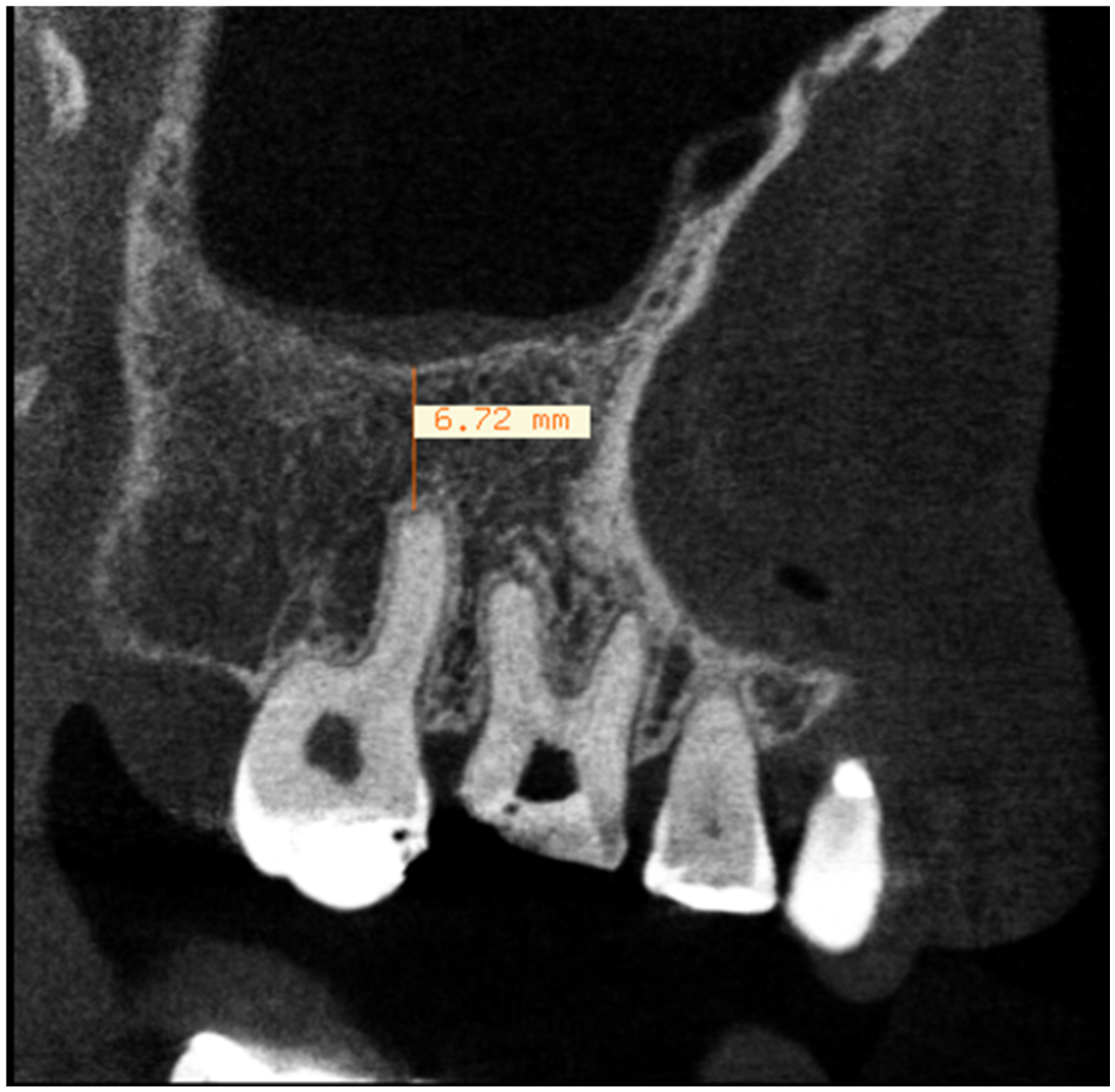
2.1. Evaluation of Dental Parameters
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBCT | Cone Beam Computed Tomography |
| MT | Mucosal thickening |
| OS | Odontogenic sinusitis |
| EPOS | European Position Paper on Rhinosinusitis and Nasal Polyps |
| CT | Computed tomography |
| ESE | European Society of Endodontology |
| CAP | Chronic apical periodontitis |
References
- Phothikhun, S.; Suphanantachat, S.; Chuenchompoonut, V.; Nisapakultorn, K. Cone-Beam Computed Tomographic Evidence of the Association Between Periodontal Bone Loss and Mucosal Thickening of the Maxillary Sinus. J. Periodontol. 2012, 83, 557–564. [Google Scholar] [CrossRef]
- Schneider, A.C.; Bragger, U.; Sendi, P.; Caversaccio, M.D.; Buser, D.; Bornstein, M.M. Characteristics and Dimensions of the Sinus Membrane in Patients Referred for Single-Implant Treatment in the Posterior Maxilla: A Cone Beam Computed Tomographic Analysis. Int. J. Oral Maxillofac. Implant. 2013, 28, 587–596. Available online: http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=08822786&AN=86217694&h=kIJ9v9tFXs%2FvIdK7K00x51uU6bZLmsUO6o%2BOK4r7JHqiJWnZC%2Bfd4QyVEtH7A4pF7lksMZ%2BUYAj971Y6sWcQbg%3D%3D&crl=c (accessed on 12 November 2024). [CrossRef] [PubMed]
- Shanbhag, S.; Karnik, P.; Shirke, P.; Shanbhag, V. Association between periapical lesions and maxillary sinus mucosal thickening: A retrospective cone-beam computed tomographic study. J. Endod. 2013, 39, 853–857. Available online: https://www.sciencedirect.com/science/article/pii/S0099239913003221 (accessed on 12 November 2024). [CrossRef]
- Nunes, C.A.B.C.M.; Guedes, O.A.; Alencar, A.H.G.; Peters, O.A.; Estrela, C.R.A.; Estrela, C. Evaluation of Periapical Lesions and Their Association with Maxillary Sinus Abnormalities on Cone-beam Computed Tomographic Images. J. Endod. 2016, 42, 42–46. [Google Scholar] [CrossRef]
- Siddiqui, F.; Smith, R.V.; Yom, S.S.; Beitler, J.J.; Busse, P.M.; Cooper, J.S.; Hanna, E.Y.; Jones, C.U.; Koyfman, S.A.; Quon, H.; et al. ACR appropriateness criteria® nasal cavity and paranasal sinus cancers. Head Neck 2017, 39, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Pazera, P.; Bornstein, M.M.; Pazera, A.; Sendi, P.; Katsaros, C. Incidental maxillary sinus findings in orthodontic patients: A radiographic analysis using cone-beam computed tomography (CBCT). Orthod. Craniofacial Res. 2011, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.L.; Sholapurkar, A.A.; Pai, K.M. Maxillary sinus findings in the elderly: A panoramic radiographic study. J. Contemp. Dent. Pract. 2009, 10, 41–48. Available online: http://www.thejcdp.com/journal/ (accessed on 24 October 2024).
- Zhang, J.; Liu, L.; Yang, L.; Wang, J.; Tan, X.; Huang, D. Diagnosis of Odontogenic Maxillary Sinusitis by Cone-beam Computed Tomography: A Critical Review. J. Endod. 2023, 49, 1445–1456. [Google Scholar] [CrossRef]
- Savolainen, S.; Eskelin, M.; Jousimies-Somer, H.; Ylikoski, J. Radiological findings in the maxillary sinuses of symptomless young men. Acta Otolaryngol. 1997, 117 (Suppl. S529), 153–157. [Google Scholar] [CrossRef]
- Ferguson, M. Rhinosinusitis in oral medicine and dentistry. Aust. Dent. J. 2014, 59, 289–295. [Google Scholar] [CrossRef]
- Chen, H.J.; Chen HSen Chang, Y.L.; Huang, Y.C. Complete unilateral maxillary sinus opacity in computed tomography. J. Formos. Med. Assoc. 2010, 109, 709–715. Available online: https://www.sciencedirect.com/science/article/pii/S0929664610601155 (accessed on 7 September 2024). [CrossRef]
- Longhini, A.B.; Branstetter, B.F.; Ferguson, B.J. Otolaryngologists’ perceptions of odontogenic maxillary sinusitis. Laryngoscope 2012, 122, 1910–1914. [Google Scholar] [CrossRef]
- Pokorny, A.; Tataryn, R. Clinical and radiologic findings in a case series of maxillary sinusitis of dental origin. Int. Forum Allergy Rhinol. 2013, 3, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.A.; Ferguson, B.J. Odontogenic sinusitis: An ancient but under-appreciated cause of maxillary sinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 24–28. [Google Scholar] [CrossRef]
- Vidal, F.; Coutinho, T.M.; Carvalho Ferreira Dde Souza RCde Gonçalves, L.S. Odontogenic sinusitis: A comprehensive review. Acta Odontol. Scand. 2017, 75, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Simuntis, R.; Kubilius, R.; Vaitkus, S. Odontogenic maxillary sinusitis: A review. Stomatologija 2014, 16, 39–43. [Google Scholar] [PubMed]
- Matsumoto, Y.; Ikeda, T.; Yokoi, H.; Kohno, N. Association between odontogenic infections and unilateral sinus opacification. Auris Nasus Larynx 2015, 42, 288–293. Available online: https://www.sciencedirect.com/science/article/pii/S0385814615000048 (accessed on 7 September 2024). [CrossRef] [PubMed]
- Troeltzsch, M.; Pache, C.; Troeltzsch, M.; Kaeppler, G.; Ehrenfeld, M.; Otto, S.; Probst, F. Etiology and clinical characteristics of symptomatic unilateral maxillary sinusitis: A review of 174 cases. J. Cranio-Maxillofac. Surg. 2015, 43, 1522–1529. Available online: https://www.sciencedirect.com/science/article/pii/S1010518215002474 (accessed on 10 September 2024). [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Ly, D.; Hellgren, J. Is dental evaluation considered in unilateral maxillary sinusitis? A retrospective case series. Acta Odontol. Scand. 2018, 76, 600–604. Available online: https://www.tandfonline.com/action/journalInformation?journalCode=iode20 (accessed on 2 October 2024). [CrossRef]
- Mehra, P.; Murad, H. Maxillary sinus disease of odontogenic origin. Otolaryngol. Clin. N. Am. 2004, 37, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Maloney, P.L.; Doku, H.C. Maxillary sinusitis of odontogenic origin. J. Can. Dent. Assoc. 1968, 34, 591. [Google Scholar]
- Maillet, M.; Bowles, W.R.; McClanahan, S.L.; John, M.T.; Ahmad, M. Cone-beam computed tomography evaluation of maxillary sinusitis. J. Endod. 2011, 37, 753–757. Available online: https://www.sciencedirect.com/science/article/pii/S0099239911002524 (accessed on 2 October 2024). [CrossRef]
- Kruse, C.; Spin-Neto, R.; Wenzel, A.; Kirkevang, L.L. Cone beam computed tomography and periapical lesions: A systematic review analysing studies on diagnostic efficacy by a hierarchical model. Int. Endod. J. 2015, 48, 815–828. [Google Scholar] [CrossRef]
- Shahbazian, M.; Vandewoude, C.; Wyatt, J.; Jacobs, R. Comparative assessment of periapical radiography and CBCT imaging for radiodiagnostics in the posterior maxilla. Odontology 2015, 103, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Campello, A.; Gonçalves, L.; Guedes, F.; Marques, F. Cone-beam computed tomography versus digital periapical radiography in the detection of artificially created periapical lesions: A pilot study of the diagnostic accuracy of endodontists using both techniques. Imaging Sci. Dent. 2017, 47, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Brown, J.; Semper, M.; Abella, F.; Mannocci, F. European society of endodontology position statement: Use of cone beam computed tomoraphy in endodontics. Int. Endod. J. 2019, 52, 1675–1678. [Google Scholar] [CrossRef]
- de Lima, C.O.; Devito, K.L.; Baraky Vasconcelos, L.R.; Prado Mdo Campos, C.N. Correlation between Endodontic Infection and Periodontal Disease and Their Association with Chronic Sinusitis: A Clinical-tomographic Study. J. Endod. 2017, 43, 1978–1983. [Google Scholar] [CrossRef]
- Brook, I. Sinusitis of odontogenic origin. Otolaryngol. Head Neck Surg. 2006, 135, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ok, E.; Güngör, E.; Colak, M.; Altunsoy, M.; Nur, B.G.; Ağlarci, O.S. Evaluation of the relationship between the maxillary posterior teeth and the sinus floor using cone-beam computed tomography. Surg. Radiol. Anat. 2014, 36, 907–914. [Google Scholar] [CrossRef]
- Sheikhi, M.; Pozve, N.J.; Khorrami, L. Using cone beam computed tomography to detect the relationship between the periodontal bone loss and mucosal thickening of the maxillary sinus. Dent. Res. J. 2014, 11, 495–501. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmc4163829/ (accessed on 15 October 2024).
- Vallo, J.; Suominen-Taipale, L.; Huumonen, S.; Soikkonen, K.; Norblad, A. Prevalence of mucosal abnormalities of the maxillary sinus and their relationship to dental disease in panoramic radiography: Results from the Health 2000 Health Examination Survey. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e80–e87. [Google Scholar] [CrossRef]
- Hoskison, E.; Daniel, M.; Rowson, J.E.; Jones, N.S. Evidence of an increase in the incidence of odontogenic sinusitis over the last decade in the UK. J. Laryngol. Otol. 2012, 126, 43–46. Available online: https://www.researchgate.net/publication/51657360 (accessed on 20 October 2024). [CrossRef]
- Costa, F.; Emanuelli, E.; Robiony, M.; Zerman, N.; Polini, F.; Politi, M. Endoscopic Surgical Treatment of Chronic Maxillary Sinusitis of Dental Origin. J. Oral Maxillofac. Surg. 2007, 65, 223–228. Available online: https://www.sciencedirect.com/science/article/pii/S0278239106013991 (accessed on 20 October 2024). [CrossRef]
- Akhlaghi, F.; Esmaeelinejad, M.; Safai, P. Etiologies and treatments of odontogenic maxillary sinusitis: A systematic review. Iran. Red Crescent Med. J. 2015, 17, e25536. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmc4706849 (accessed on 20 October 2024). [CrossRef]
- Badarne, O.; Koudstaal, M.J.; van Elswijk, J.F.; Wolvius, E.B. Odontogenic maxillary sinusitis based on overextension of root canal filling material. Ned. Tijdschr. Tandheelkd. 2012, 119, 480–483. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23126175 (accessed on 21 October 2024). [CrossRef]
- Lee, K.C.; Lee, S.J. Clinical features and treatments of odontogenic sinusitis. Yonsei Med. J. 2010, 51, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Longhini, A.B.; Branstetter, B.F.; Ferguson, B.J. Unrecognized odontogenic maxillary sinusitis: A cause of endoscopic sinus surgery failure. Am. J. Rhinol. Allergy 2010, 24, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Akhaddar, A.; Elasri, F.; Elouennass, M.; Mahi, M.; Elomari, N.; Elmostarchid, B.; Oubaaz, A.; Boucetta, M. Orbital abscess associated with sinusitis from odontogenic origin. Intern. Med. 2010, 49, 523–524. Available online: http://www.naika.or.jp/imindex.html (accessed on 21 October 2024). [CrossRef]
- Hoxworth, J.M.; Glastonbury, C.M. Orbital and intracranial complications of acute sinusitis. Neuroimaging Clin. N. Am. 2010, 20, 511–526. Available online: https://www.neuroimaging.theclinics.com/article/S1052-5149(10)00076-6/abstract (accessed on 24 October 2024). [CrossRef] [PubMed]
- Martines, F.; Salvago, P.; Ferrara, S.; Mucia, M.; Gambino, A.; Sireci, F. Parietal subdural empyema as complication of acute odontogenic sinusitis: A case report. J. Med. Case Rep. 2014, 8, 282. [Google Scholar] [CrossRef]
- Vestin Fredriksson, M.; Öhman, A.; Flygare, L.; Tano, K. When Maxillary Sinusitis Does Not Heal: Findings on CBCT Scans of the Sinuses with a Particular Focus on the Occurrence of Odontogenic Causes of Maxillary Sinusitis. Laryngoscope Investig. Otolaryngol. 2017, 2, 442–446. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Wasmer, J.; Sendi, P.; Janner, S.F.M.; Buser, D.; Von Arx, T. Characteristics and dimensions of the Schneiderian membrane and apical bone in maxillary molars referred for apical surgery: A comparative radiographic analysis using limited cone beam computed tomography. J. Endod. 2012, 38, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Nurbakhsh, B.; Friedman, S.; Kulkarni, G.V.; Basrani, B.; Lam, E. Resolution of maxillary sinus mucositis after endodontic treatment of maxillary teeth with apical periodontitis: A cone-beam computed tomography pilot study. J. Endod. 2011, 37, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitis. Rhinology 1993, 31, 183. [Google Scholar] [PubMed]
- Little, R.E.; Long, C.M.; Loehrl, T.A.; Poetker, D.M. Odontogenic sinusitis: A review of the current literature. Laryngoscope Investig. Otolaryngol. 2018, 3, 110–114. [Google Scholar] [CrossRef]
- Souza-Nunes, L.A.; Verner, L.S.; Rosado, L.P.L.; Aquino, S.N.; Carvalho, A.C.P.; Junqueira, R.B. Periapical and endodontic status scale for endodontically treated teeth and their association with maxillary sinus abnormalities: A cone-beam computed tomographic study. J. Endod. 2019, 45, 1479–1488. [Google Scholar] [CrossRef]
- Psillas, G.; Papaioannou, D.; Petsali, S.; Dimas, G.G.; Constantinidis, J.J. Odontogenic maxillary sinusitis: A comprehensive review. J. Dent. Sci. 2021, 16, 474–481. [Google Scholar] [CrossRef]
- Lima, A.D.; Benetti, F.; Ferreira, L.L.; Dezan-Júnior, E.; Gomes-Filho, J.E.; Cintra, L.T.A. Endodontic applications of cone-beam computed tomography. Braz. J. Surg. Clin. Res. 2014, 6, 30–39. [Google Scholar]
- Ponce, J.B.; Guimarães, B.M.; Pinto, L.C.; NIshiyama, C.K.; Almeida, A.L.P.F. Tamanho do voxel no diagnóstico tomográfico em endodontia. Salusvita 2014, 33, 257–267. [Google Scholar]
- Hopkins, C.; Browne, J.P.; Slack, R.; Lund, V.; Brown, P. The Lund-Mackay staging system for chronic rhinosinisitis: How is it used and what does it predict? Otolaryngol. Head Neck Surg. 2007, 137, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.J.M.; Junqueira, J.L.C.; Rodrigues, C.D.; Santos, G.N.M.; Martinez, C.R.; Panzarella, F.K. Dental maxillary sinus pathology: A CBCT-based case-control study. Odontology 2025, 113, 1269–1277. [Google Scholar] [CrossRef] [PubMed]


| Gender | Male | Female | ||
| 38% | 62% | |||
| Age (years/SD) | 54.66/12.02 | |||
| years old | ||||
| Maxillary sinus | Right side | Left side | ||
| Mucositis | Sinusitis | Mucositis | Sinusitis | |
| (22.03%) | (35.59%) | (10.76%) | (55.38%) |
| IP1 * | IP2 ** | IP3 ** | |
|---|---|---|---|
| MUCOSITIS | 7.25% | 4.03% | 2.41% |
| SINUSITIS | 9.67% | 20.16% | 12.09% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutinho, T.; Gonçalves, L.; Marceliano-Alves, M.F.V.; Figueiredo, V.R.; Lima Junior, J.d.C.; Peres, R.V.; Vidal, F. Apical Periodontitis and Maxillary Sinus Alterations: Results of an Exploratory Cross-Sectional Tomographic In Vivo Study. Sinusitis 2025, 9, 16. https://doi.org/10.3390/sinusitis9020016
Coutinho T, Gonçalves L, Marceliano-Alves MFV, Figueiredo VR, Lima Junior JdC, Peres RV, Vidal F. Apical Periodontitis and Maxillary Sinus Alterations: Results of an Exploratory Cross-Sectional Tomographic In Vivo Study. Sinusitis. 2025; 9(2):16. https://doi.org/10.3390/sinusitis9020016
Chicago/Turabian StyleCoutinho, Thaïs, Lucio Gonçalves, Marilia Fagury Videira Marceliano-Alves, Vivian Ronquete Figueiredo, Josué da Costa Lima Junior, Rafael Vidal Peres, and Fábio Vidal. 2025. "Apical Periodontitis and Maxillary Sinus Alterations: Results of an Exploratory Cross-Sectional Tomographic In Vivo Study" Sinusitis 9, no. 2: 16. https://doi.org/10.3390/sinusitis9020016
APA StyleCoutinho, T., Gonçalves, L., Marceliano-Alves, M. F. V., Figueiredo, V. R., Lima Junior, J. d. C., Peres, R. V., & Vidal, F. (2025). Apical Periodontitis and Maxillary Sinus Alterations: Results of an Exploratory Cross-Sectional Tomographic In Vivo Study. Sinusitis, 9(2), 16. https://doi.org/10.3390/sinusitis9020016






