1. Introduction
Allergic fungal rhinosinusitis (AFRS) is a non-invasive fungal sinusitis associated with chronic non-IgE-mediated allergic mucosal inflammation [
1,
2,
3]. It is a subtype of eosinophilic Type 2 chronic rhinosinusitis (CRS), where inflammation results from the activation of innate immune cells, with eosinophilic chemotaxis acting in response to extra-mucosal fungi, rather than an IgE-mediated reaction [
3,
4,
5]. AFRS constitutes up to 72% of fungal rhinosinusitis cases, occurring in immunocompetent individuals [
1,
4,
6,
7].
Diagnosing AFRS relies on the Bent–Kuhn criteria, where nasal polyposis, extra-mucosal fungal hyphae, eosinophilic mucin, Type 1 hypersensitivity to fungi, and characteristic radiological findings encompass the major criteria [
1,
5]. This criterion is limited as only 90% of patients have IgE-mediated hypersensitivity to fungal antigens. Additional features include bony erosion, Charcot–Leyden crystals, peripheral eosinophilia, disease unilaterality, immunocompetence, and atopy [
1]. The clinical presentation may mimic other unilateral sinonasal masses, such as juvenile nasopharyngeal angiofibroma (JNA), particularly in cases of peri-adolescent males presenting with recurrent epistaxis. This case examines an 11-year-old male who presented with a unilateral sinonasal mass.
2. Detailed Case Description
An 11-year-old male presented to the Otorhinolaryngology outpatient clinic at Tygerberg Hospital with a 1-year history of left-sided nasal obstruction, hemifacial asymmetry, and recurrent self-aborting epistaxis. There was no significant medical history, including no mucopurulent nasal discharge, facial pain, or cough; no exposure to environmental allergens; and no family history of atopy.
On examination, he was a healthy child with facial asymmetry, including left pre-maxillary fullness and proptosis, but preserved visual acuity, pupil reactivity, and extra-ocular muscle function. Anterior rhinoscopy revealed nasal polyps filling the left nasal cavity, deviating the nasal septum to the right lateral nasal wall.
Due to the slowly progressive nature, patient age, and history of epistaxis, there was concern for a JNA. Imaging was therefore performed to investigate the nature of the lesion prior to tissue sampling due to risk of excessive bleeding, should it be a highly vascular tumour.
2.1. Imaging
Computed tomography (CT) imaging confirmed unilateral sinonasal opacification with bone expansion and remodelling, as well as left cribriform plate and lamina papyracea dehiscence—see
Figure 1. Axial CT images showed symmetry of the pterygopalatine fossa bilaterally—see
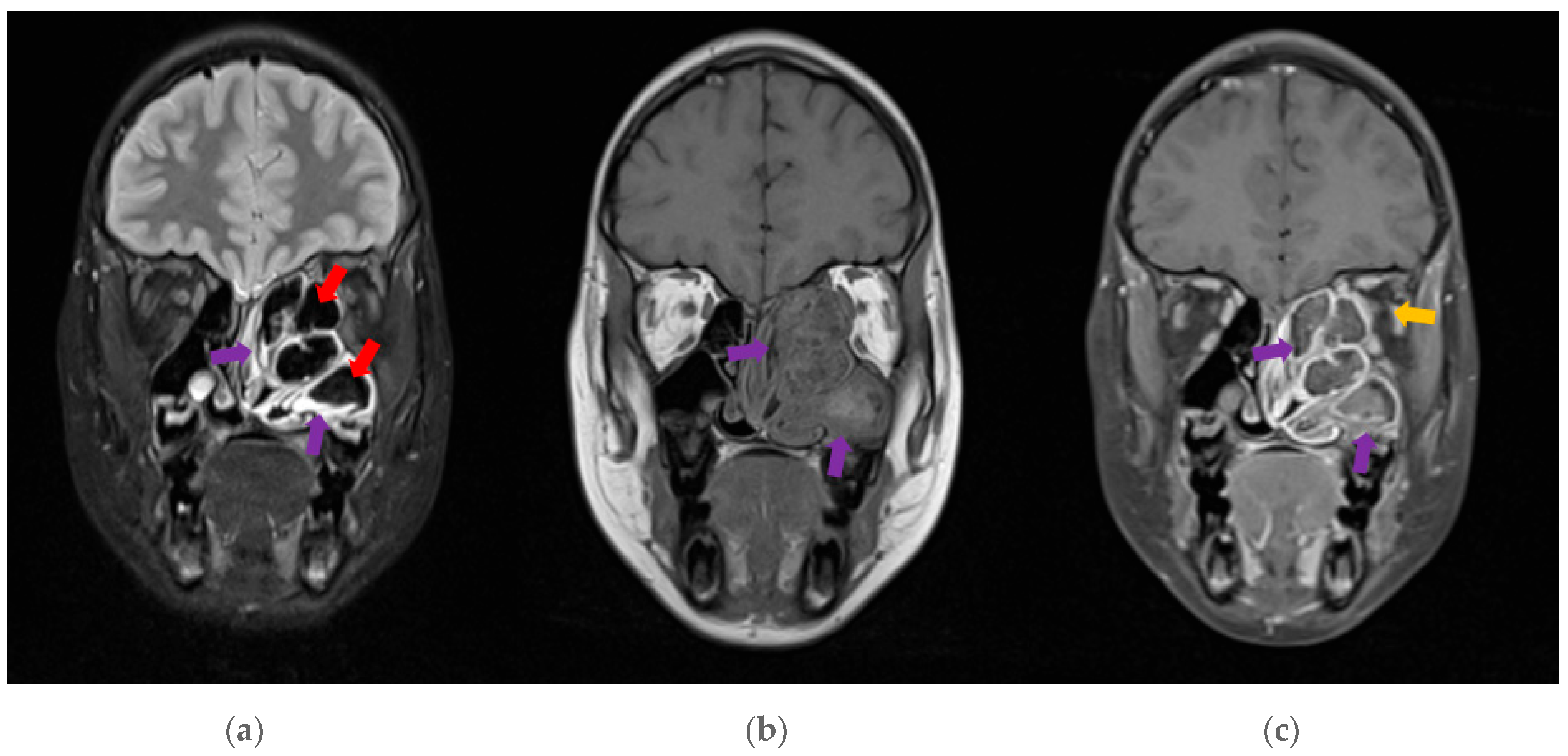
Figure 2. A subsequent MRI revealed typical features of AFRS—see
Figure 3. These findings, alongside the clinical presentation guided the decision for surgical intervention.
2.2. Management and Outcome
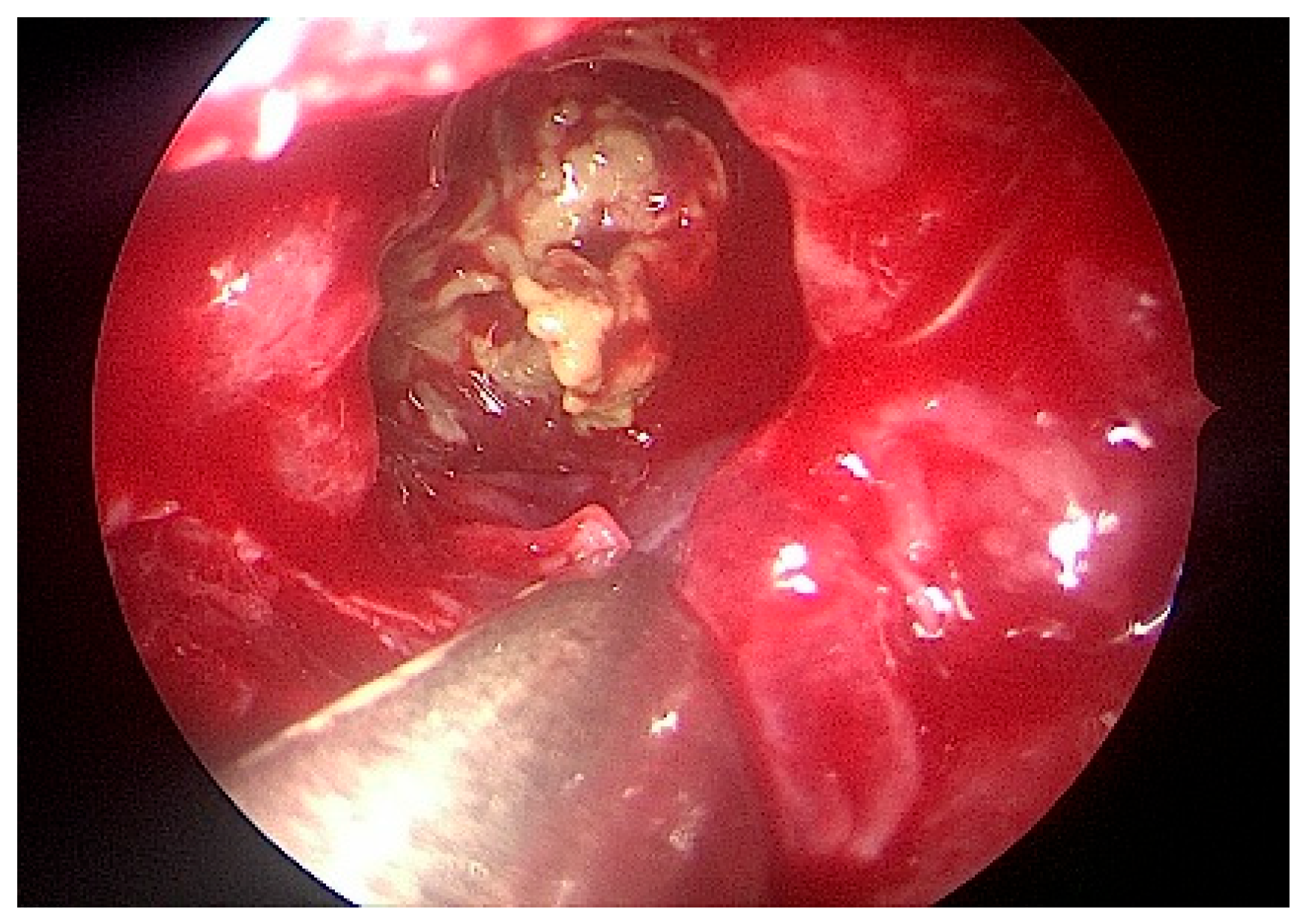
The patient underwent left-sided endoscopic sinus surgery, where copious inspissated allergic mucin was identified (
Figure 4 and
Figure 5). A histopathologic examination of the polyps confirmed eosinophilic polypoid respiratory mucosa with Charcot–Leyden crystals and non-invasive Aspergillus and Candida species on histochemical staining. These confirmed the diagnosis of allergic fungal rhinosinusitis. The patient was initiated on systemic corticosteroids and intranasal corticosteroids in saline rinses to manage chronic inflammation. Postoperative follow-up showed the resolution of nasal obstruction and rhinorrhoea, as well as improved facial symmetry. Although skin prick testing excluded hypersensitivity to fungal antigens, the clinical and histological findings were definitive for AFRS.
3. Discussion
Nasal polyps in children are rare, with a prevalence of 0.1% in individuals under the age of 10 [
2]. Their presence should encourage a thorough evaluation to exclude midline congenital skull-base defects, benign tumours, cystic fibrosis, and primary ciliary dyskinesia [
2,
8].
AFRS is a subtype of chronic rhinosinusitis, with nasal polyps (CRSwNP) being associated with Type 2 inflammation. The aetiology of CRSwNP is multifactorial, involving an impaired interaction between environmental factors including fungi, mucosal barrier breakdown, and chronic inflammation. This chronic inflammatory pattern is categorised into 2 endotypes—Type 2 and non-Type 2 [
9].
Type 2 inflammation involves specific cytokines IL4, IL5, IL13, and IgE, which initiate the infiltration of eosinophils and mast cells into the sinonasal mucosa [
1,
9]. This leads to goblet cell hyperplasia, epithelial barrier disruption, and fibrin deposition, producing polyps [
1]. Individuals with Type 2 CRS have a higher propensity for resistance to standard treatments and exhibit polyp-related phenotypes [
1].
AFRS has a higher incidence and earlier age onset in warmer climates [
4,
5,
6]. The American literature reports a mean onset age of 13–21.9 years [
5], whereas the European literature reports an older age of 37.88–45.71 years [
6]. There is male predominance in younger patients, shifting to female predominance in adults [
5]. Although this patient was a male, he falls outside of this traditionally described age group for AFRS.
Radiographic imaging aids in diagnosis and surgical planning. Non-contrast CT scans show hyperdensities in the sinuses with bony expansion, and unilaterality in 70% of paediatric cases [
1,
5,
7]. The hyperdensities result from the calcium, iron, magnesium, and manganese that are found in fungal deposits [
1,
7]. Gadolinium-contrasted MRI is useful in assessing intracranial or intra-orbital extension and may show characteristic hypo-intensities from allergic mucin on T2W sequences [
1,
7].
The management of AFRS is primarily surgical [
1,
5,
6]. Surgery clears allergic mucin containing the inciting fungal debris and opens sinus drainage pathways while preserving normal tissue to maintain mucociliary clearance [
1,
5]. Medical treatments aim to suppress eosinophilic inflammation, utilising oral corticosteroids followed by intranasal saline douches with topical corticosteroids [
6]. Long-term management may include both corticosteroids and immunotherapy, although the role of immunotherapy in AFRS requires further research [
1,
5,
6].
This case underscores the importance of comprehensive diagnostic evaluation for sinonasal masses in paediatric patients, emphasising the need to consider AFRS as a differential in younger age groups.
Author Contributions
Conceptualization: T.K.S. and S.E.A.; investigation and research: T.K.S.; writing—original draft preparation: T.K.S.; writing—evaluation, selection, and description of radiological images: U.L., R.D. and L.J.v.R.; writing—review and editing: T.K.S., S.E.A. and J.G.; supervision: S.E.A. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Health Research Ethics Committee of Stellenbosch University (protocol code: C24/06/021; date of approval: 11 July 2024).
Informed Consent Statement
Written informed consent has been obtained from the patient’s mother, and assent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The authors declare that they have no financial interests relating to this article.
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salami, S.; Bernal-Sprekelesen, M.; Mullol, J.; Alobid, I.; et al. Epos 2020. Rhinology 2020, 58, 82–111. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, E.; Santarsiero, S.; Marini, G.; Majo, F.; De Vincentiis, M.; Cristalli, G.; Artesani, M.C.; Fiocchi, A.G. Endotypes of Nasal Polyps in Children: A Multidisciplinary Approach. J. Pers. Med. 2023, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Olivier, C.E. Allergic Sinusitis, Allergic Migraine, and Sinus Headaches. Online J. Otolaryngol. Rhinol. 2024, 7, 652. [Google Scholar] [CrossRef]
- Mancuso, R.F. Pediatric allergic fungal sinusitis: A clinical review. Pediatr. Ann. 2021, 50, e297–e303. [Google Scholar] [CrossRef] [PubMed]
- Thorp, B.D.; McKinney, K.A.; Rose, A.S.; Ebert, C.S. Allergic Fungal Sinusitis in Children. Otolaryngol. Clin. N. Am. 2012, 45, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, M.; Stryjewska-Makuch, G.; Kantczak, A.; Górny, D.; Glück, J. Allergic Fungal Rhinosinusitis in Europe: Literature Review and Own Experience. Int. Arch. Allergy Immunol. 2023, 184, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. Fungal Rhinosinusitis: Unravelling the Disease Spectrum. J. Maxillofac. Oral Surg. 2019, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Schramm, V.L.; Effron, M.Z. Nasal polyps in children. Laryngoscope 1980, 90, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Peters, A.T.; Stevens, W.W.; Schleimer, R.P.; Tan, B.K.; Kern, R.C. Endotypes of chronic rhinosinusitis: Relationships to disease phenotypes, pathogenesis, clinical findings, and treatment approaches. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 812–826. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).